Summary
Action potential propagation along myelinated nerve fibers requires high-density protein complexes that include voltage-gated Na+ channels at the nodes of Ranvier. Several complementary mechanisms may be involved in node assembly including: 1) interaction of nodal cell adhesion molecules with the extracellular matrix; 2) restriction of membrane protein mobility by paranodal junctions; and 3) stabilization of ion channel clusters by axonal cytoskeletal scaffolds. In the peripheral nervous system, a secreted glial protein at the nodal extracellular matrix interacts with axonal cell adhesion molecules to initiate node formation. In the central nervous system, both glial soluble factors and paranodal axoglial junctions may function in a complementary manner to contribute to node formation.
Introduction
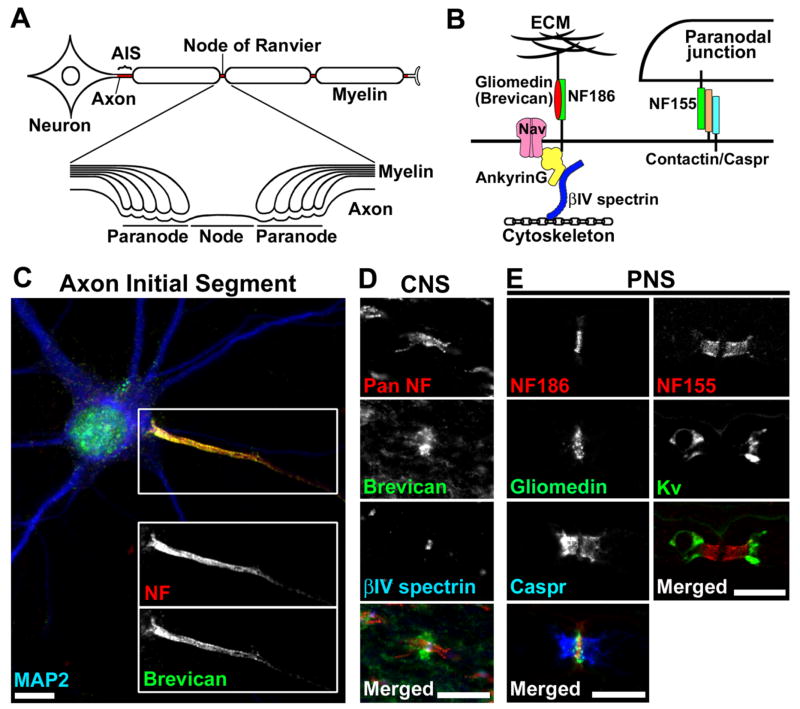
In vertebrate nervous systems, a highly integrated network of neurons and glial cells works together to transmit information throughout the entire body. One characteristic structure of neurons is their long process called the axon. The axon transmits information as an electrical impulse and transports cytoplasmic materials. Axons are often ensheathed along their entire length by myelin, a multilamellar lipid rich structure that is produced by Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS) [1]. Myelin increases membrane resistance and decreases membrane capacitance, thereby conserving ionic charge as the axolemma is depolarized during action potential propagation. Voltage-gated Na+ (Nav) channels are highly accumulated at the axon initial segment (AIS), an approximately 20–40 μm long segment devoid of myelin and adjacent to the cell soma (Figs. 1A and 1C) [2]. These channels are required for action potential generation. Clusters of Nav channels are also located at the nodes of Ranvier: ~1 μm long gaps between two adjacent myelin segments (Figs. 1A and 1B) [3,4]. Nodes regenerate the action potential as it is conducted along the axon. Thus, the myelinated nerve fibers enable rapid and efficient action potential propagation over long distances from neuronal cell soma to target cells and tissues.
Figure 1.
(A) Cartoon illustrating the structures of the myelinated nerve fiber and the specific subdomains: AIS, nodes of Ranvier, and paranodes.
(B) Schematic presentation showing molecular organization at nodes and paranodes. At nodes, gliomedin (PNS) and brevican (CNS) are present at the ECM, and interact with NF186 at the nodal axolemma. A scaffolding protein ankyrinG interacts with NF186, Nav channels, and the cytoskeletal protein βIV spectrin. At paranodes, axonal Caspr and contactin, and glial NF155 form a tripartite CAM complex, and mediate assembly of the septate-like junctions between the myelin sheath and the axon.
(C) NF (red) and brevican (green) are highly accumulated at the AIS in cultured rat hippocampal neurons. The somatodentritic domain is characterized by the staining of microtubule-associated protein 2 (MAP2; blue). Scale bar = 10 μm.
(D) Pan NF antibody (red) labels both node and paranode in rat optic nerve. Brevican (green) is localized at and around nodes of Ranvier in the ECM. Nodes are shown in blue by anti-βIV spectrin antibody. Scale bar = 10 μm.
(E) Nodal and paranodal components in the PNS. Left column shows rat sciatic nerve stained by antibodies to NF186 (red), gliomedin (green), and Caspr (blue). The short gap between two Caspr clusters is filled with nodal NF186 staining. Note that the gliomedin staining extends outside of the nodal NF186 staining. The right column shows mouse ventral root stained by antibodies to NF155 (red) and Kv channels (green). Note the nodal gap between the two paranodal NF155 clusters. Scale bars = 10 μm.
How do neurons and glia interact to establish such a complicated structure? Recent data demonstrate that in the PNS, 1) interactions between nodal axonal cell adhesion molecules (CAMs) and the glial-derived extracellular matrix (ECM) mediates initial Nav channel clustering; then the Nav channel clusters are further stabilized by 2) restriction of their lateral mobility by paranodal junctions; and 3) links to the axonal cytoskeletal scaffolds (Fig. 2A). In the CNS, however, much less is known about how node formation is initiated. Similar to the PNS, glial-derived soluble factors may promote node formation in the CNS, but recent studies indicate that paranodal junctions may also be able to initiate CNS node formation. In this review, we highlight complementary mechanisms mediated by CAMs and their ECM ligands that can initiate node formation.
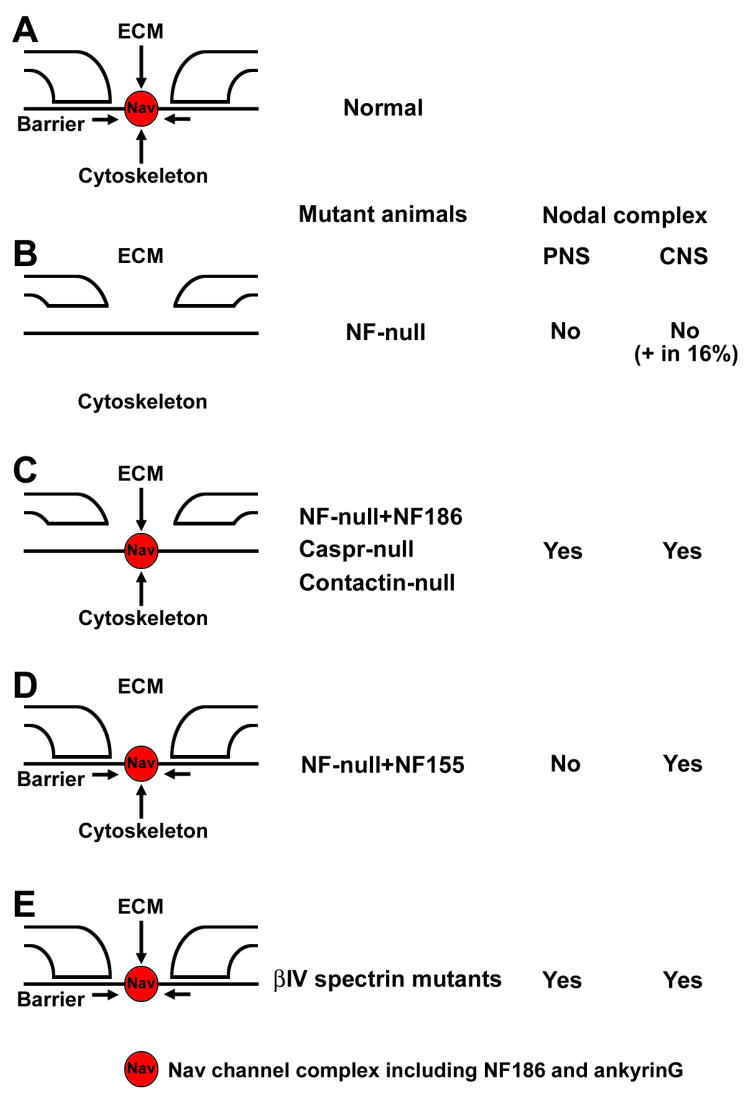
Figure 2. Schematic representation of nodal or paranodal disruption in mutant animals.
(A) Three mechanisms, interaction between the nodal ECM, a paranodal diffusion barrier, and the axonal cytoskeleton, are proposed to be involved in formation or maintenance of nodal Nav channel complexes under normal conditions.
(B) By disruption of NF gene, nodal and paranodal molecules are absent [20••]. However, small numbers (16%) of CNS nodal Nav channel clusters persist.
(C) Paranodal junctions do not assemble without glial NF155 [20••], or axonal Caspr [24,25] or contactin [26]. Nodal proteins still cluster in both PNS and CNS.
(D) Paranodal junctions are reconstituted in NF-null mice with transgenic NF155 [20••]. Nodal components are rescued in the CNS, but not in the PNS.
(E) Interactions with the axonal cytoskeleton are disrupted in βIV spectrin mutant animals [29–31]. Nav channel clusters are still present, despite reduced numbers or a change in shape.
Myelinated nerve fibers have distinct domains
Myelinated axons are divided into AIS, nodes, paranodes, juxtaparanodes, and internodes (Fig. 1). The AIS and the nodes have a similar molecular composition. In addition to Nav channels, molecular components at these sites include voltage-gated K+ (Kv) channels, and the CAMs neurofascin (NF)-186 and neuron glia-related CAM (NrCAM) [3,4]. At the paranodes flanking both sides of the nodes, the axonal contactin-associated protein (Caspr) and contactin, and the glial 155 kDa isoform of NF forms a tripartite CAM complex, and mediates formation of the septate-like junctions between myelin and the axon [3,4]. The juxtaparanodes begin at the innermost axo-glial junction of the paranodes and extend for 5–15 μm. Juxtaparanodes have high densities of Kv channels and the CAMs Caspr2 and Tag1 [3,4]. In addition to ion channels and CAMs, several classes of scaffolding and cytoskeletal proteins including ankyrins, spectrins, 4.1 proteins, and PDZ-domain containing proteins are highly enriched at the AIS, nodes, paranodes, and juxtaparanodes and interact with CAMs or ion channels [5]. For example, nodes are highly enriched in ankyrinG and βIV spectrin, paranodes enriched in ankyrinB, βII spectrin and protein 4.1B, and juxtaparanodes enriched in PSD-95 and protein 4.1B. Finally, internodes are the intervening fiber segments between two juxtaparanodes under a single compact myelin sheath. At the internodes, the axolemma is closely apposed to the glial adaxonal membrane. Two recent studies [6,7] demonstrate that the nectin-like (Necl) CAMs Necl1 (axon) and Necl4 (Schwann cell) at the internodes, mediate a specific axon-glial interaction to initiate PNS myelination.
The Glial ECM molecule gliomedin mediates initial NF186 clustering in PNS
How are Nav channel complexes assembled at nodes? Recent studies [8,9••] suggest that interactions between an ECM molecule produced by glial cells and axonal CAMs provide a critical mechanism for initiation of node formation. In the PNS, the nodal axolemma is contacted by microvilli that project from the outer collar of myelinating Schwann cells. The microvilli are embedded in a specialized ECM around nodes containing proteoglycans syndecan-3 [10], V1 isoform of versican [10], and NG2 [11]. Dystroglycan is also abundantly expressed at PNS nodes, and its specific ablation in Schwann cells results in the disorganization of the microvilli and a marked reduction in nodal Nav channels [12]. A glial ligand for NF and NrCAM was recently identified and termed gliomedin [8]. Gliomedin is expressed by myelinating Schwann cells and accumulates at the Schwann cell microvilli and surrounding nodal ECM (Fig. 1E). At the perinodal space, the majority of gliomedin exists as a secreted protein that is cleaved from the cell surface by a furin protease [9••, 13]. After cleavage, gliomedin assembles into high-molecular weight multimers, and is incorporated into the Schwann cell ECM by binding to heparan sulfate proteoglycans. The olfactomedin domain of gliomedin mediates its interaction with NF186 and NrCAM.
The ability of gliomedin to promote nodal molecule clustering was clearly demonstrated by knockdown and clustering experiments using myelinated Schwann cell-dorsal root ganglion (DRG) co-cultures. By adding a soluble extracellular domain of NF186 to myelinating cultures, gliomedin was aberrantly distributed along the internodes and node formation was inhibited [8, 14]. Similarly, silencing gliomedin expression by RNA interference disturbed Nav channel and NF186 clustering at the axolemma. Furthermore, a soluble olfactomedin domain of gliomedin induced NF186 clustering and assembled node-like clusters containing Nav channels, ankyrinG, and βIV spectrin in the absence of Schwann cells. In agreement, the ectodomain of NF186 (immunoglobulin domain) is necessary for its localization to the PNS node [15••]. These results suggest that gliomedin first positions NF186, then NF186 recruits Nav channels and other nodal molecules.
NF186 assembles the nodal complex by recruiting ankyrinG
How does NF186 promote node formation? NF is a member of the L1 subgroup of the immunoglobulin superfamily. NF has six immunoglobulin domains, several fibronectin type III repeats, a single transmembrane region and a cytoplasmic domain. There are two alternatively spliced products of the NF gene, NF186 at the nodal axon and NF155 at the paranodal glia. The mature neuronal form NF186 is dramatically upregulated in E16 as axons are ensheathed, whereas NF155 expression in the Schwann cells coincides with the onset of PNS myelination [16•]. NF186 binds the nodal Nav channel binding and scaffolding protein ankyrinG via its cytoplasmic FIGQY motif [17] (see below). NF186 may also interact with Nav channel β subunits [18].
The essential role of NF186 for node assembly was demonstrated by production of NF mutant mice [19,20••]. NF-null mice express neither NF186 nor NF155, resulting in loss of both nodal interactions with gliomedin and the disruption of paranodal axo-glial junctions (Fig. 2B). Consequently, the mutant mice fail to accumulate both nodal and paranodal molecules. The role of individual NF isoforms was further examined through a transgenic approach. The expression of transgenic NF186 on a NF-null background (i.e. no paranodal NF155 but preserved nodal NF186) rescued clustering of nodal components, but did not reconstitute the paranodal junctions (Fig. 2C). Furthermore, Nav channels were not clustered at PNS nodes by transgenic expression of NF155 on a NF-null background (i.e. no nodal NF186 but preserved paranodal NF155), despite rescue of the paranodal junction (Fig. 2D). In contrast, in the absence of NrCAM, Nav channels can still cluster at PNS nodes despite a significant delay [21], suggesting that NF186 has more critical roles than NrCAM for PNS node assembly. Consistent with this idea, ankyrinG and Nav channels failed to cluster at the nodes in NF186 shRNA-treated DRG neurons in myelinating cultures [15••]. Furthermore, knockdown of NF186 combined with expression of NF186 without its ankyrin-binding domain also disrupted nodal assembly in myelinating cultures. Thus, NF186 is critical for node assembly and recruits ankyrinG, which in turn is required for accumulation of the nodal protein complex including Nav channels.
NF155 is required for paranodal junction assembly and contributes to CNS node formation
In the CNS, some data indicate that paranodal junctions are important in the initial clustering of Nav channels [22,23]. In contrast, data from paranodal mutant mice suggested that these junctions are dispensable for node assembly. For example, Nav channels accumulate at nodes in NF-null mice rescued with transgenic NF186 (no reconstitution of the paranodal junctions) [20••]. Similarly, in mutant mice without axonal Caspr [24,25] or contactin [26], Nav channels still accumulated at nodes (Fig. 2C), emphasizing the importance of paranode independent Nav channel clustering mechanisms. Finally, in UDP-galactose/ceramide galactosyltransferase (CGT)-deficient mice, which also show disrupted paranodal axoglial junctions, Nav channels can cluster at nodes but these become more diffuse and less concentrated with time [27]. Nevertheless, paranodes may function as a diffusion barrier to restrict the mobility of membrane molecules that may be associated with stabilization of nodal Nav channels or CAMs [25]. In addition, in support of a role for paranodal junctions in the assembly of CNS nodes, Zonta et al., [20••] reconstituted paranodes by transgenic expression of glial NF155 on a NF-null background. They found that this rescued the correct assembly of CNS nodes of Ranvier in the absence of NF186 [20••], but PNS nodes were not rescued [19] (Fig. 2D). Thus, paranodal junctions can also facilitate the assembly of Nav channel clusters at CNS nodes. This study convincingly demonstrated that glial NF155 is essential for the assembly of the paranodal axoglial junction [20••]. The expression of transgenic NF186 on a NF-null background (i.e. no paranodal NF155 but preserved nodal NF186) was not associated with recruitment of axonal Caspr or contactin (Fig. 2C). Consistent with this, knockdown of NF155 in Schwann cells inhibits Caspr clustering when co-cultured with DRG neurons [16•]. In contrast, NF155 was still expressed at glial paranodes in mutants of paranodal axonal CAMs, Caspr [24] or contactin [26]. These results show that NF155 is required for paranodal junctions and that these junctions can initiate CNS node formation.
AnkyrinG recruits βIV spectrin for further nodal stabilization
The organization of nodes depends on a specialized cytoskeleton consisting of two submembranous cytoskeletal and scaffolding proteins, ankyrinG and βIV spectrin [5]. Ankyrins are large scaffolding proteins that link the spectrin-based membrane skeleton to various membrane proteins including Nav channels and NF186. AnkyrinG is recruited to nodes by NF186, and plays a central role in organizing the Nav channel complex [15••]. Knockdown of ankyrinG in DRG neurons eliminated the great majority of Nav channels, NF186, NrCAM, and βIV spectrin at nodes in myelinating co-culture. Furthermore, neurons transfected with an NF186 construct lacking the ankyrin-binding domain show accelerated protein turnover of the introduced NF186. The interactions between ankyrinG and NF186 may promote their stable and restricted localization at nodes. AnkyrinG recruits βIV spectrin to nodes, which in turn provides further linkage to the axonal cytoskeleton [28•]. Localization of βIV spectrin to CNS nodes depends on binding to ankyrinG via spectrin repeat 15. In βIV spectrin mutant mice, Nav channel clusters are still present at the nodes, but the density is reduced and the shape of clusters is changed [29–31] (Fig. 2E). Thus, the βIV spectrin and ankyrinG based axonal cytoskeleton are important for further stabilization of nodal Nav channel complexes rather than initiation of clustering.
NF186 assembles a specialized ECM at the AIS
Observations on the mechanism of AIS formation may provide some clues about how CNS nodes form because of their similar molecular organization. However, compared to the nodes, the AIS is intrinsically organized by neurons without the requirement of glial cells [2,15••]. At the AIS, NF186 and ankyrinG have different roles for protein complex assembly. Silencing expression of each AIS protein in cultured hippocampal neuron by RNA interference revealed that ankyrinG was required for Nav channel clustering and the molecular assembly of the AIS [32•]. In contrast, both NF186 and NrCAM were dispensible for ion channel clustering [15••,32•]. Consistent with these data in culture, loss of ankyrinG, but not NF186, inhibits AIS assembly in vivo [32•,33]. Then, what is the function of NF186 at the AIS? NF186, but not NrCAM, interacts with the chondroitin sulfate proteoglycan brevican that forms a specialized ECM around the AIS [32•]. Silencing NF186, but not NrCAM, resulted in loss of brevican from the AIS in both culture and in vivo. In addition, NF186 overexpression caused ectopic brevican clustering at neuronal cell soma and dendrites in culture. These results suggest that NF186 assembles and links a specialized brevican-based ECM to the intracellular cytoskeleton at the AIS. Although the function of the brevican-based AIS ECM is unknown, it suggests that a similar ECM may exist at CNS nodes, and that by analogy with gliomedin, brevican may contribute to CNS node assembly.
Model for PNS node formation
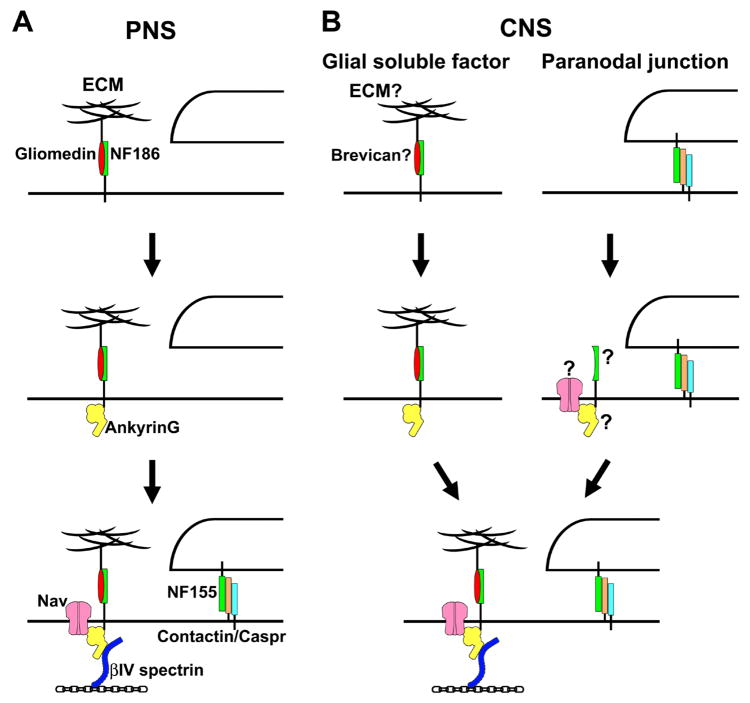
Node of Ranvier formation requires extrinsic interactions supplied by myelinating glia [2,15••]. Development of PNS nodes as well as myelin is better understood than in the CNS (Fig. 3A), because the system is much more tractable and in vitro models such as myelinating DRG neuron co-cultures allow a dissection of the molecular mechanisms. In developing rat sciatic nerves, clusters of Nav channels and gliomedin appear at the edge of elongating Schwann cells [8,34,35]. Clustering of NF and NrCAM precedes that of ankyrinG and Nav channels in the myelinating sciatic nerve [36]. At these sites, binding of gliomedin to NF186 and NrCAM causes their initial clustering. In contrast, NF186 is cleared from the internode by interactions between its ectodomain and myelinating Schwann cells [15••]. NF186 is thought to act as pioneer molecule, serving as a nucleation site for recruitment of ankyrinG [2,15••,37]. Nav channels bind to ankyrinG and mediate the currents necessaryfor action potential propagation. Caspr accumulates at the paranodes shortly after gliomedin, ankyrinG, and Nav channels cluster at the nodes [8]. Similarly, clustering of nodal NF186 precedes accumulation of NF155 at paranodes [35]. Paranodal junctions act as a diffusion barrier to restrict mobility of the complex for further stabilization. Finally, βIV spectrin is recruited to the initial clusters by ankyrinG, and links them to axon cytoskeleton [28•].
Figure 3.
(A) Current model for formation of PNS nodes. Schwann cell derived gliomedin is incorporated into the nodal ECM, and initiates NF186 clustering. NF186 recruits ankyrinG. Then, Nav channels bind to ankyrinG. βIV spectrin is recruited to nodes by binding to ankyrinG, and links the complex to the axon cytoskeleton. Nodal complexes are further stabilized by paranodal junctions formed by glial NF155 and axonal Caspr and contactin.
(B) Two possible mechanisms for initiation of CNS node formation. Soluble factors secreted by oligodendrocytes may initiate nodal assembly. A possible candidate is brevican in the ECM since it can interact with NF186. Paranodal junctions may also be able to initiate CNS node formation. It remains unclear which molecule, CAM, Nav channels, or ankyrinG, is directly restricted by paranodes.
Models for CNS node formation
CNS node formation is poorly understood because of the lack of well-developed in vitro models for the CNS nodes and because of the relatively few proteins identified at these sites. As described above, CNS paranodal junctions may play more important roles for node assembly than in the PNS (Fig. 3B). In particular, in the CNS, paranodal proteins appear to cluster before localization of nodal proteins [23,38]. The reconstitution of paranodal complexes in NF-null mice could initiate the correct assembly of nodal Nav channel clusters in the CNS [20••], but not in the PNS [19] (Fig 2D). How paranodal junctions promote nodal clustering remains unclear. Paranodal junctions may restrict the NF186 or some unknown CAM, or Nav channels, and then ankyrinG may be recruited. Alternatively, the spectrin-based cytoskeleton at paranodes [5] may restrict the localization of ankyrinG, which recruits Nav channels. Nevertheless, even without paranodal junctions, nodal molecules can still cluster at CNS nodes (Fig 2C). Thus, other compensatory mechanisms must also exist in the CNS.
It has been reported that, similar to gliomedin in the PNS [8], yet to be identified soluble factors secreted by oligodendrocytes promote Nav channel clustering in CNS axons in culture in the absence of axoglial contact [39]. Thus, soluble glial factors may be able to promote CNS node formation, although gliomedin is not present at CNS nodes [8]. One possibility is that a specialized ECM surrounding CNS nodes including brevican may function in a similar wayto gliomedin in the PNS to initiate CNS node formation. Indeed, brevican is found in the ECM surrounding CNS nodes as well as at the AIS, and interacts with NF186 [32•] (Fig 1C and 1D). In addition, there are several other ECM molecules at CNS nodes including tenascin-R [40], oligodendrocyte-myelin glycoprotein (OMgp) [41,42], V2 isoform of versican [10], and a brain-specific hyaluronan-binding protein, Bral1 [43]. However, the mutant mice lacking brevican [44] or tenascin-R [40] show no apparent neurological phenotype. At CNS nodes from tenascin-R-deficient mice, there was no apparent change in expression or distribution of Nav channels [40]. OMgp-null mice did not exhibit obvious behavioral abnormalities, although CNS nodes were abnormally elongated [41]. Other OMgp mutant mice demonstrated that Nav channels still cluster at CNS nodes, despite hypo-myelination and disorganized nodal and paranodal architectures [42]. The presence of multiple different ECM proteins at CNS nodes suggests that it may be necessary to generate knockouts for multiple nodal ECM proteins to determine if they contribute to CNS node formation. Otherwise, nodal ECM in the CNS may act to stabilize Nav channel complexes rather than initiate their assembly. In developing optic nerves, OMgp staining appears after Nav channel clustering [42]. Alternatively, collateral axonal sprouting at nodes was observed in OMgp-null mice, suggesting that nodal ECM in the CNS may stabilize the node and prevent axonal sprouting [41].
Even in NF-null mice (i.e. no interaction of NF186 with ECM and paranodal junctions), small numbers of Nav channel clusters were still observed at nodes [20••] (Fig 2B). This suggests that there are additional unknown mechanisms involved in CNS node formation that are not associated with NF. These may be related to soluble factors secreted by oligodendrocytes. Are there still unknown CAMs in CNS nodes that can promote Nav channel clustering in response to soluble factors? Can soluble factors directly recruit Nav channels, which in turn recruit ankyrinG and cytoskeletal proteins? Many questions still remain regarding the mechanisms underlying formation of these critical domains in CNS myelinated nerve fibers.
Conclusions
Recent progress demonstrates that CAMs and their ligands within the ECM play instructive roles for formation of PNS nodes of Ranvier. Ongoing experiments will determine if the ECM at CNS nodes has similar or compensatory functions. One surprising conclusion from the recent work described here is that despite their similar molecular and structural features, the mechanisms for node formation are likely different between the PNS and CNS. Future studies on neuron-glial interactions will no doubt provide important clues about these differences.
Acknowledgments
This work was supported by NIH grant NS044916, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and Mission Connect. MNR is a Harry Weaver Neuroscience Scholar of the National Multiple Sclerosis Society. We apologize for the omission of citations or discussion of relevant papers that was due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 2.Hedstrom KL, Rasband MN. Intrinsic and extrinsic determinants of ion channel localization in neurons. J Neurochem. 2006;98:1345–1352. doi: 10.1111/j.1471-4159.2006.04001.x. [DOI] [PubMed] [Google Scholar]
- 3.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 4.Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- 5.Susuki K, Rasband MN. Spectrin and ankyrin-based cytoskeletons at polarized domains in myelinated axons. Exp Biol Med. 2008;233:394–400. doi: 10.3181/0709-MR-243. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel I, Adamsky K, Eshed Y, Milo R, Sabanay H, Sarig-Nadir O, Horresh I, Scherer SS, Rasband MN, Peles E. A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat Neurosci. 2007;10:861–869. doi: 10.1038/nn1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurel P, Einheber S, Galinska J, Thaker P, Lam I, Rubin MB, Scherer SS, Murakami Y, Gutmann DH, Salzer JL. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol. 2007;178:861–874. doi: 10.1083/jcb.200705132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR, Jr, Peles E. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47:215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 9••.Eshed Y, Feinberg K, Carey DJ, Peles E. Secreted gliomedin is a perinodal matrix component of peripheral nerves. J Cell Biol. 2007;177:551–562. doi: 10.1083/jcb.200612139. This study demonstrates that a secreted glial protein gliomedin is incorporated into nodal ECM and promotes the initial clustering of NF186 in PNS nodes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melendez-Vasquez C, Carey DJ, Zanazzi G, Reizes O, Maurel P, Salzer JL. Differential expression of proteoglycans at central and peripheral nodes of Ranvier. Glia. 2005;52:301–308. doi: 10.1002/glia.20245. [DOI] [PubMed] [Google Scholar]
- 11.Martin S, Levine AK, Chen ZJ, Ughrin Y, Levine JM. Deposition of the NG2 proteoglycan at nodes of Ranvier in the peripheral nervous system. J Neurosci. 2001;21:8119–8128. doi: 10.1523/JNEUROSCI.21-20-08119.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, Brophy PJ, Schmelzer JD, Low PA, Wrabetz L, Feltri ML, Campbell KP. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 13.Maertens B, Hopkins D, Franzke CW, Keene DR, Bruckner-Tuderman L, Greenspan DS, Koch M. Cleavage and oligomerization of gliomedin, a transmembrane collagen required for node of Ranvier formation. J Biol Chem. 2007;282:10647–10659. doi: 10.1074/jbc.M611339200. [DOI] [PubMed] [Google Scholar]
- 14.Koticha D, Maurel P, Zanazzi G, Kane-Goldsmith N, Basak S, Babiarz J, Salzer J, Grumet M. Neurofascin interactions play a critical role in clustering sodium channels, ankyrin G and βIV spectrin at peripheral nodes of Ranvier. Dev Biol. 2006;293:1–12. doi: 10.1016/j.ydbio.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 15••.Dzhashiashvili Y, Zhang Y, Galinska J, Lam I, Grumet M, Salzer JL. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J Cell Biol. 2007;177:857–870. doi: 10.1083/jcb.200612012. This study demonstrates the role of each domain in NF186 and ankyrinG for PNS node or AIS formation in detail, suggesting different mechanisms of formation for each of these domains. It also provides the first evidence to suggest that ankyrinG is necessary for node of Ranvier formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Basak S, Raju K, Babiarz J, Kane-Goldsmith N, Koticha D, Grumet M. Differential expression and functions of neuronal and glial neurofascin isoforms and splice variants during PNS development. Dev Biol. 2007;311:408–422. doi: 10.1016/j.ydbio.2007.08.045. This study shows the temporal regulation of each NF isoform in neurons and Schwann cells during the early developmental period. [DOI] [PubMed] [Google Scholar]
- 17.Garver TD, Ren Q, Tuvia S, Bennett V. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J Cell Biol. 1997;137:703–714. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen DP, Isom LL. Heterophilic interactions of sodium channel beta1 subunits with axonal and glial cell adhesion molecules. J Biol Chem. 2004;279:52744–52752. doi: 10.1074/jbc.M405990200. [DOI] [PubMed] [Google Scholar]
- 19.Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJ, Cottrell DF, Brophy PJ. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 20••.Zonta B, Tait S, Melrose S, Anderson H, Harroch S, Higginson J, Sherman DL, Brophy PJ. Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. J Cell Biol. 2008;181:1169–1177. doi: 10.1083/jcb.200712154. This study demonstrates that the single NF gene is required for node formation: nodal and paranodal components are absent in NF-null mice. A transgenic approach for rescue experiments revealed the role of each NF isoform, and proved for the first time that paranodal junctions are sufficient to assemble CNS nodal Nav channel clusters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Custer AW, Kazarinova-Noyes K, Sakurai T, Xu X, Simon W, Grumet M, Shrager P. The role of the ankyrin-binding protein NrCAM in node of Ranvier formation. J Neurosci. 2003;23:10032–10039. doi: 10.1523/JNEUROSCI.23-31-10032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbluth J. Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axons of the frog brain. J Neurocytol. 1976;5:731–745. doi: 10.1007/BF01181584. [DOI] [PubMed] [Google Scholar]
- 23.Rasband MN, Peles E, Trimmer JS, Levinson SR, Lux SE, Shrager P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- 25.Rios JC, Rubin M, St Martin M, Downey RT, Einheber S, Rosenbluth J, Levinson SR, Bhat M, Salzer JL. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. J Neurosci. 2003;23:7001–7011. doi: 10.1523/JNEUROSCI.23-18-07001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 27.Rasband MN, Taylor CM, Bansal R. Paranodal transverse bands are required for maintenance but not initiation of Nav1.6 sodium channel clustering in CNS optic nerve axons. Glia. 2003;44:173–182. doi: 10.1002/glia.10284. [DOI] [PubMed] [Google Scholar]
- 28•.Yang Y, Ogawa Y, Hedstrom KL, Rasband MN. βIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J Cell Biol. 2007;176:509–519. doi: 10.1083/jcb.200610128. This study shows the ankyrinG-binding site of βIV spectrin is required for βIV spectrin’s localization to nodes and the AIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komada M, Soriano P. βIV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156:337–348. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacas-Gervais S, Guo J, Strenzke N, Scarfone E, Kolpe M, Jahkel M, De Camilli P, Moser T, Rasband MN, Solimena M. βIVΣ1 spectrin stabilizes the nodes of Ranvier and axon initial segments. J Cell Biol. 2004;166:983–990. doi: 10.1083/jcb.200408007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Lacas-Gervais S, Morest DK, Solimena M, Rasband MN. βIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J Neurosci. 2004;24:7230–7240. doi: 10.1523/JNEUROSCI.2125-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Hedstrom KL, Xu X, Ogawa Y, Frischknecht R, Seidenbecher CI, Shrager P, Rasband MN. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol. 2007;178:875–886. doi: 10.1083/jcb.200705119. This study reports that brevican is a binding partner of NF186 in the ECM surrounding AIS and CNS nodes of Ranvier. It also demonstrates that NF186 is dispensable for AIS assembly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vabnick I, Novakovic SD, Levinson SR, Schachner M, Shrager P. The clustering of axonal sodium channels during development of the peripheral nervous system. J Neurosci. 1996;16:4914–4922. doi: 10.1523/JNEUROSCI.16-16-04914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schafer DP, Custer AW, Shrager P, Rasband MN. Early events in node of Ranvier formation during myelination and remyelination in the PNS. Neuron Glia Biol. 2006;2:69–79. doi: 10.1017/S1740925X06000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert S, Davis JQ, Bennett V. Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J Neurosci. 1997;17:7025–7036. doi: 10.1523/JNEUROSCI.17-18-07025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schafer DP, Rasband MN. Glial regulation of the axonal membrane at nodes of Ranvier. Curr Opin Neurobiol. 2006;16:508–514. doi: 10.1016/j.conb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Schafer DP, Bansal R, Hedstrom KL, Pfeiffer SE, Rasband MN. Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts? J Neurosci. 2004;24:3176–3185. doi: 10.1523/JNEUROSCI.5427-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan MR, Meyer-Franke A, Lambert S, Bennett V, Duncan ID, Levinson SR, Barres BA. Induction of sodium channel clustering by oligodendrocytes. Nature. 1997;386:724–728. doi: 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- 40.Weber P, Bartsch U, Rasband MN, Czaniera R, Lang Y, Bluethmann H, Margolis RU, Levinson SR, Shrager P, Montag D, Schachner M. Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J Neurosci. 1999;19:4245–62. doi: 10.1523/JNEUROSCI.19-11-04245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang JK, Phillips GR, Roth AD, Pedraza L, Shan W, Belkaid W, Mi S, Fex-Svenningsen A, Florens L, Yates JR, III, Colman DR. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- 42.Nie DY, Ma QH, Law JW, Chia CP, Dhingra NK, Shimoda Y, Yang WL, Gong N, Chen QW, Xu G, Hu QD, Chow PK, Ng YK, Ling EA, Watanabe K, Xu TL, Habib AA, Schachner M, Xiao ZC. Oligodendrocytes regulate formation of nodes of Ranvier via the recognition molecule OMgp. Neuron Glia Biol. 2006;2:151–164. doi: 10.1017/S1740925X06000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oohashi T, Hirakawa S, Bekku Y, Rauch U, Zimmermann DR, Su WD, Ohtsuka A, Murakami T, Ninomiya Y. Bral1, a brain-specific link protein, colocalizing with the versican V2 isoform at the nodes of Ranvier in developing and adult mouse central nervous systems. Mol Cell Neurosci. 2002;19:43–57. doi: 10.1006/mcne.2001.1061. [DOI] [PubMed] [Google Scholar]
- 44.Brakebusch C, Seidenbecher CI, Asztely F, Rauch U, Matthies H, Meyer H, Krug M, Böckers TM, Zhou X, Kreutz MR, Montag D, Gundelfinger ED, Fässler R. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol. 2002;22:7417–7427. doi: 10.1128/MCB.22.21.7417-7427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]