Abstract
Soluble epoxide hydrolase (sEH) is the major enzyme responsible for the metabolism and inactivation of epoxyeicosatrienoic acids (EETs). EETs are produced by the cytochrome P450 (CYP) epoxygenase pathway of arachidonic acid (AA) metabolism and tend to be anti-hypertensive, anti-inflammatory and protective against ischemic injury. Since the metabolism of EETs by sEH reduces or eliminates their bioactivity, inhibition of sEH has become a therapeutic strategy for hypertension and inflammation. sEH is found in nearly all tissues so the systemic application of inhibitors is likely to affect more than blood pressure and inflammation. In the central nervous system, EETs are thought to play a role in the regulation of local blood flow, protection from ischemic injury, inhibition of inflammation, the release of peptide hormones and modulation of fever. However, little is known about region- and cell-specific expression of sEH in the brain. In the mouse brain, expression of sEH was found widely in cortical and hippocampal astrocytes and also in a few specific neuron-types in the cortex, cerebellum, and medulla. To assess the functional significance of neuronal sEH, we generated a transgenic mouse model, which over-expresses sEH specifically in neurons. Transgenic mice showed increased neuron labeling in cortex and hippocampus with little change in labeling of other brain regions. Despite a 3-fold increase in sEH activity in the brain, there was no change in arterial pressure. This data provides new information required for studying the central roles of the cytochrome P450 epoxygenase pathway.
1. Introduction
Recently soluble epoxide hydrolase (sEH) has attracted interest as a potential therapeutic target for renal, cardiovascular and inflammatory disease. Inhibition of sEH results in an increase in epoxyeicosatrienoic acid (EET) levels. In the CNS, EETs have been shown to be involved in regulation of cerebral blood flow (Iliff et al., 2007; Zhang et al., 2008), inflammation (Inceoglu et al., 2008; Schmelzer et al., 2005; Schmelzer et al., 2006), pain (Inceoglu et al., 2006; Terashvili et al., 2008), angiogenesis (Medhora et al., 2003; Munzenmaier et al., 2000; Zhang et al., 2002), and release of peptide hormones (Junier et al., 1990; Snyder et al., 1989; Zeldin et al., 1997). EETs are generally protective. For example, inhibition of sEH reduced infarct size in a stroke model (Zhang et al., 2007), lowered blood pressure and reduced renal damage in angiotensin dependent salt sensitive hypertension (Imig et al., 2005), and was cardioprotective in a ischemia-reperfusion injury model (Motoki et al., 2008). In most cases, hydration of the EET epoxide moiety by sEH eliminates its bioactivity (Chacos et al., 1983). The inhibition of sEH is therefore an attractive strategy for increasing EET levels (Spector et al., 2007).
While sEH is found in all tissues, its expression is regional and cell specific. For example in the human kidney, sEH is highly expressed in the vasculature, predominantly in the smooth muscle with much lesser expression in the endothelium (Yu et al., 2004). Expression is also found in renal tubules but to a much lesser extent than observed in the vasculature (Yu et al., 2004). Other tissues also exhibit cell-restricted expression of sEH. Expression of sEH was found to be predominantly located in cells along the periphery of the pancreatic islets, the germinal centers of lymphoid follicles, and to be variably distributed in cells of the anterior pituitary (Enayetallah et al., 2006). Regulation of sEH expression can also be tissue- and strain-specific. For example, angiotensin-II induced hypertension results in an increase in sEH expression in the vasculature of the rat kidney (Zhao et al., 2004). Moreover, sEH expression is markedly increased in the brain of SHR compared with WKY, and this correlated with increased expression in neurons cultured from the hypothalamus and brain stem (Sellers et al., 2005).
Recent in vivo experiments have utilized the systemic application of sEH inhibitors to elucidate anti-hypertensive (Imig et al., 2002), anti-inflammatory (Zhao et al., 2004) and ischemic protective (Zhang et al., 2007) roles of the CYP epoxygenase pathway. Since sEH is regulated in a tissue-specific manner and is found in nearly all tissues, the systemic application of these inhibitors could have unexpected physiologic results in non-renal and vascular systems. Little is known about the cell-specific expression of sEH in the brain. However, the presence of CYP epoxygenase pathway components in the CNS has been established. Rat astrocyte cultures were shown to express sEH (Rawal et al., 2009), CYP 2C11, produce EETs, and rapidly breakdown EETs (Alkayed et al., 1996; Amruthesh et al., 1993). Rat primary neuronal cell cultures also express sEH (Sellers et al., 2005). In addition, immunochemical detection of sEH in brain sections (Zhang et al., 2007) indicated that the enzyme is present in cortical neurons and axons but surprisingly not in astrocytes as determined by co-localization with glial fibrillary acidic protein (GFAP).
We have undertaken a detailed immunohistochemical study of sEH in the mouse brain. We found neuron-specific expression in several brain regions. These data in addition to the increased expression of sEH observed in neurons in the SHR (Sellers et al., 2005) prompted us to generate a transgenic mouse that expresses sEH specifically in neurons to further examine the role of sEH in the CNS. Herein we describe the cell and regional specific expression of sEH as well as the characterization of Syn1-EPHX2 mice.
2. Results
2.1 Specificity of sEH antibody
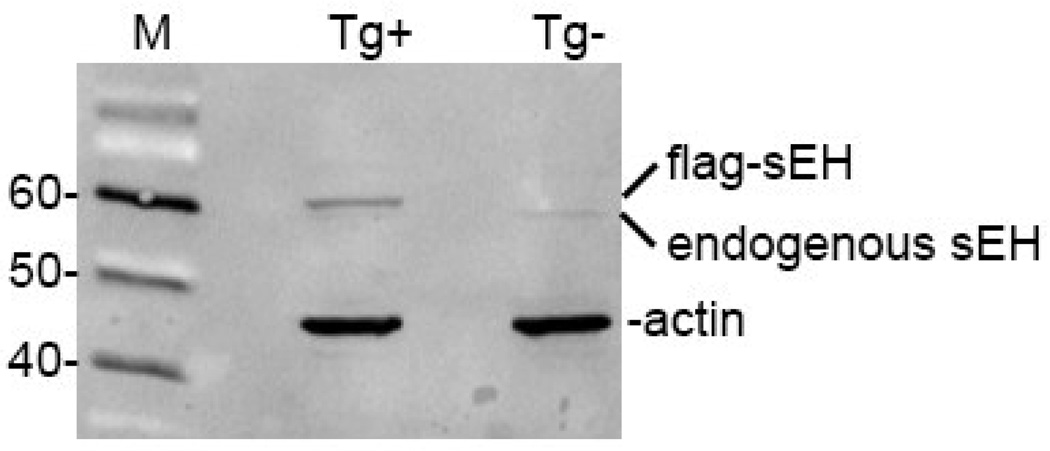
The antibody directed to mouse sEH exclusively recognized endogenous and FLAG epitope tagged sEH in non-transgenic and transgenic mouse brain homogenates (Figure 1). As expected, the FLAG-sEH band was slightly larger in size than the endogenous sEH band.
Figure 1. Western blot demonstrating specificity of the anti-sEH antisera.
The antisera detects a single band of approximately 60 kD molecular weight in non-transgenic brain homogenate and an additional band of slight larger molecular in transgenic mouse brain expressing Flag-tagged sEH.
2.2 Immunohistochemistry of wild type (non-transgenic) brain
Immunohistochemical examination of non-transgenic mouse brain regions identified sEH localized in a cell- and region-specific manner.
Cortex
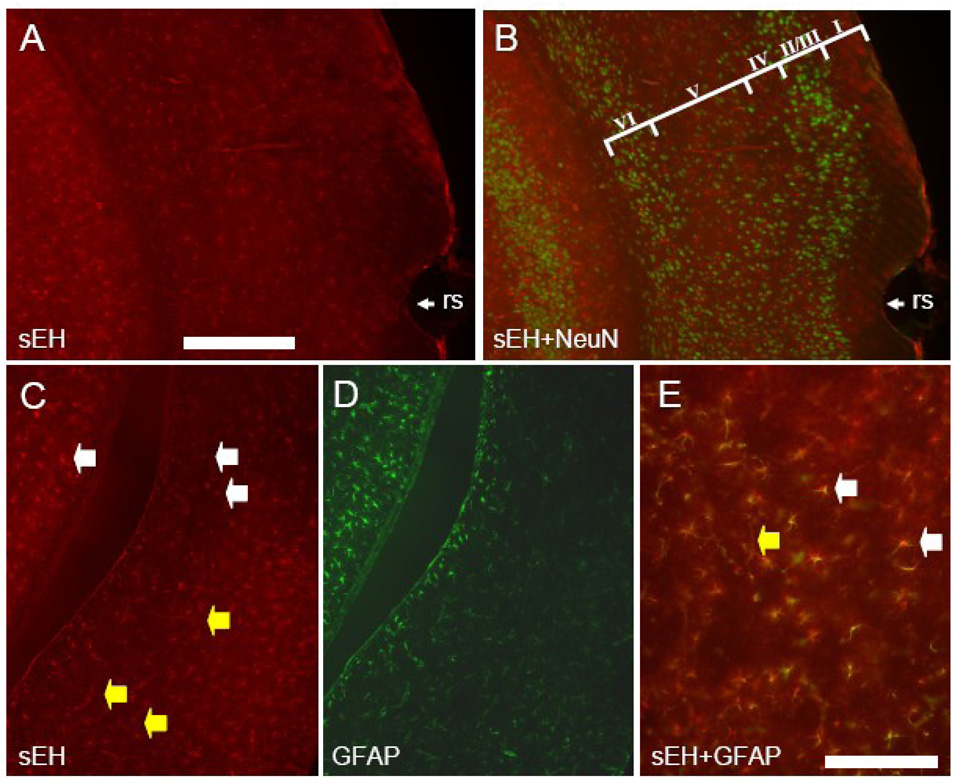
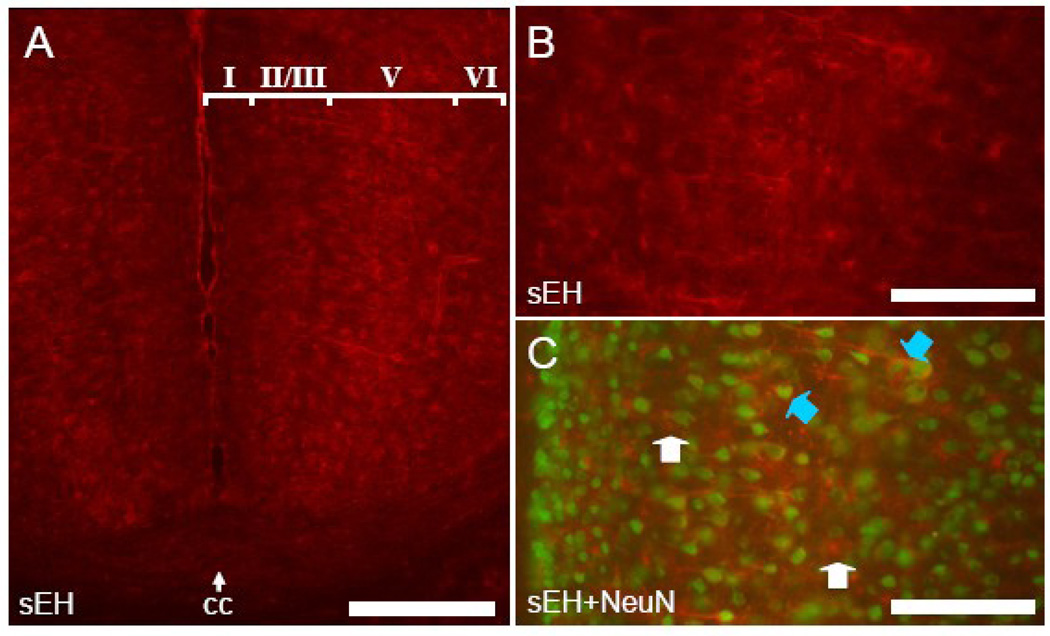
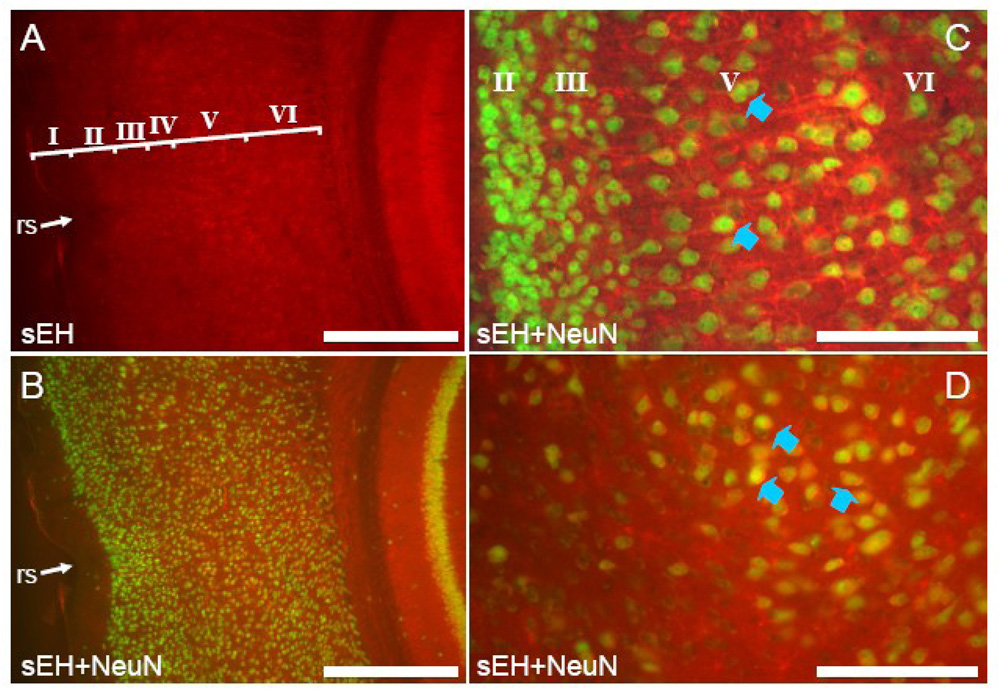
Anti-mouse sEH antibodies recognized only astrocytes, capillaries and arterioles throughout most of the cerebral cortex (Figure 2 and Figure 3). Co-labeling of astrocytes is apparent in slices incubated with antibodies directed to both GFAP and sEH (Figure 2C–E). Neuronal labeling was limited to pyramidal neurons of layer V in dorsomedial cortical regions in particular the cingulate cortex (Figure 3). In this area, sEH immunolabeled dendrites could be observed originating in layer V and running through the superficial layers almost to the cortical surface. Co-labeling with antibodies directed to both NeuN and sEH clearly showed sEH immunoreactive dendrites arising from NeuN stained perikarya (Figure 3C). Laterally, neuronal sEH labeling progressively diminished until only astrocytes and the microvasculature were immunostained in the dorsolateral, lateral and ventral regions of the cortex (Figure 3A).
Figure 2. sEH is expressed in astrocytes and vascular cells in the lateral and ventral cerebral cortex.
Coronal section showing the lateral cerebral cortex dorsal to the rhinal sulcus (rs) labeled with anti-sEH (A) and anti-NeuN (B). Note no double labeled cells are present. Cortical layers are identified by roman numerals in panel B. Coronal section through the cerebral cortex ventral to the rhinal sulcus stained with anti-sEH (C) and GFAP (D). Higher magnification of merged C and D (E) shows extensive co-localization of sEH and GFAP (yellow cells). White arrowheads identify sEH positive astrocytes whereas yellow arrowheads identify sEH positive blood vessels. Scale bars: A–D = 500 µm; E = 100 µm
Figure 3. sEH is expressed in astrocytes, vascular cells and neurons in dorsomedial cerebral cortex.
A. Mouse cingulate cortex (note midline and corpus callosum (cc)) labeled with anti-sEH antibody. sEH positive neurons can be seen in layer V. B. High power view of mouse cingulated cortex showing layer V neurons labeled with anti-sEH.. C. Co-labeling of pyramidal neurons stained with anti-NeuN and anti-sEH (blue arrowheads) is apparent while astrocytes (white arrowheads) are only labeled by anti-sEH. Scale bars: A = 500 µm; B,C =100 µm
Hippocampus
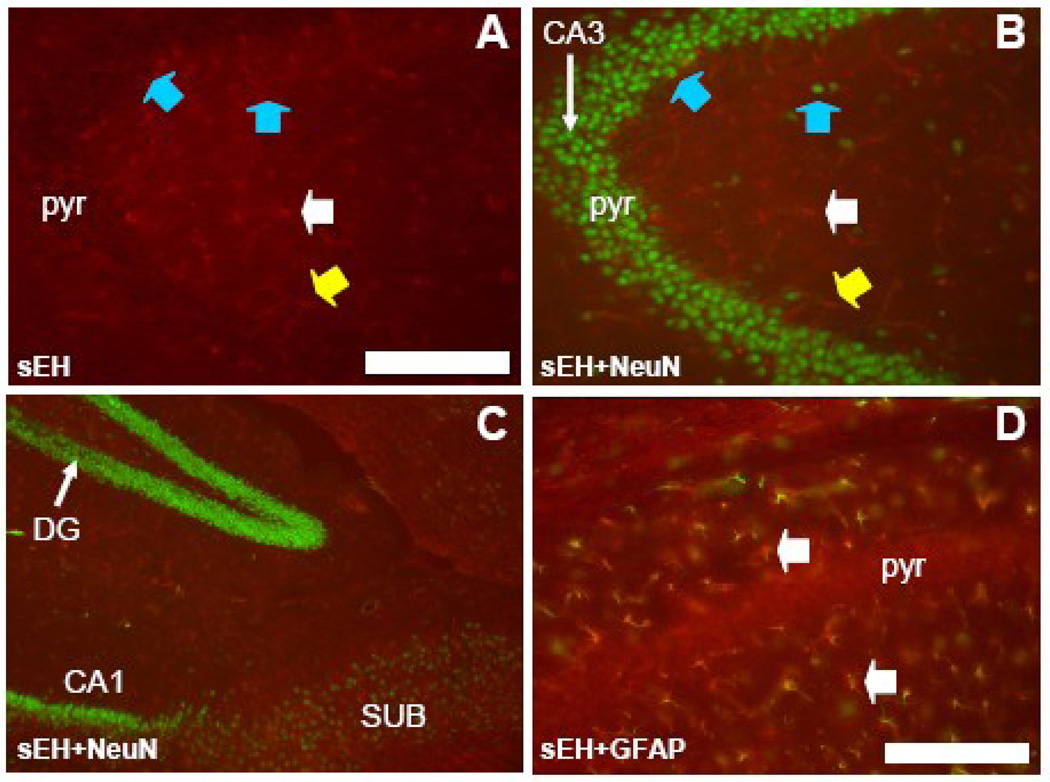
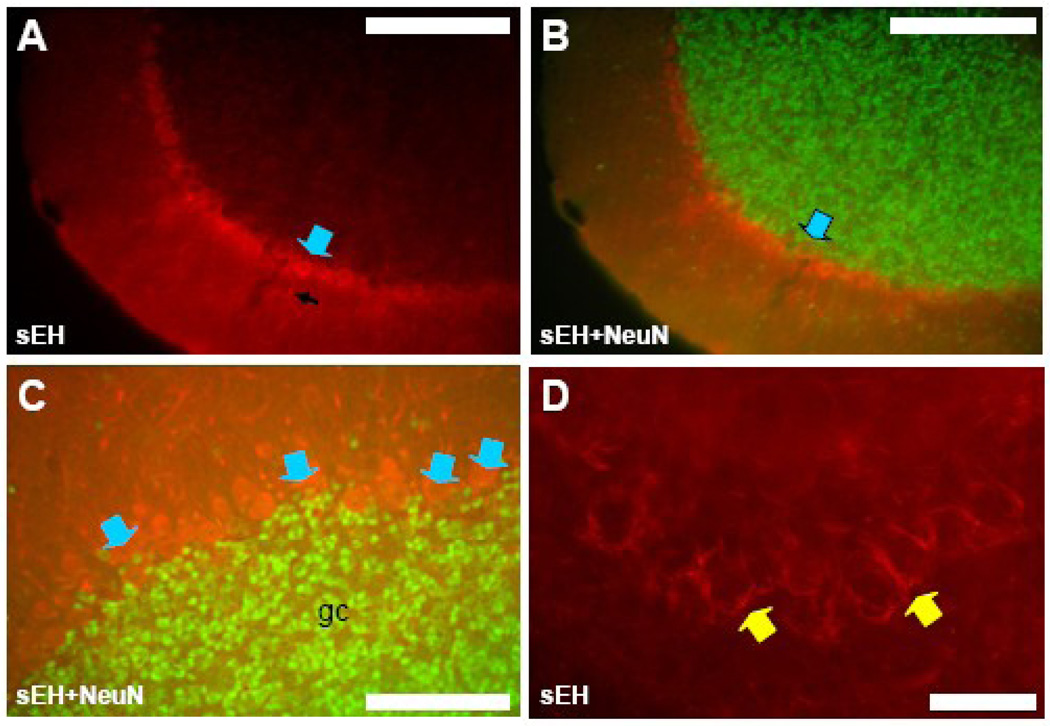
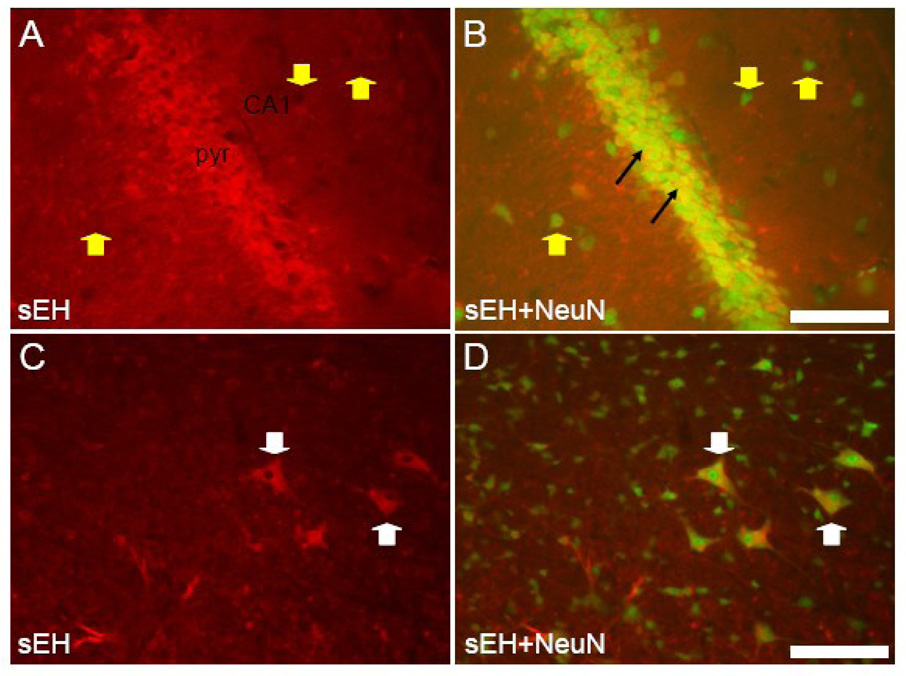
Antibodies specific for mouse sEH labeled only astrocytes and capillaries and arterioles in the dentate gyrus, hippocampus and subiculum (Figure 4). Hippocampal pyramidal cell somata in all subfields (CA1 through CA4) had no labeling, in fact neuronal cell bodies in the stratum pyramidale and other layers could be seen as black unstained areas standing out against labeled astrocytes and vascular labeling (Figure 4A). NeuN staining produced no co-labeling in either the hippocampus, dentate gyrus or subiculum (Figure 4B and C). Co-labeling of astrocytes was evident in slices incubated with both GFAP and sEH antibodies (Figure 4D). These double-labeled astrocytes were particularly conspicuous in the non-pyramidal cell layers of the hippocampus (white arrows in Fig. 4D).
Figure 4. sEH is expressed in astrocytes in the hippocampus.
A. Coronal section through the CA3 subfield of the hippocampus labeled with anti-sEH antibodies, and B with both anti-sEH and anti-NeuN. Note the absence of any sEH staining in pyramidal neurons of the pyramidal cell (pyr) and extrapyramidal layers (blue arrowheads) but positive staining of blood vessels (yellow arrowhead) and astrocytes (white arrowhead). C. Coronal section through the dentate gyrus (DG), hippocampal subfield CA1, and the subiculum (SUB) immunostained for both sEH and NeuN showing lack of co-localization in these components of the hippocampus. D. High power view of a portion of hippocampal subfield CA3 stained with both anti-sEH and anti-GFAP. Co-localization can be seen in astrocytes (white arrowhead) in hippocampal layers outside of the pyramidal cell layer (pyr). Scale bars: A–C = 250 µm; D = 100 µm.
Cerebellum
There was robust sEH labeling of Purkinje cells, cerebellar basket cells and capillaries and arterioles. Immunostaining of Purkinje cell bodies and most of their dendritic trees was present in most cerebellar folia (Figure 5A–C) though not all Purkinje cells were labeled by anti-sEH. Cerebellar Purkinje cells did not label with NeuN antibodies (Figure 5A–C) so co-localization could not be confirmed, though their size and location made positive staining obvious (Figure 5C). In areas where Purkinje cell staining was absent, cerebellar basket cell axonal processes were visible (Figure 5D). No sEH-astrocyte co-labeling was observed in the cerebellum.
Figure 5. sEH is expressed in some neurons in cerebellum.
A, B and C. Coronal section through a folium of the cerebellum labeled with anti-sEH antibodies (A) and anti-sEH and anti-NeuN (B and C). Purkinje cell bodies (blue arrowheads) and dendritic trees (black arrow in A) are sEH immunoreactive whereas granule cells (gc) are not. D. Basket cells and their processes (yellow arrowhead) surrounding Purkinje cells were also labeled by anti-sEH in some areas of the cerebellum. Scale bars: A and B = 400 µm; C = 100 µm; D = 50 µm
Medulla
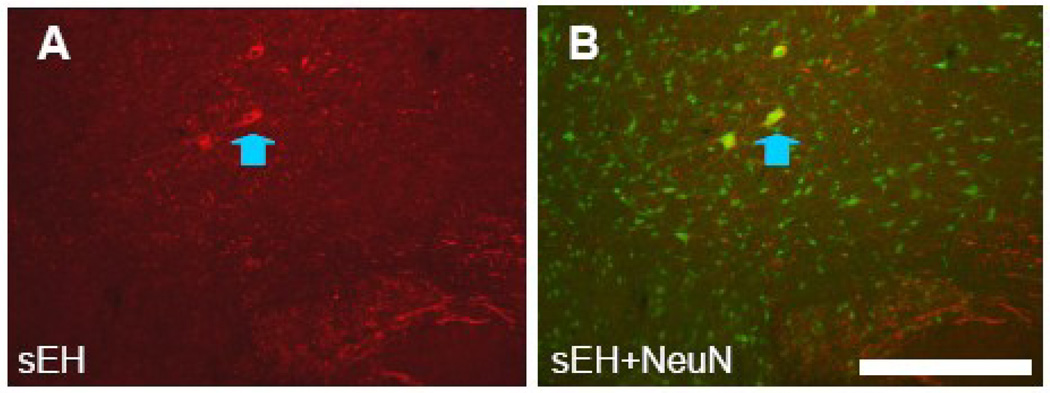
The only neuronal labeling in the brainstem was very distinct sEH labeling of gigantocellular neurons of the ventrally situated gigantocellular reticular nucleus. Neuron somata and dendrites were clearly visible with sEH immunostaining (Figure 6A) and co-labeling of these neurons was apparent in slices incubated with both sEH and NeuN antibodies (Figure 6B). No other neuron types were definitively labeled and no astrocyte labeling was detected in the medulla.
Figure 6. sEH is expressed in a restricted population of neurons in medulla.
A and B. Coronal section through the ventral medulla incubated with anti-sEH (A), and anti-sEH and anti-NeuN (B). Large neurons gigantocellular reticular formation (blue arrowhead) are labeled by both anti-sEH and NeuN antibodies. Scale bar = 250 µm
Hypothalamus
The hypothalamus had virtually no sEH labeling. Occasionally labeling of the microvasculature could be seen but otherwise this region looked no different from the controls in which the primary antibody to sEH was omitted (data not shown).
2.3 Development of Syn1-FLAH-sEH transgenic mice
The detection of sEH expression in select neurons coupled with the observation by Sellers et al (Sellers et al., 2005) that sEH expression is increased in neuronal cultures derived from the hypothalamus and brain stem of SHR, promoted us to assess the consequences on arterial pressure of neuron-specific over-expression of sEH. Consequently, we tested the hypothesis that neuronal over-expression of sEH would cause hypertension.
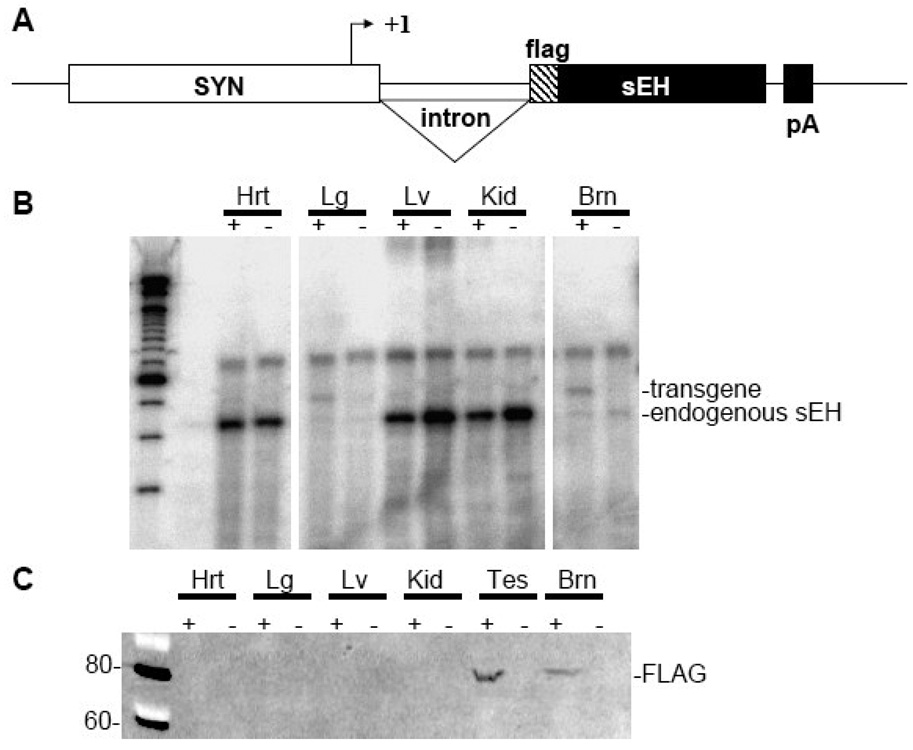
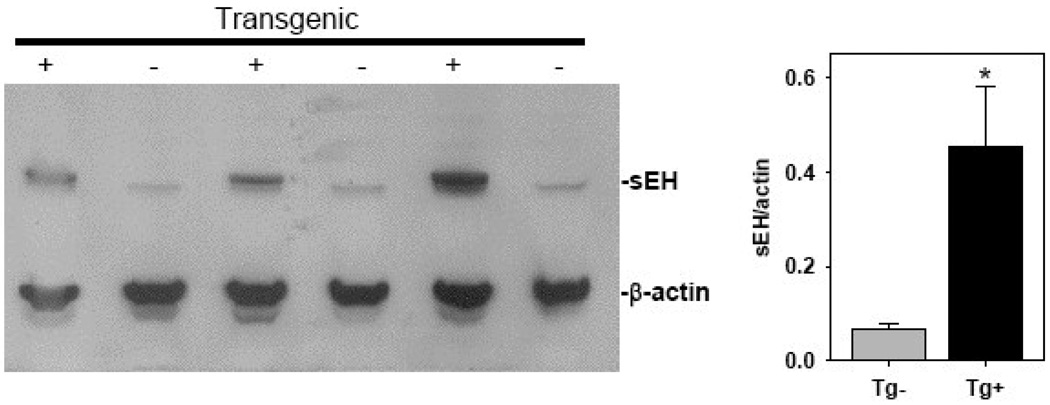
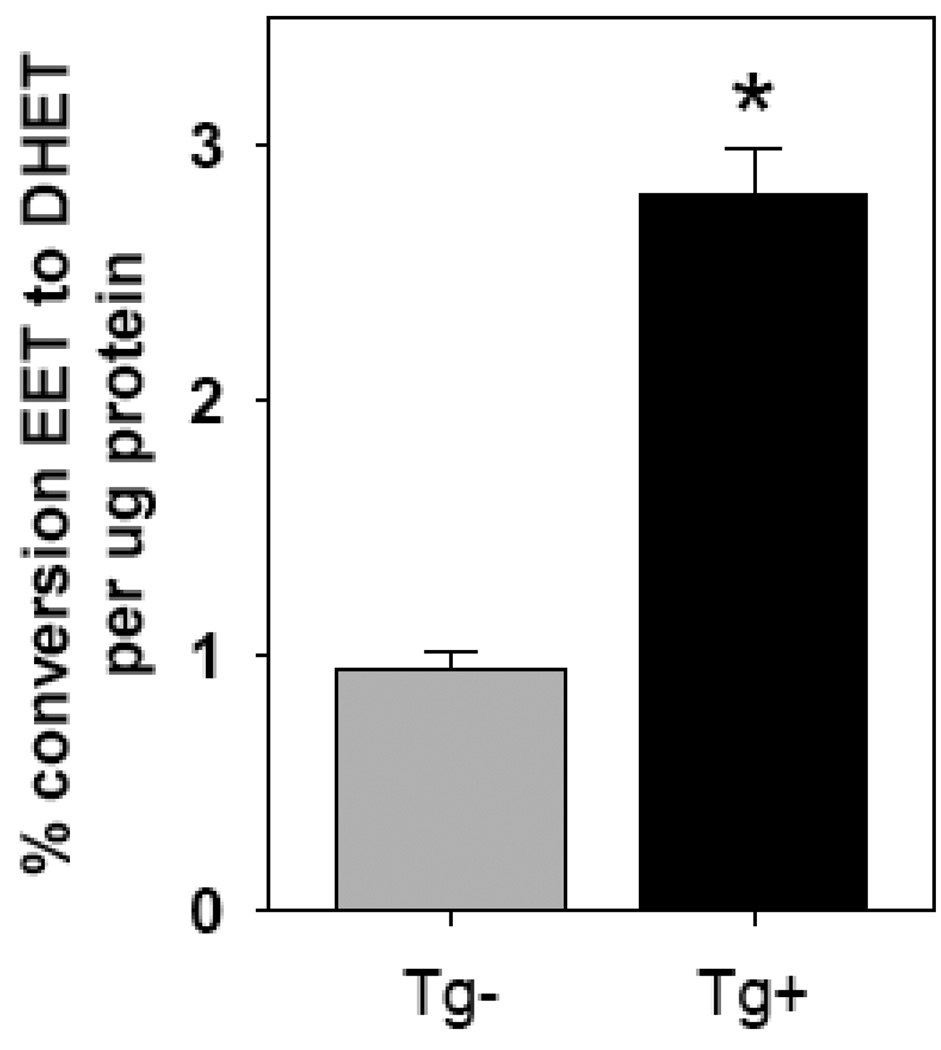
Syn1-FLAG-sEH transgenic mice were generated by fusing the rat synapsin 1 promoter to a N-terminally FLAG tagged cDNA for mouse sEH (Figure 7A). The FLAG epitope tag was essential to differentiate between endogenous and transgenic sEH. Four transgenic founders were identified and two of these were successfully bred to establish transgenic lines. An RNAse protection assay was performed to examine tissue specific expression of the transgene (Figure 7B). Line 24849/1 had significant transgene expression in the brain with some ectopic expression seen in lung (Figure 7B) and testes (data not shown). Western blots using antibodies directed against sEH or the FLAG epitope tag showed that expression was limited to the brain and testes of transgenic mice (Figure 7C). An additional Western blot was performed to quantify total sEH protein in transgenic brain homogenates (Figure 8). This result shows an approximately 5-fold increase in total brain sEH compared to non-transgenic littermates (Figure 8). sEH Enzyme activity in transgenic and non-transgenic brain homogenates was compared by HPLC quantitation of the conversion of 14,15-[3H]EET to 14,15-[3H]DHET. Transgenic brains had 2.8±0.2 vs 1.0±0.07% conversion per µg protein in non-transgenic littermates for an approximately 3-fold increase in sEH activity (Figure 9).
Figure 7. Development of neuronal sEH expressing transgenic mice.
A. Transgene map. B. RNase protection assay showing expression of endogenous and transgenic sEH as indicated. C. Western blot showing flag-tagged sEH protein in tissue extracts. Key: + vs − indicates the presence of the transgene. Hrt, heart; Lg, lung; Lv, liver; Kid, kidney; Brn, brain; Tes, testes.
Figure 8. sEH protein is expressed in the brain of transgenic mice.
sEH protein expression in the brain of transgenic and non-transgenic mice is shown by Western blot (n=3). The right panel shows quantification of the blot *, P<0.05 vs non-transgenic.
Figure 9. sEH enzymatic activity in the brain of transgenic mice.
sEH enzymatic activity in brain was measured as described in the methods. n=9 for Tg+; n=12 for Tg−; *, P<0.001).
2.4 Immunohistochemistry of Syn1-FLAG-sEH brain
Immunohistochemical examination of transgenic mouse brain regions identified several differences in sEH expression when compared to non-transgenic controls.
Cortex
Antibodies specific for mouse sEH recognized neurons throughout the cortex of Syn1-EPHX2 mice (Figure 10). Co-labeling of these neurons is evident in slices incubated with antibodies directed to NeuN and sEH (Figure 10B–D). Neuronal labeling was particularly evident in the lateral and ventral regions of the cerebral cortex, in contrast to non-transgenic littermates in which labeling was limited to dorsal cortical areas (i.e. Figure 2A and B). sEH positive neurons were most prominent in layer V of all neocortical areas (Figure 10A and B) though sEH positive cells were evident in layers II, III, IV and VI (Figure 10A). In the cingulated cortex, sEH was most heavily expressed in layer V, as in non-transgenic animals, though dendritic labeling was much more pronounced (Figure 10C). Ventral to the rhinal sulcus, both piriform and entorhinal (Figure 10D) areas had prominent expression of sEH in deep layers.
Figure 10. sEH is expressed in neurons of the lateral cerebral cortex in Syn-FLAG-sEH transgenic mice.
A. Coronal section through the lateral cerebral cortex of a Syn-FLAG-sEH transgenic mouse labeled with anti-sEH (A) and both anti-sEH and anti-NeuN (B). Compared to the section from a non-transgenic animal shown in Figure 2, expression of sEH in pyramidal neurons, in particular in layer V, is evident. C. Section through the cingulate cortex from a transgenic mouse labeled with both anti-sEH (red) and anti-NeuN (green) shows expression of sEH in virtually all pyramidal neurons (blue arrowhead) in layer V (cf. Figure 3A). D. Section through the entorhinal cortex ventral to the rhinal sulcus labeled with both anti- sEH and anti-NeuN. Again, note numerous double-labeled neurons (blue arrowheads) in deep layers. Scale bar: A and B = 500 µm; C and D 100 µm
Hippocampus
Anti-sEH antibodies labeled hippocampal pyramidal neurons in addition to astrocytes and vasculature (Figure 11A and B). Co-labeling of pyramidal neurons was evident in all hippocampal subfields and the subiculum in slices incubated with antibodies directed to NeuN and sEH (Figure 11B). However, many hippocampal neurons in layers other than the stratum pyramidale (Figure 11B) and the granule cells of the dentate gyrus did not express sEH.
Figure 11. sEH is expressed in neurons of hippocampus and medulla in Syn-FLAG-sEH transgenic mice.
A and B. Coronal section through the CA1 region of a transgenic mouse hippocampus labeled with anti-sEH (A) and both anti-sEH anti-NeuN (B). Note the dramatic increase in sEH expression in pyramidal neurons (black arrows) compared to non-transgenic animals (e.g. Figure 4) though some neurons outside the pyramidal cell layer (yellow arrowheads) do not express sEH. C and D. Coronal section through the ventral medulla from a transgenic animal. Note the expression of sEH is still restricted to gigantocellular neurons (white arrowheads) which are clearly labeled by both anti sEH and NeuN antibodies. Scale bars = 100 µm
Cerebellum, Hypothalamus, and Medulla
There were no differences in labeling between Syn1-EPHX2 transgenic mice and non-transgenic littermates in the cerebellum and hypothalamus, whereas in the medulla there was scant neuronal expression of sEH throughout. In particular large neurons of the gigantocellular reticular formation reproducibly expressed sEH (Figure 11C and D).
2.5 Blood pressure
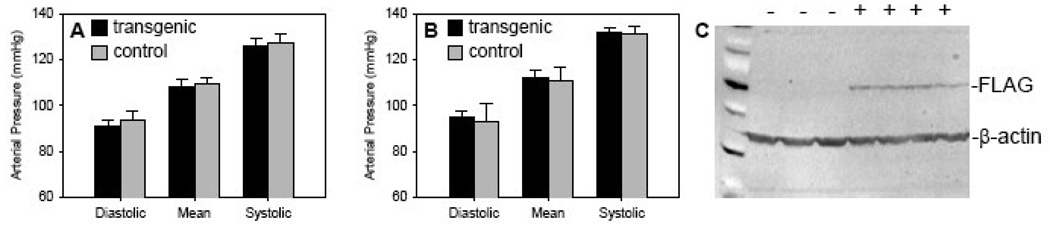
The effect of neuronal over expression of sEH on blood pressure was examined by radiotelemetry in conscious mice fed either normal or high salt diet (Figure 12A–B). Over expression of sEH in neurons had no effect on daily systolic pressure, diastolic pressure, and mean arterial pressure when compared to non-transgenic littermates when fed either normal or a high salt diet. Heart rate was unchanged comparing transgenic (553±18 bpm) with non-transgenic controls (557±25 bpm). We confirmed that each transgenic mouse expressed the transgene by monitoring expression of the flag epitope in the brain (Figure 12C).
Figure 12. Arterial pressure in Syn-FLAG-sEH transgenic mice.
Blood pressure was measured by radiotelemetry (n=4 per group). After 7 days of normal salt diet (A), mice were switched to a high salt diet. Mice were given two days to acclimate to the new diet and then blood pressure was measured for and additional 7 days (B). C. Expression of the transgene in these experimental mice was verified by western blot.
3. Discussion
There has been increased interest in the EET epoxygenase pathway because systemic inhibition of sEH has both cardiovascular and renal protective properties in some pathological states (Imig et al., 2005; Motoki et al., 2008; Zhang et al., 2007; Zhao et al., 2004). In general, these studies have shown that EETs, and their regulation by sEH, have a role in the regulation of vascular tone and regional blood flow. Similar to what is seen in other tissues (Motoki et al., 2008; Zhao et al., 2004), the systemic inhibition of sEH results in increased cerebral blood flow and a decrease in ischemic injury (Zhang et al., 2007). However, the increase in neuronal sEH expression seen in the SHR (Sellers et al., 2005) and the regional expression of sEH in the brain suggests their may be a central blood pressure role for the epoxidase pathway. This underscores the importance of understanding where in the brain sEH is expressed and what roles the epoxidase pathway is playing in the CNS. For this purpose, we have generated transgenic mice over-expressing sEH under the control of the rat synapsin 1 promoter. The synapsin 1 promoter drives neuron-specific expression of sEH in these mice. Transgene mRNA and protein expression was essentially limited to the brain and its expression was about 5-fold elevated as compared with non-transgenic littermate controls. This correlated well with a 3-fold increase in sEH activity in transgenic brain homogenates. The activity data clearly demonstrates that the over-expressed protein was functional and that the epitope tag did not effect enzymatic activity.
Immunohistochemical labeling of sEH in transgenic mice identified considerable labeling of neurons in the cortex and hippocampus. Labeling of soma and dendrites was clearly visible. In contrast, the only definitive identification of sEH-containing neurons in non-transgenic cortex was the pyramidal neurons of the fifth cortical layer (layer V). These glutamatergic neurons are the principal source of cortical efferents to motor and sensory related subcortical structures. Pyramidal neurons are the dominant type of neuron in the cortex, but subtypes of layer V pyramidal cells are being identified through differential expression of certain genes (reviewed in (Molnar et al., 2006)). The hippocampus also contains pyramidal neurons but no neuronal staining was seen in this region in the non-transgenic animal. Such limited sEH expression in the non-transgenic mouse suggests a specific role for the CYP epoxygenase pathway in the cortical layer V subpopulation of neurons. It is possible that, similar to their role in the vasculature, EETs produced by neurons are involved in modulating Ca2+ gated K+ channels. Ca2+ gated K+ channels are involved in regulating neuronal membrane potentials and intrinsic firing rates (Edgerton et al., 2003). If EETs are produced by these neurons, the presence of sEH may be necessary to regulate the level of EETs. Alternatively, neuronal sEH may be acting to prevent EETs produced by neighboring astrocytes from acting on neurons and affecting their membrane potential. Recently Iliff et al identified subcellular specific expression of sEH in nerve fibers extending along conduit arteries in the cortex (Iliff et al., 2007). They hypothesize that sEH in these fibers are involved in the regulation of vascular flow. It is likely that sEH is playing multiple roles within the different signaling microenvironments of the cortex.
It had been previously hypothesized that EETs produced by cortical and hippocampal astrocytes were involved in the regulation of vascular tone and contributed to protection via actions on the vasculature (Alkayed et al., 1996; Amruthesh et al., 1993). In support of this hypothesis, the authors presented evidence of astrocyte conversion of EET to DHET, presumably by sEH. However, immunochemical studies failed to find sEH in cortical astrocytes (Zhang et al., 2007). Here, we report the presence of sEH in both cortical and hippocampal astrocytes.
In cerebellum, sEH expression is limited to Purkinje neurons and the axonal processes of basket neurons. Purkinje neurons are the sole source of cerebellar efferents for motor coordination. Staining for sEH was visible in the soma and dendrites of many but not all Purkinje cells. Interestingly, the neuron-specific over-expression of sEH did not result in additional sEH positive neurons in the cerebellum as labeling remained limited to Purkinje neurons and basket cell processes. This would suggest a tight regulation of sEH in this region. Purkinje neurons have an intrinsic firing rate that is modulated in part by Ca2+ gated K+ channels (Edgerton et al., 2003). It is unclear if the epoxide hydrolase pathway plays a role in modulating these channels and membrane potential. The axonal basket processes of basket neurons form inhibitory synapses on the cell body of Purkinje neurons. Only the basket processes were labeled by sEH antibodies. Such a limited localization suggests but certainly does not prove a role in the regulation of neurotransmitter release. Interestingly, inhibition of sEH increases the firing rate of SHR neurons (Sellers et al., 2005). Ultimately the role for sEH in cerebellum neurons is speculative since the source or even the presence of EETs in the cerebellum is unknown.
CYP450 inhibitor studies suggested that the epoxide hydrolase pathway might be involved in the central regulation of fever (reviewed in (Kozak et al., 2004)). When we looked for expression of sEH in the non-transgenic hypothalamus however, we found no labeling, except in the vasculature. Fever is mediated by prostaglandin E2 produced in the endothelium of vessels in the anterior hypothalamus (Ek et al., 2001) so the regulatory role of sEH may be at the level of the vasculature. If sEH is present in neurons in this region of the brain, it is below the level of detection by this assay. Further investigation will be necessary to see if sEH is induced in this region of the brain during fever.
Expression of sEH was previously reported in primary neuron cultures from rat brainstem (Sellers et al., 2005). We found definitive neuron labeling in gigantocellular reticular neuron cell bodies and dendrites of the brainstem from wildtype mice. The neurons in the gigantocellular region are thought to be involved in baroreflex control (Aicher et al., 1997). Previous work has shown that intracranial ventricular application of sEH inhibitors caused a significant decrease in baroreceptor reflex gain (Sellers et al., 2005). These data are consistent with our identification of gigantocellular neurons as expressing sEH and the hypothesis that these neurons are involved in baroreflex regulation. Neuronal over expression of sEH did not result in definitive differences in neuronal labeling in the medulla.
The spontaneously hypertensive rat model has an increased sEH expression in the brain (Sellers et al., 2005). The variant blood pressure in this model is multi-factorial, however it was possible that sEH could be playing a role. We examined blood pressures in mice on standard and high salt diets, but no differences were observed between transgenic and non-transgenic mice. These results suggest that sEH over-expression alone is not sufficient to affect systemic blood pressure. Nevertheless, we recognize that sEH may not have been over-expressed specifically in those neurons in the medulla and hypothalamus regulating blood pressure.
Astrocytes and some perivascular nerve fibers in the cortex are known to express CYP450 capable of producing EETs (Iliff et al., 2007), but for the rest of the sEH expressing cells identified in this study, the source of EET substrate is not known. EETs may be produced by the sEH expressing neurons or they could be produced and released by neighboring cells and taken up by these neurons. It is noteworthy that such a small subset of neurons express sEH and that they are in regions involved in the control of motor function. Motor function is not a role that has been associated with the CYP epoxygenase pathway.
This study has identified specific neurons expressing sEH in the cortex, cerebellum, and medulla of non-transgenic mice. We also verified in vivo expression of sEH in cortical and hippocampal astrocytes and blood vessels. In general, expression of sEH in the central nervous system appears to be both regional and cell specific, but its over-expression in neurons does not alter arterial pressure. Additional studies will be necessary to determine the roles the CYP epoxygenase pathway is playing in these areas.
4. Experimental Procedures
4.1
All animal procedures were approved by the University of Iowa Animal Use Committee and were consistent with NIH guidelines.
4.2 Immunohistochemistry
Immediately after CO2 asphyxiation, a syringe pump was used to transcardially perfuse mice with 25 ml sterile saline at 5.0 ml/minute and then fix with 50 ml 4% paraformaldehyde/0.5% glutaraldehyde at 2.5 ml/minute. The brain was carefully removed and post fixed over night in the same fixative. Brains were rinsed 5 times in TBS and then suspended in 30% sucrose/TBS for 2–3 hours at room temperature with gentle rotation. The sucrose solution was changed and the brains incubated at 4°C over night. Brains were sliced into 25 µm thick sections on a vibratome (Sectioning System 3000) and the sections washed 3 times for 10 minutes in TBS followed by an over night wash at 4°C. Sections were transferred to a 15×45 mm glass vial and incubated in blocking solution (TBS, 2% normal goat serum, 1% BSA, 0.05% Triton X-100) for 30 minutes followed by incubation in Superblock (Pierce: 37535) for 30 minutes at room temperature. Sections were dual labeled with rabbit anti mouse sEH (1:800 dilution, gift by Dr. B.D. Hammock, UC Davis, CA) and either mouse anti Glial Fibrillary Acidic Protein (GFAP 1:500 dilution, Chemicon: MAB3402) or mouse anti Neuron-Specific Nuclear Protein (NeuN 1:500 dilution, Chemicon: MAB337). Sections were incubated for 24 hours in primary antibodies diluted in blocking solution followed by three 15-minute washes in wash solution (TBS + 0.05% Triton X-100). As a negative control, we omitted the primary antibody directed to sEH. No signal was detected when the primary antibody was omitted. Sections were incubated with secondary antibodies goat anti-Rabbit conjugated to Alexa Flour 568 (Invitrogen: A21069) and goat anti mouse conjugated to Alexa Fluor 488 (Invitrogen: A21121) diluted 1:400 in blocking solution for 2 hours at room temperature. Sections were washed two times for 15-minutes in wash solution followed by two 15-minute washes in TBS. Sections were mounted on slides with Vectishield (Vector Laboratories Inc.: H-1000). Slides were examined with a Bio-Rad fluorescent microscope and pictures captured using Spot advanced software.
4.3 Western blots
Tissues were homogenized in homogenization buffer [PBS, 5mM EDTA, 1mM DTT, EDTA free protease inhibitor pellet (Roche: 11836170001), centrifuged at 9,000×g for 12 minutes, and the supernatant recovered for analysis. Fifty µg of supernatant protein was loaded in each lane. Primary antibodies consisted of rabbit anti mouse sEH polyclonal antibody (gift of Dr. B.D. Hammock, UC Davis, CA) at a dilution of 1:6000 in antibody buffer (1% dry milk dissolved in TBS) or a goat anti FLAG polyclonal antibody (Abcam: ab1257) at a dilution of 1:5000. A rabbit anti β-actin polyclonal antibody (Abcam: ab8227) was used as an internal loading control. Horseradish peroxidase conjugated secondary antibodies consisted of donkey anti rabbit IgG diluted 1:4000 (Amersham: NA934V) or rabbit anti goat IgG diluted 1:10,000 (Santa Cruz: 2768). Chemiluminescence using the ECL+ kit (Amersham) was detected on the Storm 840 (Molecular Dynamics) or by exposure to X-ray film. Densitometry of Western blot films was used to determine the total protein in transgenic vs. non-transgenic brain. Gel loading was normalized to β-actin.
4.4 Generation of Syn1-FLAG-sEH expression vector
The mouse cDNA for soluble epoxide hydrolase was cloned by reverse transcriptase PCR from strain C57BL/6J and inserted into pCR2.1TOPO (Invitrogen: K4500-40). The sEH cDNA was PCR amplified with a 5’ primer containing the sequence for FLAG epitope tag (5-gccgccatgGACTACAAGGACGACGATGACAAAatggcgctgcgtgtagccgcgttcg-3’) and inserted into pCR2.1TOPO to yield pCR2.1-FLAG-sEH. The FLAG sEH cDNA was removed from pCR2.1-FLAG-sEH by digestion with SpeI and EcoRV. The cDNA for human renin was removed from the pSYN1-hREN expression construct described earlier by digestion with NheI and SmaI (Morimoto et al., 2002). FLAG-sEH cDNA and the pSYN1 expression vector were then ligated together to yield pSYN1-FLAG-sEH. The SYN1-FLAG-sEH construct was removed from the plasmid backbone by digestion with XhoI-SpeI and agarose gel purified prior to injection into one cell stage fertilized mouse embryo.
4.5 Ribonuclease protection assay (RPA)
Tissues were homogenized using individual RNAse free plastic pestles (VWR: KT749520-0090) in 0.5 ml Tri-Reagent (MRC Inc.: TR 118). Total RNA was isolated using the Tri-reagent manufacturer’s instructions. The RPA probe consisted of the 26 nucleotide (nuc) FLAG tag sequence and the first 257 nuc of the sEH cDNA. This probe hybridized with both the transgene mRNA (284 nuc band) and endogenous sEH mRNA (257 nuc band). The RPA was performed using the RPA III kit (Ambion inc.) as per the manufacturer’s instructions. Twenty five µg total RNA and 50,000 CPM of 32P labeled probe were hybridized in each sample. A probe recognizing 28S RNA was used as an internal loading control.
4.6 Soluble epoxide hydrolase activity
Relative sEH activity was determined by HPLC quantitation of the conversion of 14,15-[3H]EET to 14,15-[3H]DHET by mouse brain homogenates. Ten µl of homogenate was incubated with 2.0 µM 14,15-[3H]EET and 0.1 mM BSA in PO4- buffer at 37°C for 10 minutes. The reaction was stopped by adjusting the pH to 4.0 with 20 µl 1:10 formic acid. The reactions were extracted twice with three volumes of water-saturated ethyl acetate. After the solvent was evaporated under N2, the lipid residue was dissolved in acetonitrile for separation by reverse-phase HPLC using a Gilson 332 gradient system with a Supelco C18 5.0µM 4.6×150 mm Discovery™ column. The percent conversion of EET to DHET was normalized to 20 µg total homogenate protein.
4.7 Blood pressure
Mice were anesthetized with a solution of Ketamine (87.5 mg/kg) and Xylazine (12.5 mg/kg) and then implanted with TA11PA-C20 radiotelemeters (Data Sciences International) in the left carotid artery for direct measurement of arterial pressure (AP) and heart rate (HR) as described previously (Halabi et al., 2008). Mice were given 7 days to recover before HR and AP were recorded for 10 sec every 5 minutes for 7 days. The mice were switched to a high salt diet, allowed two days to adjust to the diet, and then recordings were continued for an additional 7 days. Sampling segments for each hour were averaged and these averages used to determine the 12 hour light and dark cycle average blood pressures or 24 hour blood pressure. All of the hemodynamic data were collected and analyzed on a computer using Dataquest ART.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aicher SA, Reis DJ. Gigantocellular vasodepressor area is tonically active and distinct from caudal ventrolateral vasodepressor area. Am. J. Physiol. 1997;272:R731–R742. doi: 10.1152/ajpregu.1997.272.3.R731. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- Amruthesh SC, Boerschel MF, McKinney JS, Willoughby KA, Ellis EF. Metabolism of arachidonic acid to epoxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and prostaglandins in cultured rat hippocampal astrocytes. J. Neurochem. 1993;61:150–159. doi: 10.1111/j.1471-4159.1993.tb03550.x. [DOI] [PubMed] [Google Scholar]
- Chacos N, Capdevila J, Falck JR, Manna S, Martin-Wixtrom C, Gill SS, Hammock BD, Estabrook RW. The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch. Biochem. Biophys. 1983;223:639–648. doi: 10.1016/0003-9861(83)90628-8. [DOI] [PubMed] [Google Scholar]
- Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J. Physiol. 2003;548:53–69. doi: 10.1113/jphysiol.2002.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A. Inflammatory response: pathway across the blood-brain barrier. Nature. 2001;410:430–431. doi: 10.1038/35068632. [DOI] [PubMed] [Google Scholar]
- Enayetallah AE, French RA, Barber M, Grant DF. Cell-specific subcellular localization of soluble epoxide hydrolase in human tissues. J. Histochem. Cytochem. 2006;54:329–335. doi: 10.1369/jhc.5A6808.2005. [DOI] [PubMed] [Google Scholar]
- Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARγ Function in Smooth Muscle Causes Vascular Dysfunction and Hypertension. Cell Metabolism. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Close LN, Selden NR, Alkayed NJ. A novel role for P450 eicosanoids in the neurogenic control of cerebral blood flow in the rat. Exp. Physiol. 2007;92:653–658. doi: 10.1113/expphysiol.2006/036889. [DOI] [PubMed] [Google Scholar]
- Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79:2311–2319. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, Wagner K, Jones PD, Morisseau C, Hammock BD. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junier MP, Dray F, Blair I, Capdevila J, Dishman E, Falck JR, Ojeda SR. Epoxygenase products of arachidonic acid are endogenous constituents of the hypothalamus involved in D2 receptor-mediated, dopamine-induced release of somatostatin. Endocrinology. 1990;126:1534–1540. doi: 10.1210/endo-126-3-1534. [DOI] [PubMed] [Google Scholar]
- Kozak W, Fraifeld V. Non-prostaglandin eicosanoids in fever and anapyrexia. Front Biosci. 2004;9:3339–3355. doi: 10.2741/1486. [DOI] [PubMed] [Google Scholar]
- Medhora M, Daniels J, Mundey K, Fisslthaler B, Busse R, Jacobs ER, Harder DR. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am. J. Physiol Heart Circ. Physiol. 2003;284:H215–H224. doi: 10.1152/ajpheart.01118.2001. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci. Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Cassell MD, Sigmund CD. Glial- and neuronal-specific expression of the renin-angiotensin system in brain alters blood pressure, water intake, and salt preference. J. Biol. Chem. 2002;277:33235–33241. doi: 10.1074/jbc.M204309200. [DOI] [PubMed] [Google Scholar]
- Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, Alkayed NJ, Van Winkle DM. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Physiol Heart Circ. Physiol. 2008;295:H2128–H2134. doi: 10.1152/ajpheart.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am. J. Physiol Heart Circ. Physiol. 2000;278:H1163–H1167. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- Rawal S, Morisseau C, Hammock BD, Shivachar AC. Differential subcellular distribution and colocalization of the microsomal and soluble epoxide hydrolases in cultured neonatal rat brain cortical astrocytes. J Neurosci. Res. 2009;87:218–227. doi: 10.1002/jnr.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, Hammock BD. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers KW, Sun C, ez-Freire C, Waki H, Morisseau C, Falck JR, Hammock BD, Paton JF, Raizada MK. Novel mechanism of brain soluble epoxide hydrolase-mediated blood pressure regulation in the spontaneously hypertensive rat. FASEB J. 2005;19:626–628. doi: 10.1096/fj.04-3128fje. [DOI] [PubMed] [Google Scholar]
- Snyder GD, Yadagiri P, Falck JR. Effect of epoxyeicosatrienoic acids on growth hormone release from somatotrophs. Am. J. Physiol. 1989;256:E221–E226. doi: 10.1152/ajpendo.1989.256.2.E221. [DOI] [PubMed] [Google Scholar]
- Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- Terashvili M, Tseng LF, Wu HE, Narayanan J, Hart LM, Falck JR, Pratt PF, Harder DR. Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of beta-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol. Exp. Ther. 2008;326:614–622. doi: 10.1124/jpet.108.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Davis BB, Morisseau C, Hammock BD, Olson JL, Kroetz DL, Weiss RH. Vascular localization of soluble epoxide hydrolase in the human kidney. Am. J. Physiol Renal Physiol. 2004;286:F720–F726. doi: 10.1152/ajprenal.00165.2003. [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Foley J, Boyle JE, Moomaw CR, Tomer KB, Parker C, Steenbergen C, Wu S. Predominant expression of an arachidonate epoxygenase in islets of Langerhans cells in human and rat pancreas. Endocrinology. 1997;138:1338–1346. doi: 10.1210/endo.138.3.4970. [DOI] [PubMed] [Google Scholar]
- Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke. 2002;33:2957–2964. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J. Cereb. Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J. Am. Soc. Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]