Abstract
Purpose
Present study was undertaken to gain insights into the mechanism of cell cycle arrest by ginseng saponin ginsenoside Rh2 (Rh2) using MCF-7 and MDA-MB-231 breast cancer cells.
Methods
Cell viability and cell cycle distribution was determined by trypan blue dye exclusion assay and flow cytometry, respectively. Immunoblotting was performed to determine changes in protein levels. Knockdown of desired protein was achieved by transfection with small interfering RNA (siRNA).
Results
Rh2 treatment significantly inhibited viability of both cells in a concentration-dependent manner, which correlated with G0/G1 phase cell cycle arrest. Rh2-mediated cell cycle arrest was accompanied by down-regulation of cyclin-dependent kinases (Cdk) and cyclins leading to decreased interaction between cyclin D1 and Cdk4/Cdk6 and increased recruitment of p15Ink4B and p27Kip1 to cyclin D1/Cdk4 and cyclin D1/Cdk6 complexes. In addition, Rh2 treatment markedly reduced the levels of phosphorylated retinoblastoma protein (P-Rb) and decreased transcriptional activity of E2F1 in luciferase reporter assay. Rh2-induced cell cycle arrest was significantly attenuated by knockdown of p15Ink4B and/or p27Kip1 proteins.
Conclusions
Rh2-mediated cell cycle arrest in human breast cancer cells is caused by p15Ink4B and p27Kip1-dependent inhibition of kinase activities of G1-S specific Cdks/cyclin complexes.
Keywords: Ginsenoside Rh2, Breast Cancer, p15 and p27, Cell cycle arrest
INTRODUCTION
Breast cancer continues to claim thousands of lives each year despite significant advances in screening techniques and treatment modalities (1). The known risk factors for breast cancer include family history, Li-Fraumeni syndrome, atypical hyperplasia of the breast, late age at first full-term pregnancy, early menarche, and late menopause (2–4). Because some of these risk factors are not easily modifiable (e.g., genetic predisposition), other strategies for reduction of the breast cancer risk must be considered. Selective estrogen-receptor (ER) modulators (e.g., tamoxifen) appear promising for prevention of breast cancer, but this strategy is largely ineffective against ER negative breast cancers and ER modulators have serious side effects including increased risk of uterine cancer, thromboembolism, cataracts, and perimenopausal symptoms (5,6). Therefore, novel agents for prevention and treatment of human breast cancer, especially hormone-independent breast cancer, are highly desirable. Natural products have received increasing attention in recent years for the discovery of novel cancer preventive and therapeutic agents (7).
The root of Panax ginseng has been used for thousands of years in Korean alternative medicine for treatment of diverse ailments including liver dysfunction, hypertension, atherosclerosis, and post-menopausal symptoms (8). More recent studies have indicated that purified ginsenoside saponins isolated from the root of Panax ginseng C. A. Meyer can inhibit growth of cancer cells in culture and in vivo (9–17).For example, crude ginsenosides caused phenotypic reverse transformation in Morris hepatoma cells and purified ginsenoside Rh2 (Rh2) inhibited growth of B16 melanoma cells in association with increased melanogenesis (9,10). Treatment with Rh2 caused repression of matrix metalloproteinase genes in human astroglioma cells (18). The Rh2 and paclitaxel combination synergistically inhibited growth of human prostate cancer cells (19). Furthermore, Rh2 enhanced in vivo antitumor activity of cyclophosphamide against B16 melanoma cells (20).
The Rh2-mediated suppression of cancer cell proliferation correlates with G0/G1 phase cell cycle arrest or apoptosis induction (10–17). Elucidation of the mechanism responsible for Rh2-mediated apoptosis and cell cycle arrest has been the topic of intense research in the past few years (11–17). The Rh2-induced apoptotic cell death in neuroblastoma cells was caused by activation of caspase-1 and -3 and up-regulation of Bax (13). Apoptosis induction resulting from Rh2 exposure in PC-3 and LNCaP human prostate cells correlated with modulation of mitogen-activated protein kinases (14). The Rh2 treatment blocked cell cycle progression of SK-HEP-1 cells at the G1/S boundary by selectively inducing expression of p27kip1 but without affecting levels of cyclin E, cyclin-dependent kinase 2 (Cdk2), and p21WAF1 (11). The G0/G1 phase arrest caused by Rh2 treatment in MCF-7 human breast cancer cells was accompanied by induction of p21WAF1 (12).
The present study extends these findings and now demonstrates that Rh2 causes G0/G1 phase cell cycle arrest in human breast cancer cells (MCF-7 and MDA-MB-231) regardless of their estrogen responsiveness and p15Ink4B or p53 status by inhibiting kinase activities of G1-S specific Cdk/cyclin complexes, reducing phosphorylation of retinoblastoma (Rb), and suppressing transcriptional activity of E2F1. Moreover, knockdown of p15Ink4B and p27Kip1 proteins confer significant protection against Rh2-mediated cell cycle arrest.
MATERIALS AND METHODS
Reagents
Ginsenoside Rh2 (purity ~97%) was purchased from LKT Laboratories (St. Paul, MN). Stock solution of Rh2 was prepared in dimethyl sulfoxide (DMSO), stored at −20 °C, and diluted with fresh complete medium immediately before use. An equal volume of DMSO (final concentration, <0.1%) was added to the controls. Tissue culture media, fetal bovine serum (FBS), trypsin-EDTA solution, antibiotic mixture, sodium pyruvate, HEPES, and nonessential amino acids were obtained from GIBCO (Grand Island, NY, USA). The HiPerFect transfection reagent was from Qiagen (Germantown, MD, USA). Propidium iodide, RNaseA and phosphatase inhibitors were from Sigma (St. Louis, MO). Protease inhibitor cocktail was from BD Biosciences PharMingen (San Diego, CA). Antibodies against cyclin A, cyclin D1, cyclin E, Cdk2, Cdk6, and p15Ink4B were from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies against total Rb and phospho-(Ser 807/811)-Rb were from Cell Signaling Technology (Beverly, MA). The antibodies against Cdk4 and p27Kip1 were from BD Biosciences PharMingen. Anti-actin antibody was from Sigma. Protein A/G Plus-Agarose was from Santa Cruz Biotechnology. A control nonspecific siRNA was from Qiagen. The p15Ink4B- and 27Kip1-targeted siRNA were purchased from Santa Cruz Biotechnology.
Cell Culture and Cell Viability Assay
Monolayer cultures of MCF-7 cells were maintained in MEM supplemented with 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 1.5 g/L sodium bicarbonate, 10% (v/v) FBS, and antibiotics. The MDA-MB-231 cultures were maintained in RPMI1640 medium supplemented with 10% (v/v) FBS and antibiotics. Each cell line was maintained in an atmosphere of 95% air and 5% CO2 at 37°C. The effect of Rh2 on cell viability was determined by trypan blue dye exclusion assay as described previously (21).
Analysis of Cell Cycle Distribution
The effect of Rh2 treatment on cell cycle distribution was determined by flow cytometry following staining the cells with propidium iodide as described previously (22). Briefly, 5×105 cells were plated and treated with Rh2 or DMSO (control) for specified time periods, and both floating and attached cells were collected. The cells were then stained with propidium iodide, and the cell cycle distribution was determined using a Coulter Epics XL flow cytometer (22).
Immunoblotting
Cells were treated with desired concentrations of Rh2 for specified time intervals and lysed as described by us previously (23). The cell lysate was cleared by centrifugation at 20,000 rpm for 20 min, and the supernatant fraction was used for immunoblotting. Proteins were resolved by sodium-dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto membrane. After blocking with 5% non-fat dry milk in Tris buffered saline containing 0.05% Tween-20, the membrane was incubated with the desired primary antibody for 1 h at room temperature or overnight at 4°C. The membrane was then treated with appropriate secondary antibody, and the immunoreactive bands were visualized by enhanced chemiluminescence method.
Immunoprecipitation-Immunoblotting Assay
The MCF-7 cells were treated with 40 μM Rh2 for the indicated time periods, washed twice with ice-cold phosphate buffered saline (PBS), and lysed with 10 mM HEPES buffer containing 1% CHAPS. Equal amounts of lysate proteins were incubated with desired primary antibody (anti-Cdk4, anti-cyclin D1 or anti-Cdk2). Pulled down immune complexes with Protein A/G-agarose were subjected to electrophoresis followed by immunoblotting using anti-cyclin D1, anti-Cdk6, anti-cyclin E, anti-p15Ink4B or anti-p27Kip1 antibody as described above.
Kinase Assay
Cdk and cyclin-associated kinase activity was determined as described (24) with some modifications. After the indicated treatments, MCF-7 cells were lysed in 20 mM HEPES buffer containing 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 10 mM β-glycerophosphate, and protease and phosphatase inhibitors. Aliquots containing 500 μg of lysate proteins were incubated overnight at 4°C with 10 μg of desired antibody. Protein A-agarose (50 μl) was subsequently added to each sample and the incubation was continued for an additional 2 h at 4°C with gentle shaking. Immunoprecipitated Cdk2, Cdk4, Cyclin D1, and Cyclin E were incubated with the substrates Histone H1 or Rb in kinase reaction buffer containing [γ-32P] for 30 minute at 30°C. The immunoprecipitates were subjected to SDS-PAGE followed by imaging.
Luciferase Reporter Assay
The E2F1 transcriptional activity was measured using E2F1 luciferase reporter construct as described by us previously (25). Briefly, cells were transiently co-transfected with pGL2-E2F1-Luc and pRL-CMV plasmids. The E2F1-luciferase reporter construct (−728/+77 promoter region) was a generous gift from Dr. Stephen Safe (Texas A&M University, College Station, TX). After transfection, cells were treated with Rh2 for desired time periods. A 20 μl supernatant fraction was used for measurement of dual luciferase activity (Promega) using a luminometer. The luciferase activity normalized against protein concentration was expressed as a ratio of firefly luciferase to Renilla luciferase units.
RNA Interference
The MCF-7 cells were seeded in 60-mm plates and treated at 50% confluency with a control nonspecific siRNA and p15Ink4B and/or p27Kip1 siRNA using HiPerFect transfection reagent according to the manufacturer’s recommendations. Twenty-four hours after transfection, the cells were treated with DMSO or Rh2 (40 or 60 μM) for the indicated time periods. The cells were then collected and processed for immunoblotting and analysis of cell cycle distribution.
RESULTS
Ginsenoside Rh2 Treatment Decreased Viability of Human Breast Cancer Cells
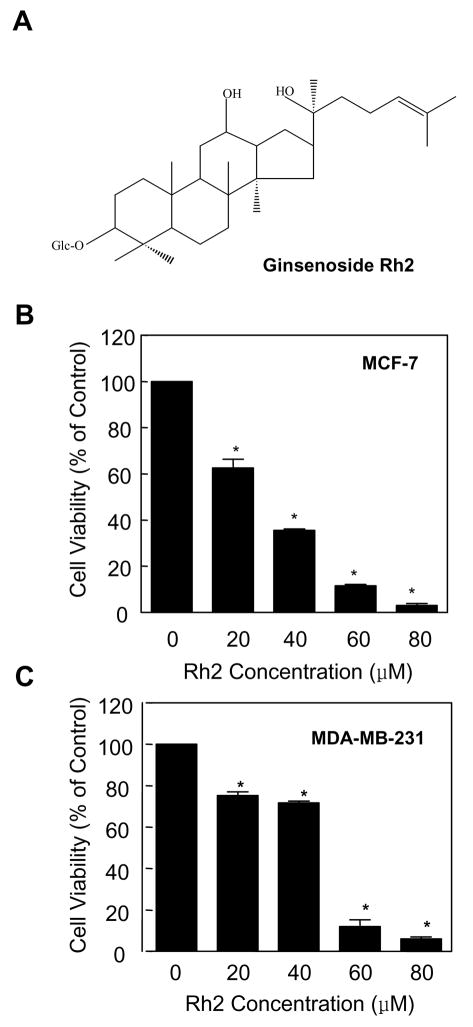
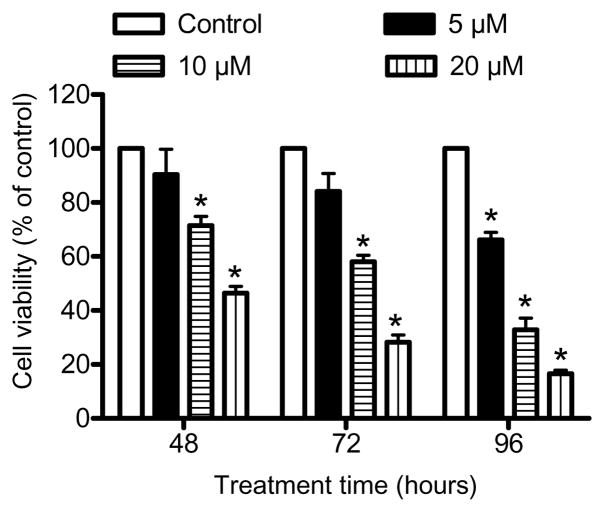
Initially, we determined the effect of ginsenoside Rh2 (Fig. 1A for the structure of Rh2) treatment (24 h exposure) on cell viability using MCF-7 and MDA-MB-231 cell lines, which respectively are well characterized representatives of estrogen-responsive and estrogen-independent human breast cancers. Ginsenoside Rh2 decreased the viability of both MCF-7 (Fig. 1B) and MDA-MB-231 cells (Fig. 1C) in a dose-dependent manner. In a separate experiment, we determined antiproliferative effect of extended treatment duration (48, 72, and 96 h) with lower concentrations of Rh2 using MCF-7 cells. As can be seen in Fig. 2, statistically significant decrease in MCF-7 cell viability was evident in the presence of 5 μM Rh2 at the 96 h time point. Collectively, these results indicated that ginsenoside Rh2 treatment decreased viability of breast cancer cells irrespective of their estrogen responsiveness, albeit more effectively in the MCF-7 cell line.
Fig. 1.
Rh2 treatment inhibits viability of human breast cancer cells. A chemical structure of Rh2. Effect of Rh2 treatment on viability of (B) MCF-7 and (C) MDA-MB-231 cells as determined by trypan blue dye exclusion assay. The desired cell line was treated with DMSO (control) or different concentrations of Rh2 (20, 40, 60, and 80 μM) for 24 h. Columns, mean (n=3); bars, SE. *, p < 0.05, significantly different compared with DMSO-treated control by one-way ANOVA followed by Dunnett’s test. Similar results were observed in replicate experiments.
Fig. 2.
Effect of Rh2 treatment on viability of MCF-7 cells. The MCF-7 cells were treated with DMSO (control) or Rh2 (5, 10, and 20 μM) for the indicated time periods. Columns, mean (n=3); bars, SE. *, p < 0.05, significantly different compared with DMSO-treated control by one-way ANOVA followed by Dunnett’s test. Similar results were observed in replicate experiments.
Ginsenoside Rh2 Caused G0/G1 Phase Cell Cycle Arrest in Human Breast Cancer Cells
Previous studies have revealed G0/G1 phase cell cycle arrest by Rh2 treatment in MCF-7 cells (12), which express wild type p15Ink4B and p53. It was of interest to determine whether Rh2-mediated cell cycle arrest is dependent on p15 Ink4B and/or p53 status. This association is plausible considering important roles of p15Ink4B and p53 in regulation of cell cycle progression (26,27). Exposure of MCF-7 cells to 40 μM Rh2 for 12–24 h resulted in significant G0/G1 arrest, which was accompanied by a decrease in both S phase and G2/M phase cells (Table I). The Rh2-mediated cell cycle arrest in MCF-7 cells was evident as early as 8 h and peaked between 24 h and 48 h post-treatment. At the 48 h time point, the Rh2 treatment resulted in a significant increase in fraction of sub-diploid apoptotic cells (Table I). The Rh2-mediated enrichment of G0/G1 fraction was also observed in the MDA-MB-231 cell line (Table I). However, this effect was relatively more pronounced in the MCF-7 cell line than in MDA-MB-231 (Table I). For example, 24 h exposure of MCF-7 cells to 40 μM Rh2 resulted in about 1.42-fold enrichment of G0/G1 fraction relative to DMSO-treated control. A similar treatment with Rh2 caused only a modest yet statistically significant enrichment of G0/G1 fraction in the MDA-MB-231 cells (Table I). These results indicated that the MCF-7 cell line was relatively more sensitive to Rh2-mediated cell cycle arrest compared with the MDA-MB-231 cell line.
Table I.
Effect of Rh2 treatment on cell cycle distribution in human breast cancer cells.
| Treatment time (hours) | (percent cell in phase) |
|||
|---|---|---|---|---|
| Sub-G0/G1 | G0/G1 | S | G2/M | |
| MCF-7 | ||||
| 0 | <1 | 51.6 ± 0.5 | 12.9 ± 0.6 | 26.7 ± 0.1 |
| 4 | <1 | 55.8 ± 0.4 | 12.4 ± 0.8 | 29.7 ± 0.1 |
| 8 | <1 | 55.6 ± 0.2* | 11.6 ± 0.2 | 30.6 ± 0.1* |
| 12 | <1 | 63.8 ± 0.5* | 10.2 ± 1.7* | 24.4 ± 0.1* |
| 24 | <1 | 73.2 ± 0.4* | 5.2 ± 0.7* | 20.1 ± 0.1* |
| 48 | 21.4 ± 1.3* | 58.7 ± 0.9* | 5.5 ± 0.2* | 12.8 ± 0.6* |
| MDA-MB-231 | ||||
| 0 | 1.2 ± 0.8 | 59.5 ± 0.2 | 8.4 ± 0.8 | 30.3 ± 0.1 |
| 4 | 1.1 ± 0.7 | 58.0 ± 0.1 | 8.9 ± 0.5 | 31.2 ± 0.1 |
| 8 | 1.5 ± 1.5 | 60.9 ± 0.5 | 10.5 ± 0.9* | 26.5 ± 0.2* |
| 12 | 1.1 ± 0.4 | 60.9 ± 0.2* | 8.6 ± 0.8 | 28.8 ± 0.1* |
| 24 | 3.0 ± 1.9 | 65.1 ± 0.5* | 6.9 ± 1.2* | 24.3 ± 0.5* |
| 48 | 7.7 ± 2.1* | 68.4 ± 0.6* | 5.4 ± 1.0* | 18.0 ± 0.9* |
Cells were treated with DMSO (control) or 40 μM Rh2 for the indicated time periods. Results are mean SE (n=3).
p < 0.05, significantly different compared with DMSO-treated control by one-way ANOVA followed by Dunnett’s test.
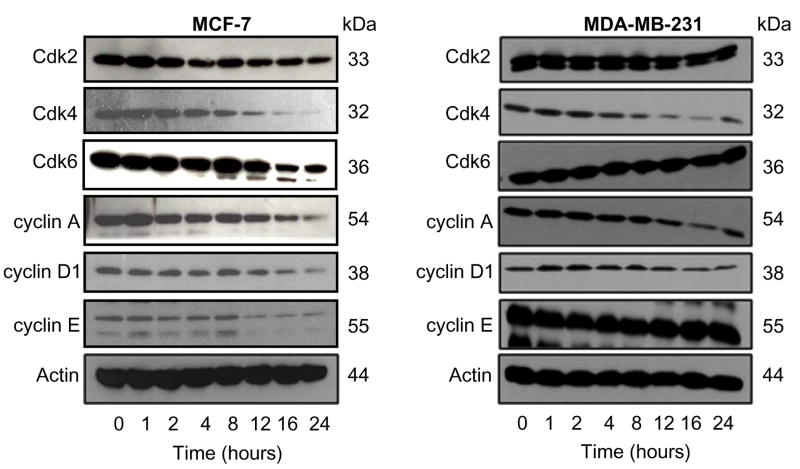
Effect of Rh2 Treatment on Protein Levels of G1-S Specific Cdks/Cyclins
Eukaryotic cell cycle progression involves sequential activation of Cdks whose association with corresponding regulatory cyclins is necessary for their activation (28,29). For instance, the G0/G1-S transition is regulated by complexes formed by cyclin D and Cdk4 or Cdk6 and cyclin E and Cdk2 (28,29). We determined the effect of Rh2 treatment on protein levels of G0/G1-S specific cyclins and Cdks by immunoblotting to gain insight into the mechanism of Rh2-induced cell cycle arrest in our model. As can be seen in Fig. 3, Rh2 treatment caused a marked decrease in protein levels of Cdk2, Cdk4, Cdk6, cyclin A, cyclin D1, and cyclin E in MCF-7 cells. The Rh2-mediated decline in levels of above mentioned proteins in MCF-7 cells was evident between 12–24 h. On the other hand, Rh2 treatment caused down-regulation of Cdk4, cyclin A, and cyclin D1, but not Cdk2, Cdk6, or cyclin E, in the MDA-MB-231 cells (Fig. 3). These results indicated that Rh2-mediated cell cycle arrest in MCF-7 and MDA-MB-231 cells was associated with a decrease in protein levels of cyclins and Cdks. However, the Rh2-mediated decline in levels of Cdks/cyclins was more severe in the MCF-7 cell line than in MDA-MB-231, which may partly explain relative resistance of the later to growth inhibition and cell cycle arrest by Rh2.
Figure 3.
Effect of Rh2 treatment on protein levels of cell cycle regulators. Immunoblotting for Cdk2, Cdk4, Cdk6, cyclin A, cyclin D1, and cyclin E using lysates from MCF-7 and MDA-MB-231 cells treated with 40 μM Rh2 for the indicated time periods. The blots were stripped and reprobed with anti-actin antibody to correct for differences in protein level. Immunoblotting for each protein was performed at least twice using independently prepared lysates and the results were similar.
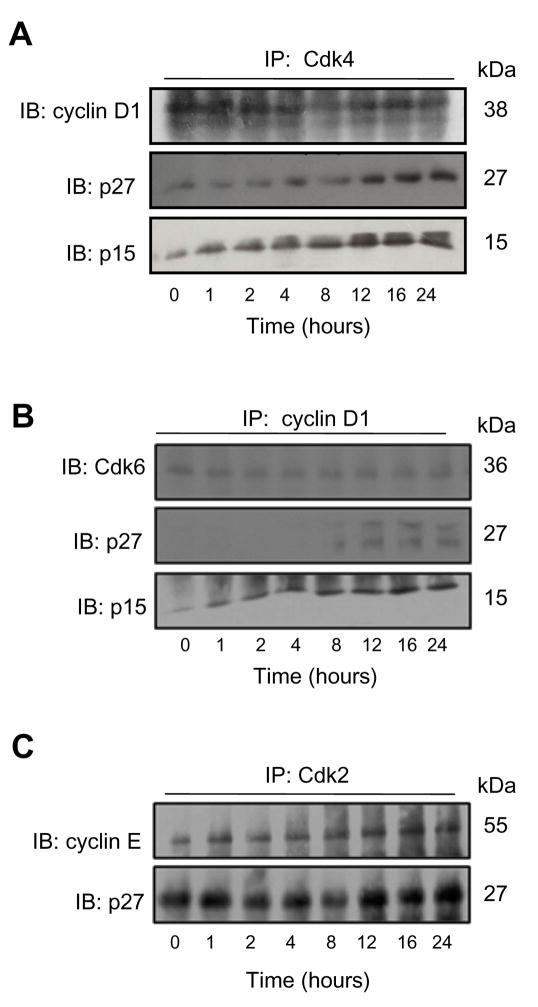
Rh2 Treatment Affected Interaction Between Cdks, Cyclins, and Cdk Inhibitors
Because the effect of Rh2 was most pronounced on cyclin D1 and Cdk4 protein expression especially in the MCF-7 cell line, we raised the question of whether Rh2 treatment affected interaction between these proteins. We addressed this question by immunoprecipitation of Cdk4, cyclin D1, or Cdk2 from equal amounts of lysate proteins from control and 40 μM Rh2-treated MCF-7 cells followed by immunoblotting using anti-cyclin D1, anti-Cdk6 or anti-cyclin E antibody. The binding of cyclin D1 with Cdk4 (Fig. 4A) or Cdk6 (Fig. 4B) was suppressed by Rh2 treatment. On the other hand, the interaction between cyclin E and Cdk2 was not inhibited by Rh2 treatment (Fig. 4C). The Cdk inhibitors can negatively regulate cell cycle progression by competing with cyclin D1 for binding with Cdk4 or Cdk6 complexes and inhibiting the kinase activities of Cdks/cyclin complexes (28,29). The MCF-7 and MDA-MB-231 cell lines have different genetic characters. The p15Ink4B and p27Kip1 are wild type in MCF-7 cells whereas p15Ink4B gene is deleted in the MDA-MB-231 cells. The expression of p27Kip1 protein is relatively lower in the MDA-MB-231 cells compared with MCF-7. To determine whether these Cdk inhibitors were involved in G0/G1 phase cell cycle arrest in our model, the immunoprecipitated complexes were also probed with anti-p15Ink4B and p27Kip1 antibodies. As can be seen in Fig. 4A-C, the Rh2 treatment caused recruitment of p15Ink4B and p27Kip1 to cyclin D1-Cdk4 complex despite reduction in the complex formation between cyclin D1 and Cdk4. Recruitment of p27Kip1 to cyclin D1/Cdk6 complex was less pronounced than that observed for Cdk4/cyclin D1. The Rh2 treatment also resulted in increased binding of p27Kip1 with cyclin E/Cdk2 complex (Fig. 4C). Collectively, these results indicated that the Rh2-mediated cell cycle arrest in MCF-7 cells was associated with reduced complex formation between cyclin D1 and Cdk4/Cdk6 and increased recruitment of Cdk inhibitors p15Ink4B and p27Kip1 to these complexes.
Figure 4.
Rh2 treatment recruits Cdk1 inhibitors. Immunoblotting for (A)cyclin D1, p27 Kip1 and p15Ink4B, (B) Cdk6, p27Kip1 and p15Ink4B, and (C) cyclin E and p27Kip1 using immunoprecipitated complexes with (A) anti-Cdk4, (B) anti-cyclin D1, and (C) anti-Cdk2 antibodies. Equal amounts of lysate proteins from MCF-7 cell treated with 40 μM Rh2 for the indicated time periods were used for immunoprecipitation. Similar results were observed in replicate experiments.
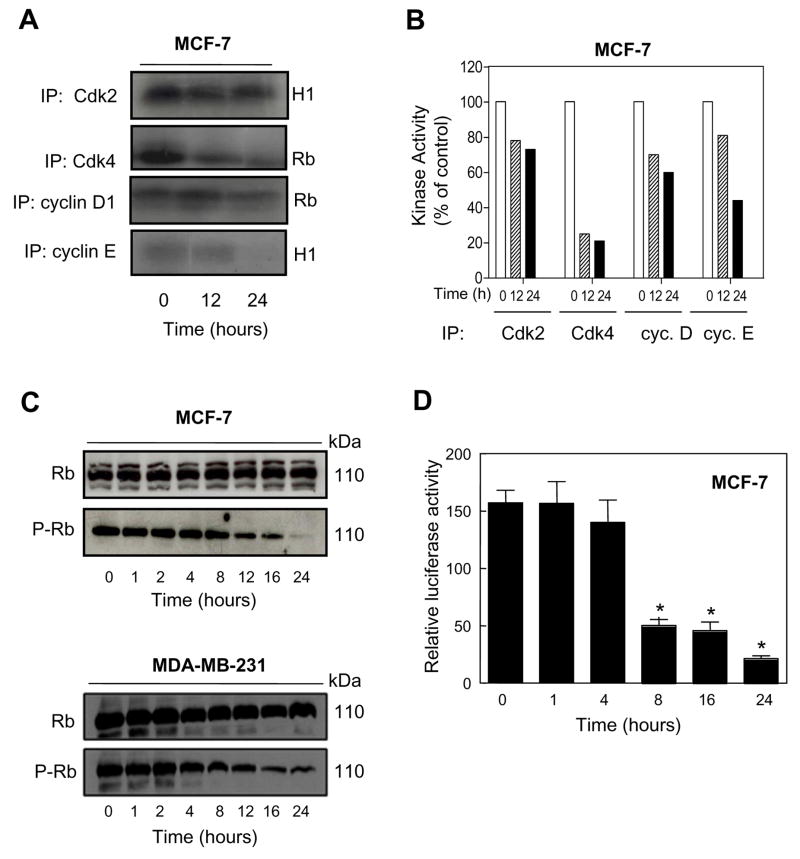
Ginsenoside Rh2 Treatment Inhibited Kinase Activities of Cdk4/cyclin D1 and Cdk2/cyclin E Complexes in MCF-7 Cells
Next, we determined the effect of Rh2 treatment on kinase activities of Cdk4/cyclin D1 and Cdk2/cyclin E complexes by immunoprecipitation-kinase assays using Rb and Histone H1 as substrates, respectively, to confirm inhibition of these kinases. Ginsenoside Rh2 treatment strongly decreased the kinase activity of Cdk4/cyclin D1 complex towards Rb (Fig. 5A). Despite retained complex formation between Cdk2 and cyclin E, the Rh2 treatment also inhibited the kinase activity of Cdk2/cyclin E in MCF-7 cells (Fig. 5A,B).
Figure 5.
Effect of Rh2 treatment on kinase activity. A In vitro kinase activity of Cdk2, Cdk4, cyclin D1 and cyclin E immunoprecipitates from MCF-7 cells treated with 40 μM Rh2 for 12 or 24 h using Rb fragment and histone H1 as substrates. B Relative kinase activity in different immunoprecipitates in MCF-7 cultures treated with 40 μM Rh2 for 12 or 24 h. C Immunoblotting for total Rb and P-Rb using lysate from MCF-7 and MDA-MB-231 cells treated with 40 μM Rh2 for the indicated time periods. Immunoblotting for each protein was preformed at least twice using independently prepared lysates and the results were similar. The blots were stripped and re-probed with anti-actin antibody to ensure equal protein loading. D Transcriptional activity of E2F1 as determined by luciferase reporter gene assay in MCF-7 cells treated with 40 μM Rh2 for the indicated time periods. Columns, mean (n= 3); bars, SE. *, p < 0.05, significantly different compared with control by t-test.
Ginsenoside Rh2 Treatment Suppressed Phosphorylation of Rb and Transcriptional Activity of E2F1
The cyclin D1/Cdk4 and cyclin D1/Cdk6 kinase complexes hyperphosphorylate Rb protein leading to its dissociation from the transcription factor E2F1, which regulates expression of genes necessary for cell cycle progression (28,29). To gain further insight into the mechanism of Rh2-induced cell cycle arrest, we determined its effect on protein levels and phosphorylation of Rb by immunoblotting. As shown in Fig. 5C, Rh2 treatment caused a decrease in levels of phosphorylated P-Rb in both MCF-7 and MDA-MB-231 cells, which was not due to a decrease in total Rb protein level. Because phosphorylation of Rb affects the transcriptional activity of E2F1 (28, 29) and Rh2 treatment resulted in suppression of Rb phosphorylation (Fig. 5C), we determined the transcriptional activity of E2F1 by luciferase reporter gene assay in Rh2-treated MCF-7 cells. As can be seen in Fig. 5D, Rh2 treatment indeed caused a significant decrease in E2F1-associated luciferase activity in MCF-7 cells, which coincided with the kinetics of cell cycle arrest (Table I). The transcriptional activity of E2F1 was also inhibited in MDA-MB-231 cells after treatment with 40 μM Rh2 for 16- and 24 h (results not shown). Collectively, these results indicated that the Rh2-mediated G1 phase cell cycle arrest in breast cancer cell lines was associated with decreased phosphorylation of Rb leading to inhibition of the transcriptional activity of E2F1.
p15Ink4B and p27Kip1 Knockdown Protected Against Rh2-mediated Cell Cycle Arrest
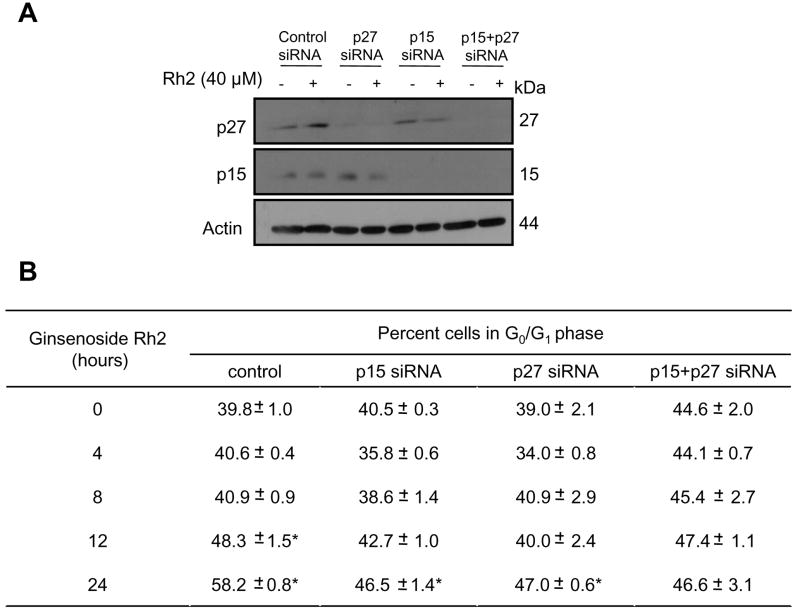
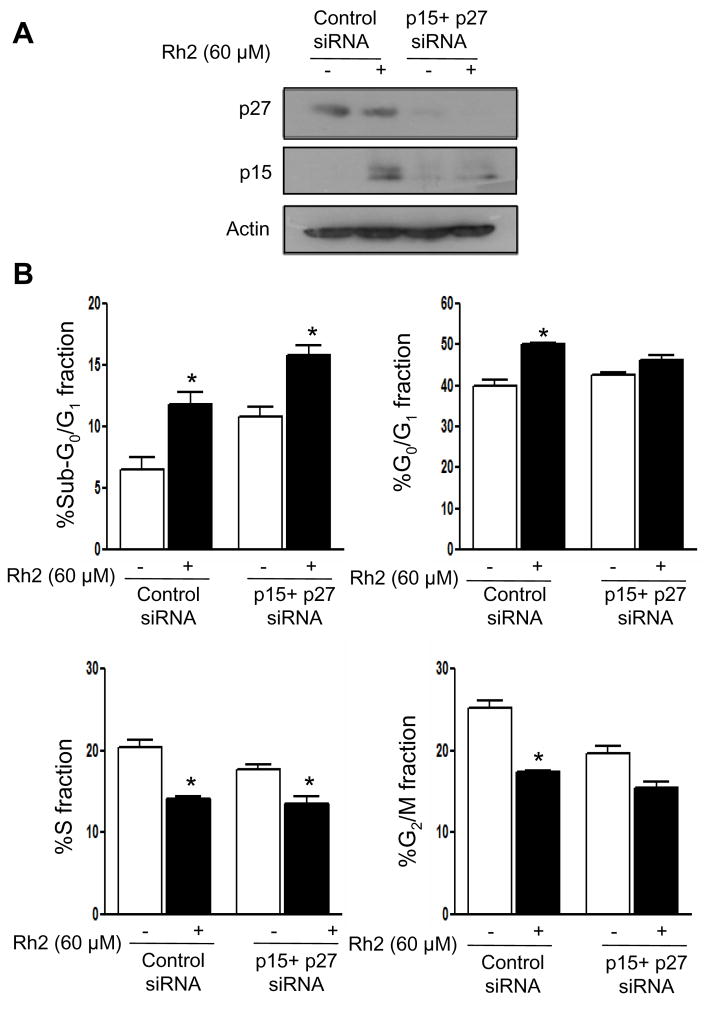
To experimentally verify the roles of Cdk inhibitors p15Ink4B and p27Kip1 in Rh2-induced cell cycle arrest, we performed siRNA knockdown studies using MCF-7 cells. As can be seen in Fig. 6A, transfection with p15Ink4B and p27Kip1-targeted siRNA resulted in near-complete knockdown of respective proteins (Fig. 6A). We next analyzed the effect of p15Ink4B and p27 Kip1 knockdown on Rh2-mediated cell cycle arrest using MCF-7 cells and the results are shown in Fig. 6B. Treatment of control nonspecific siRNA transfected MCF-7 cells with 40 μM Rh2 for 24 h resulted in about 46% increase in G0/G1 fraction relative to control. The Rh2-induced cell cycle arrest was partially but statistically significantly attenuated in MCF-7 cells transfected with p15Ink4B and p27Kip1-targeted siRNA (Fig. 6B). We also carried out double siRNA transfection to suppress both p15Ink4B and p27Kip1 simultaneously. The Rh2-induced cell cycle arrest was fully blocked in p15Ink4B and p27Kip1 depleted cells compared with control nonspecific siRNA transfected cells (Fig. 6B). In a separate experiment, we used a higher concentration of Rh2 (60 μM) to confirm roles of p15Ink4B and p27Kip1 in cell cycle arrest in our model. As can be seen in Fig. 7A, transfection with p15Ink4B and p27Kip1 targeted siRNA caused significant knockdown of these proteins in MCF-7 cells. In addition, the G0/G1 phase cell cycle arrest resulting from a 24 h treatment with 60 μM Rh2 in MCF-7 cells was significantly attenuated by combined knockdown of p15Ink4B and p27Kip1 proteins (Fig. 7B). Collectively, these results indicated that p15Ink4B and p27Kip1proteins play important roles in Rh2-induced G1 phase cell cycle arrest in breast cancer cells.
Figure 6.
Knockdown of p27 and p15 proteins confers protection against Rh2-induced cell cycle arrest. A Immunoblotting for p27Kip1 and p15Ink4B using lysates from control nonspecific siRNA-transfected MCF-7 cells and MCF-7 cells transfected with p27Kip1 and/or p15Ink4B-targeted siRNAs, and treated for 24 h with DMSO (control) or 40 μM Rh2. The blots were stripped and re-probed with anti-actin antibody to ensure equal protein loading. B Percentage of G1 fraction in MCF-7 cultures transfected with control nonspecific siRNA or p27Kip1 and/or p15Ink4B-targeted siRNAs and treated for 24 h with DMSO (control) or 40 μM Rh2. Columns, mean (n= 3); bars, SE. *, p < 0.05, significantly different compared with control by t-test.
Fig. 7.
A Immunoblotting for p27Kip1 and p15Ink4B using lysates from control nonspecific siRNA-transfected MCF-7 cells and MCF-7 cells transfected with p27Kip1 and p15Ink4B-targeted siRNAs, and treated for 24 h with DMSO (control) or 60 μM Rh2. The blots were stripped and re-probed with anti-actin antibody to ensure equal protein loading. B Cell cycle distribution in control nonspecific siRNA transfected MCF-7 cells and in MCF-7 cells transfected with p27Kip1/p15Ink4B siRNAs following 24 h treatment with DMSO (control) or 60 μM Rh2. Columns, mean (n= 3); bars, SE. *, p < 0.05, significantly different compared with control by paired t-test. Experiment was repeated with similar results.
DISCUSSION
In this study, we designed experiments to elucidate the mechanism of Rh2-induced cell cycle arrest using human breast cancer cells as a model. We found that the Rh2-mediated G0/G1 phase cell cycle arrest in breast cancer cell lines is caused by inhibition of kinase activities of G0/G1-S specific Cdk/cyclin complexes. This conclusion is supported by the following observations: (a) Rh2 treatment causes a decrease in protein levels of G0/G1-S specific Cdks and cyclins in both cell lines but more severely in the MCF-7 cells than in MDA-MB-231; (b) Rh2 treatment reduces complex formation between Cdk4/cyclin D1 and Cdk6/cyclin D1 in MCF-7 cells; and (c) the Rh2-treated breast cancer cells exhibit diminished activities of Cdk4/cyclin D1 and Cdk2/cyclin E complexes as judged by immunoprecipitation kinase assay and suppressed phosphorylation of Cdk4/cyclin D1 substrate Rb. The Rh2-mediated cell cycle arrest has also been documented in other cellular models but the mechanism may be cell line-specific. For example, unlike our data in MCF-7 cells, Rh2-mediated G0/G1 arrest in SK-HEP-1 hepatoma cells is not accompanied by decline in protein levels of cyclin E or Cdk2 but correlates with induction of p27Kip1; the levels of Cdk4, Cdk6 or cyclin D were not determined in this study (11). Likewise, the Rh2-mediated G0/G1 phase cell cycle arrest in A549 lung adenocarcinoma cells correlated with suppression of Cdk6, cyclin D1 and cyclin E protein expression, but unlike MCF-7 cells the levels of Cdk4, Cdk2, and cyclin A were not altered (15). Discrepancies are also evident between the results of the present study and literature in the MCF-7 cell line. For example, in contrast to our data, Oh et el. (12) did not observe changes in protein levels of cyclin D1, Cdk2, or Cdk4 upon treatment with 100 μM Rh2. One possibility to explain this discrepancy relates to the source/purity of Rh2 and preparation of its stock solution and the concentration of the test agent. The Rh2 used in our study was obtained from LKT laboratories with ~97% purity and solubilized in DMSO. The purity of the Rh2 preparation used by Oh et al. (12) was not described and stock solution was prepared in 75% ethanol. The possibility of differential chemical transformation/rearrangement of Rh2 in DMSO versus ethanol can’t be fully discarded.
The present study reveals that the Rh2-mediated cell cycle arrest is relatively more pronounced in the MCF-7 cell line than in MDA-MB-231. The MCF-7 cell line is also relatively more sensitive to growth suppression by Rh2, especially at lower concentrations, compared with the MDA-MB-231. Several possibilities exist to explain these results. First, the Rh2-mediated decline in protein levels of Cdk4, Cdk6, cyclin D1, Cdk2 and cyclin E is less severe or absent in the MDA-MB-231 cells compared with MCF-7 (Fig. 3). Second, the MDA-MB-231 cells have deletion of the p15Ink4B gene, whose protein product seems to be important in Rh2-mediated cell cycle arrest. Third, the MDA-MB-231 cells express mutant p53, which is transcriptional regulator of p21WAF1 (30). The p21WAF1 protein plays an important role in G0/G1-S transition (31). Even though we have not determined the effect of Rh2 treatment on p21WAF1 protein level in the present study, Rh2-mediated induction of this protein has been documented in the MCF-7 cell line (12). Finally, the possibility that ER somehow controls the Rh2-mediated cell cycle arrest can’t be discarded and requires experimental verification.
The Cdk inhibitors are considered tumor suppressors and negatively regulate cell cycle progression by binding with and inhibiting kinase activities of Cdks/cyclins (28,29). The Cdk inhibitors comprises of Ink and Cip/Kip family proteins, whose expression are up-regulated and closely related with cell cycle arrest in response to antiproliferative signal (26,28,29,31). The increased expression of G0/G1 phase specific cyclins provides an uncontrolled growth advantage because many cancer cell lines such as MDA-MB-231 either have deletion or low expression of Cdk inhibitors. We demonstrate in this study, for the first time, that both p15Ink4B and p27Kip1 play important roles in Rh2-mediated cell cycle arrest. The Rh2 treatment increases their recruitment (binding) to the Cdk/cyclin complexes (Fig. 4). Moreover, the knockdown of p15Ink4Band p27 Kip1 proteins confers near complete protection against Rh2-induced cell cycle arrest.
The Rb family proteins are critical down-stream targets of G0/G1 specific Cdk4/cyclin D complex (32). In hypophosphorylated state the Rb family proteins associate with and inhibit activity of E2F family transcription factors, which are involved in transcription of key cell cycle regulatory proteins (29,33,34). Upon growth stimulus, the G0/G1 specific Cdks/cyclins phosphorylate Rb proteins on multiple residues causing release of E2F family transcription factors (32). We found that the Rh2-mediated cell cycle arrest in breast cancer cells correlates not only with reduced phosphorylation of Rb but also with inhibition of transcriptional activity of E2F1 as revealed by luciferase reporter gene assay.
In conclusion, the present study indicates that Rh2 suppresses growth of human breast cancer cells in association with G0/G1 phase cell cycle arrest. The Rh2-induced cell cycle arrest correlates with inhibition of kinase activities of G0/G1-S specific Cdk/cyclin complexes. Furthermore, knockdown of p15Ink4B and p27Kip1 proteins confer significant protection against Rh2-mediated cell cycle arrest at least in MCF-7 cells.
Acknowledgments
Grant Support: This investigation was supported in part by the Kangwon Bio-Nuri grant, Korea Research Foundation, Korea, and US PHS grant CA129347, awarded by the National Cancer Institute.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 3.Hulka BS, Stark AT. Breast cancer: cause and prevention. Lancet. 1995;346:883–7. doi: 10.1016/s0140-6736(95)92713-1. [DOI] [PubMed] [Google Scholar]
- 4.Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Pub Health. 1996;17:47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention study (IBIS-I): a randomized prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 7.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 8.Yun TK, Meyer CA. Brief introduction of Panax ginseng. J Korean Med Sci. 2001;16(suppl):S3. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe H, Arichi S, Hayashi T, Odashima S. Ultrastructural studies of Morris hepatoma cells reversely transformed by ginsenosides. Experientia. 1979;35:1647–8. doi: 10.1007/BF01953246. [DOI] [PubMed] [Google Scholar]
- 10.Odashima S, Ohta T, Kohno H, et al. Control of phenotypic expression of cultured B16 melanoma cells by plant glycosides. Cancer Res. 1985;45:2781–4. [PubMed] [Google Scholar]
- 11.Lee KY, Park JA, Chung E, Lee YH, Kim SI, Lee SK. Ginsenoside-Rh2 blocks the cell cycle of SK-HEP-1 cells at the G1/S boundary by selectively inducing the protein expression of p27kip1. Cancer Lett. 1996;110:193–200. doi: 10.1016/s0304-3835(96)04502-8. [DOI] [PubMed] [Google Scholar]
- 12.Oh M, Choi YH, Choi SH, et al. Anti-proliferating effects of ginsenoside Rh2 on MCF-7 human breast cancer cells. Int J Oncol. 1999;14:869–75. doi: 10.3892/ijo.14.5.869. [DOI] [PubMed] [Google Scholar]
- 13.Kim YS, Jin SH. Ginsenoside Rh2 induces apoptosis via activation of caspase-1 and -3 and up-regulation of Bax in human neuroblastoma. Arch Pharm Res. 2004;27:834–9. doi: 10.1007/BF02980175. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Lee EH, Ko SR, Choi KJ, Park JH, Im DS. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch Pharm Res. 2004;27:429–35. doi: 10.1007/BF02980085. [DOI] [PubMed] [Google Scholar]
- 15.Cheng CC, Yang SM, Huang CY, Chen JC, Chang WM, Hsu SL. Molecular mechanisms of ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Cancer Chemother Pharmacol. 2005;55:531–40. doi: 10.1007/s00280-004-0919-6. [DOI] [PubMed] [Google Scholar]
- 16.Kitts DD, Popovich DG, Hu C. Characterizing the mechanism for ginsenoside-induced cytotoxicity in cultured leukemia (THP-1) cells. Can J Physiol Pharmacol. 2007;85:1173–83. doi: 10.1139/Y07-099. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Tang XH, Ikejima T, et al. A new triterpenoid from Panax ginseng exhibits cytotoxicity through p53 and the caspase signaling pathway in the HepG2 cell line. Arch Pharm Res. 2008;31:323–9. doi: 10.1007/s12272-001-1159-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim SY, Kim DH, Han SJ, Hyun JW, Kim HS. Repression of matrix metalloproteinase gene expression by ginsenoside Rh2 in human astroglioma cells. Biochem Pharmacol. 2007;74:1642–51. doi: 10.1016/j.bcp.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Xie X, Eberding A, Madera C, Guns ES, et al. Rh2 synergistically enhances paclitaxel or mitoxantrone in prostate cancer models. J Urol. 2006;175:1926–31. doi: 10.1016/S0022-5347(05)00891-8. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Zheng Q, Liu K, Li G, Zheng R. Ginsenoside Rh2 enhances antitumor activity and decreases genotoxic effect of cyclophosphamide. Basic Clin Pharmacol Toxicol. 2006;98:411–5. doi: 10.1111/j.1742-7843.2006.pto_348.x. [DOI] [PubMed] [Google Scholar]
- 21.Xiao D, Choi S, Johnson DE, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 22.Herman-Antosiewicz A, Singh SV. Checkpoint kinase 1 regulates diallyl trisulfide-induced mitotic arrest in human prostate cancer cells. J Biol Chem. 2005;280:28519–28. doi: 10.1074/jbc.M501443200. [DOI] [PubMed] [Google Scholar]
- 23.Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–7. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 24.Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25:1053–69. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- 25.Hahm ER, Singh SV. Honokiol causes G0-G1 phase cell cycle arrest in human prostate cancer cells in association with suppression of retinoblastoma protein level/phosphorylation and inhibition of E2F1 transcriptional activity. Mol Cancer Ther. 2007;6:2686–95. doi: 10.1158/1535-7163.MCT-07-0217. [DOI] [PubMed] [Google Scholar]
- 26.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–75. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Brown L, Boswell S, Raj L, Lee SW. Transcriptional targets of p53 that regulate cellular proliferation. Crit Rev Eukaryot Gene Expr. 2007;17:73–85. doi: 10.1615/critreveukargeneexpr.v17.i1.50. [DOI] [PubMed] [Google Scholar]
- 28.Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–74. doi: 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer KA. The cell cycle: a review. Vet Pathol. 1998;35:461–78. doi: 10.1177/030098589803500601. [DOI] [PubMed] [Google Scholar]
- 30.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–15. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 31.Harper JW, Elledge SJ, Keyomarsi K, et al. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin-D to the retinoblastoma gene product (pRB) and pRb phosphorylation by the cyclin D-dependent kinase cdk4. Genes Dev. 1993;7:331–42. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 33.Arroyo M, Raychaudhuri P. Retinoblastoma-repression of E2F-dependent transcription depends on the ability of the retinoblastoma protein to interact with E2F and is abrogated by the adenovirus E1A oncoprotein. Nucleic Acids Res. 1992;20:5947–54. doi: 10.1093/nar/20.22.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]