Abstract
 The syntheses and spectroscopic properties of eight new push-pull type near-infrared fluorophores that contain either isobenzofuran or isothianapthene subunits are presented. The isobenzofuran dyes demonstrate significantly red-shifted absorption from their isothianaphthene counterparts, which is attributed to isobenzofuran's more potent pro-quinoidal character.
The syntheses and spectroscopic properties of eight new push-pull type near-infrared fluorophores that contain either isobenzofuran or isothianapthene subunits are presented. The isobenzofuran dyes demonstrate significantly red-shifted absorption from their isothianaphthene counterparts, which is attributed to isobenzofuran's more potent pro-quinoidal character.
In vivo near-infrared (NIR) fluorescence imaging is rapidly emerging as a powerful diagnostic method.1 In the NIR region (650-900nm) biological chromophores exhibit low absorption and autofluorescence2 thus allowing photons to pass through the tissue and rendering this technique relatively non-invasive. With applications such as vascular mapping of the heart3 and brain4 and visualization of various pathologies including tumors5, atherosclerosis6, and β-amyloid plaques7, the demand for new NIR fluorescent contrast agents is ever increasing.
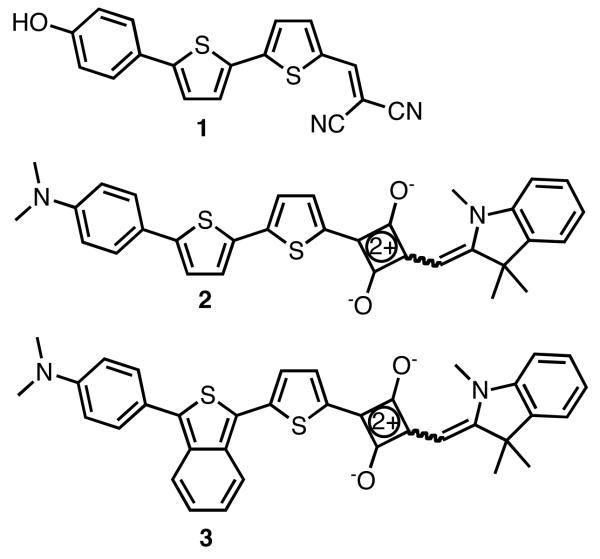
Our group resently reported the synthesis and in vivo imaging properties of NIAD-4 (1), NIM-1 (2), and NIM-2 (3), all donor-acceptor type dyes.7a,8 In the case of 2 and 3, it was found that the incorporation of benzo[c]heterocycle isothianaphthene (ITN) into the conjugated bridge red-shifted both the absorption and emission substantially. Herein we explore the use of benzo[c]heterocycle isobenzofuran (IBF) in place of ITN as a molecular component in this class of dyes.
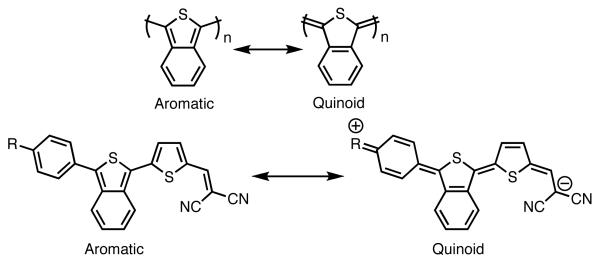
Isothianapthene has a relatively long history as a component in functional materials, with examples in fluorophores,9 OLEDs,10 photovoltaics,11 and low band-gap polmyers, of which polyisothianaphthene (PITN)12 is the progenitor. The smaller band-gap of these ITN containing materials over similar thiophene containing materials is a result of the increased contribution of the quinoid resonance structure (Figure 2) due to the stabilization achieved by aromatization of the benzene ring.13
Figure 2.
The aromatic and quinoid resonance structures of PITN and ITN donor-acceptor dyes
In contrast, only a few examples14 of functional materials containing isobenzofuran are reported in the literature, presumably due its reduced stability.15 For this reason, we predicted that IBF would prove even more pro-quinoidal and that its incorporation into donor-acceptor dyes would further reduce the band-gap of these materials.
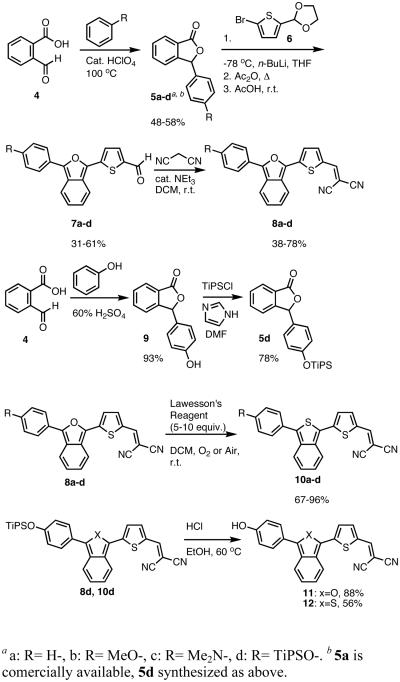
In order to test this principal, we synthesized a series of IBF and ITN dyes similar to NIAD-4 (Scheme 1). Four different electron donating (R) groups, H-, MeO-, HO-, and Me2N-, were incorporated in order to span a range of wavelengths. Appropriately substituted aryl-lactones 5a-d were either purchased or synthesized via acid mediated condensation16 of 2-carboxybenzaldehyde and substituted benzenes. In the case of the phenol group (R = OH), TiPS protection was employed. Initially, the IBF framework was constructed with a free 5 position on the thiophene, which was subsequently lithiated and the formylated with DMF. However, these unsubstituted intermediate compounds proved relatively difficult to isolate and purify, resulting in poor yields for the formylation.
Scheme 1.
Synthesis of IBF and ITN Dyes
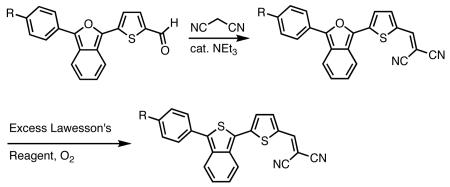
Exploiting the acetal protected aldehydes allowed us to bypass these troublesome intermediates. Protected aldehydes 7a-d were produced in one step by reaction of the lactones with the lithio-derivative of 617 followed by dehydration with acetic anhydride and deprotection with aqueous acetic acid. Knoevenegal condensation of the aldehydes with malonitrile installed the dicyanomethylene electron withdrawing group to afford dyes 8a-d in moderate yields. Deprotection of 8d was achieved under acidic conditions as fluoride was found to revert the dicyanovinyl group to the aldehyde.
In order to access the ITN counterparts to dyes 10a-d, we decided to explore methods by which the IBF dyes could be converted directly. It was found that such a transformation could be accomplished with good yields, in one pot, using a 5-10 fold excess of Lawesson's reagent in the presence of air or anhydrous oxygen. The precise role of the oxygen is unknown at this time. In the case of 8c, the dicyanovinyl group was cleaved during the reaction, presumably to a thioaldehyde, and thus treatment with excess malonitrile and base was neccesary to obtain 10c. 10d was deprotected under acid conditions in similar manner to 8d.
The spectroscopic properties of 8a-c, 10a-c, 11 and 12 are listed in Table 1. In all cases, the IBF dyes exhibited markedly red-shifted absorption from the coresponding ITN moieties, thus demonstrating the narrower band-gaps of the IBF compounds. The degree of this effect increases as the efficacy of the the electron donating group increases. The degree of red-shift in the emission proved only modest, especially in the case of 8c and 10c, indicating that perhaps the quinoid resonance structure stabilization does not factor as heavily into the excited state energies of the these molecules.
Table 1.
Spectroscopic Properties of IBF and ITN Dyesa
| IBF Dye | λmax abs. (nm) | λmax em. (nm) | Φb | ITN Dye | λmax abs. (nm) | λmax em. (nm) | Φb |
|---|---|---|---|---|---|---|---|
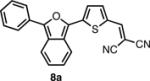 |
556c | 658c | .077c | 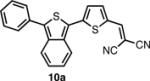 |
525c | 637c | .061c |
| 578d | 635d | .075d | 544d | 629d | .079d | ||
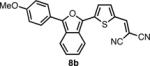 |
578c | 686c | .078c | 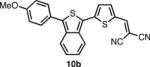 |
537c | 672c | .046c |
| 599d | 675d | .16d | 559d | 663d | .099d | ||
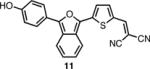 |
584c | 696c | .049c | 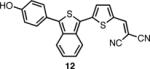 |
545c | 684c | .023c |
| 600d | 679d | .12d | 559d | 667d | .071d | ||
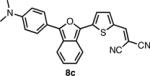 |
637c | 790c | .0055c | 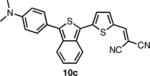 |
579c | 785c | .0015c |
| 656d | 762d | .046d | 604d | 743d | .021d |
Spectra available in Supporting Information.
Quantum yields calculated by comparison to standards, see Supporting information.
MeOH as solvent.
CHCl3 as solvent.
In conclusion, we have demonstrated the greater effectiveness of isobenzofuran over isothianaphthene as a red-shifting component in donor-acceptor type dyes, and effect attributable to the great pro-quinoidal nature of IBF. Currently, these compounds are being screened as NIR contrast agents for biomedical applications, and future investigations will explore the use alternate electron withdrawing groups and the incorporation of different heterocycles into this facinating class of compounds.
Supplementary Material
Figure 1.
Structures of NIAD-4, NIM-1, and NIM-2
Acknowledgment
This work was supported by a NIH BRP grant (R01 AG026240-01A1).
Footnotes
Supporting Information Available: Experimental procedures and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.(a) Frangioni JV. Curr. Opin. Chem. Biol. 2003;7:626. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]; (b) Rao J, Dragulescu-Andrasi A, Yao H. Curr. Opin. Chem. Biol. 2007 doi: 10.1016/j.copbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Weissleder R. Nat. Biotech. 2001;19:316. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]; Weissleder R. Nat. Biotechnol. 2001;19:316. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama A, del Monte F, Hajjar RJ, Frangioni JV. Mol. Imaging. 2002;1:365. doi: 10.1162/15353500200221333. [DOI] [PubMed] [Google Scholar]
- 4.Sankatani K, Kashiwasake-Jibu M, Taka Y, Wang S, Zou H, Yamamoto K, Shimizu K. J. Neurosurg. 1997;87:738. doi: 10.3171/jns.1997.87.5.0738. [DOI] [PubMed] [Google Scholar]
- 5.Weissleder R, Tung C, Mahmood U, Weissleder R, Tung C, Mahmood U, Bogdanov A., Jr. Nat. Biotechnol. 1999;17:375. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, Huang PL, Weissleder R. Circulation. 2002;105:2766. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- 7.(a) Nesterov EE, Skoch J, Hyman B, Klunk WE, Bacskai BJ, Swager TM. Angew. Chem. Int. Ed. 2005;44:5452. doi: 10.1002/anie.200500845. [DOI] [PubMed] [Google Scholar]; (b) Hintersteiner M, Enz A, Jaton A, Kinzy W, Kneuer R, Neumann U, Rudin M, Staufenbiel M, Stoeckli M, Wiederhold K, Gremlich H. Nat. Biotechnol. 2005;23:577. doi: 10.1038/nbt1085. [DOI] [PubMed] [Google Scholar]
- 8.Xiang Z, Nesterov EE, Skoch J, Lin T, Hyman BT, Swager TM, Bacskai BJ, Reeves SA. J. Histochem. Cytochem. 2005;53:1511. doi: 10.1369/jhc.5A6704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohanakrishnan AK, Lakshmikantham MV, McDougal C, Cava MP, Baldwin JM, Metzger RM. J. Org. Chem. 1998;63:3105. [Google Scholar]
- 10.Mitschke U, Bauerle P. J. Chem. Soc., Perkin Trans. 1. 2001;7:740. [Google Scholar]
- 11.Vangeneugden DL, Vanderzande DJM, Salbeck J, van Hal PA, Janssen RAJ, Hummelen JC, Brabec CJ, Shaheen SE, Sariciftci NS. J. Phys. Chem. B. 2001;105:11106. [Google Scholar]
- 12.Wudl F, Kobayashi M, Heeger AJ. J. Org. Chem. 1984;49:3382. [Google Scholar]
- 13.Bredas JL, Heeger AJ, Wudl F. J. Chem. Phys. 1986;85:4673. [Google Scholar]
- 14.Li JY, Hong ZR, Wang PF, Lee CS, Wong NB, Kwong HL, Lee ST. Thin Solid Films. 2004;446:111. [Google Scholar]
- 15.Jursic BS. J. Heterocycl. chem. 1996;33:1079. [Google Scholar]
- 16.(a) Barili PL, Scartoni V. J. Heterocycl. Chem. 1985;22:1199. [Google Scholar]; (b) Al-Hamdany R, Al-Rawi JM, Ibrahim S. J. Prakt. Chem. 1987;329:126. [Google Scholar]
- 17.Johnson AL. J. Org. Chem. 1976;41:1320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.