Abstract
Due to their electrical, chemical, mechanical and thermal properties, carbon nanotubes are one of the most promising materials for the electronics, computer and aerospace industries. Here, we discuss their properties in the context of future applications in biotechnology and biomedicine. The purification and chemical modification of carbon nanotubes with organic, polymeric and biological molecules are discussed. Additionally we review their uses in biosensors, assembly of structures and devices, scanning probe microscopy and as substrates for neuronal growth. We note that additional toxicity studies of carbon nanotubes are necessary so that exposure guidelines and safety regulations can be established in a timely manner.
Keywords: Carbon Nanotubes, Structure, Purification, Modification, Biosensors, Assembly of Structures and Devices, Scanning Probe Tips, Nanosurgery, Substrates for Neuronal Growth
Although nanomaterials have existed in nature long before mankind was able to identify forms at the nanoscale level, advances in synthetic chemistry have been one of the driving forces in the development of a biological nanotechnology. Nanomaterials have been designed for a variety of biomedical and biotechnological applications, including bone growth,1 enzyme encapsulation,2 biosensors3, 4 and as vesicles for DNA delivery into living cells.5, 6 Whereas nanotechnology may provide novel materials which can result in revolutionary new structures and devices, biotechnology already offers extremely sophisticated tools to precisely position molecules and assemble hierarchal structures and devices. The application of the principles of biology to nanotechnology provides a valuable route for further miniaturization and performance improvement of artificial devices. The feasibility of the bottom-up approach which is based on molecular recognition and self-assembly properties of proteins has already been proved in many inorganic-organic hybrid systems and devices. Nanodevices with biorecognition properties provide tools at a scale, which offers a tremendous opportunity to study biochemical processes and to manipulate living cells at the single molecule level. The synergetic future of nano- and bio-technologies holds great promise for further advancement in tissue engineering, prostheses, genomics, pharmacogenomics, drug delivery, surgery and general medicine.
Ever since Edison discovered that carbon changes its resistance with pressure and a carbon filament glows when an electric current is passed through it, the unique properties of carbon have intrigued scientists. After more than a century of interest, carbon has found its apogee in the fullerenes and carbon nanotubes (CNTs)—arguably the most promising of all nanomaterials. Because of their unique quasi one-dimensional structure and fascinating mechanical and electronic properties, CNTs have captured the attention of physicists, chemists and materials scientists. Biology and medicine are rapidly emerging as new areas for the application of CNTs.
This review highlights two aspects of the interaction between biology and CNTs: the use of biological principles for manipulation and self-assembly of functional structures and devices based on CNTs, and the utilization of modified CNTs to manipulate and study living cells.
1. CARBON NANOTUBES—TYPES AND STRUCTURES
The first published observation of carbon nanotubes in 19917 stimulated significant interest and research, leading to rapid progress in the field. Carbon nanotubes occur as two types: multi-walled carbon nanotubes (MWNTs) and single-walled carbon nanotubes8 (SWNTs), as illustrated in Figure 1. MWNTs consist of several coaxial cylinders, each made of a single graphene sheet surrounding a hollow core. The most common techniques for their production are electric arc9 and chemical vapor deposition (CVD).10, 11 The growth of MWNTs may require a catalyst. The outer diameter of MWNTs ranges from 2 to 100 nm, while the inner diameter is in the range of 1–3 nm, and their length is 1 to several μm.12
Fig. 1.
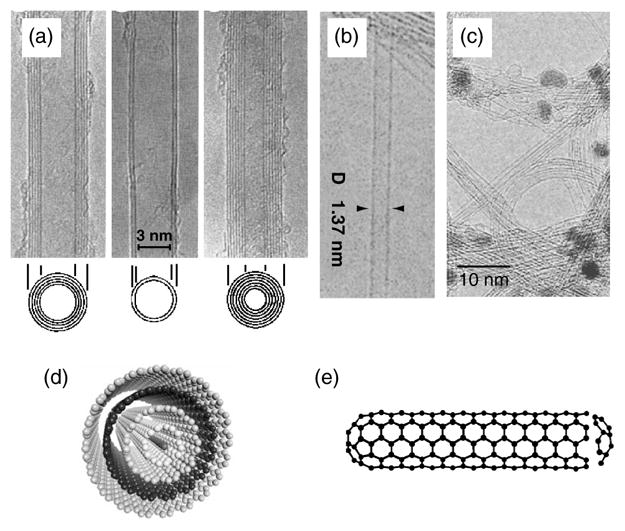
TEM micrographs of (a) MWNTs7 and (b) SWNTs8 (c) TEM micrograph showing bundles of SWNTs. The dark spots are catalyst particles used for nanotube growth. (d, e) Schematics of (d) MWNT and (e) SWNT. The TEM micrographs shown in (a) and (b) were kindly provided by Sumio Iijima, NEC, Japan and reproduced with the permission of Nature Publishing Group.
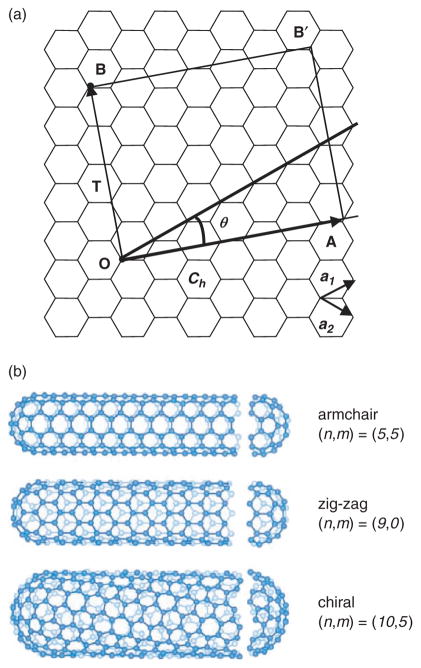
SWNTs consist of a single graphene cylinder and their diameter varies between 0.4 and 2 nm.8, 13, 14 SWNTs usually occur as hexagonal close-packed bundles in which the nanotubes are held together by van der Walls forces. SWNTs are produced by electric arc,15 laser ablation,16 CVD17–19 and gas-phase catalytic processes (HiPco).20 Their growth requires a metal catalyst, usually Fe, Ni, Co, Y or Mo. The hexagonal lattice structure of CNTs, gives rise to three types of SWNTs. Based on the unit cell of a CNT (Fig. 2) it is possible to identify armchair nanotubes, formed when n = m and the chiral angle is 30°; zig-zag nanotubes, formed when either n or m are zero and the chiral angle is 0°; and chiral tubes, with chiral angles intermediate between 0° and 30°. SWNTs are either metallic or semiconducting depending on their diameter and helicity.21–23 All armchair nanotubes are metallic, while zig-zag and chiral nanotubes can be metallic or semiconducting. As a result of the quantum confinement of electrons in the radial direction, the continuous density of states in graphite splits into a number of sharp peaks (van Hove singularities) that appear at energies that depend on the inverse of the nanotube diameter.24, 25 Electronic transitions between these singularities give rise to distinct features in the optical absorption spectra, which have been widely used to study the electronic structure of SWNTs,25–29 and have been shown to provide a diagnostic tool for the study of the effect of chemistry on the electronic structure of as-prepared and chemically modified SWNTs.30, 31
Fig. 2.
(a) Two-dimensional graphene sheet. A carbon nanotube is formed by rolling up the graphene sheet, superimposing the two ends, OA, of the chiral vector (Ch). The chiral vector is defined as Ch = na1 + ma2, where a1 and a2 are unit vectors in the two-dimensional hexagonal lattice, and n and m are integers. The pair of integers (n= m) and the chiral angle (θ—the angle between Ch and a1) define the nanotube type: armchair (n = m, and θ = 30°), zig-zag (n or m = 0, and θ = 0°) and chiral (θ between 0 and 30°). The diagram is constructed for a (5, 2) carbon nanotube. T is the basic translation vector for the tubule and the unit cell of this tubule is defined by OABB′. (b) Schematic models for the three types of SWNTs; courtesy of Riichiro Saito, Tohoku University, Japan.
2. PURIFICATION OF SWNTs
The realization of the intrinsic properties of carbon nano-tubes requires access to high purity SWNT material. As-prepared SWNTs typically contain ~30 % by weight metal catalyst, together with a substantial fraction of carbonaceous impurities which usually consist of amorphous carbon and nanoparticles. Many purification procedures have been reported (see,16, 32–37 and references therein), and these strategies involve some combination of the following steps: gas or vapor-phase oxidation, wet chemical oxidation, centrifugation, filtration or chromatography. A common purification procedure is based on nitric acid reflux, which was designed for the removal of the metal catalyst (Fig. 3),36 but also serves to incorporate carboxylic acid groups into the SWNTs that can be used for further functionalization.26, 36–41
Fig. 3.
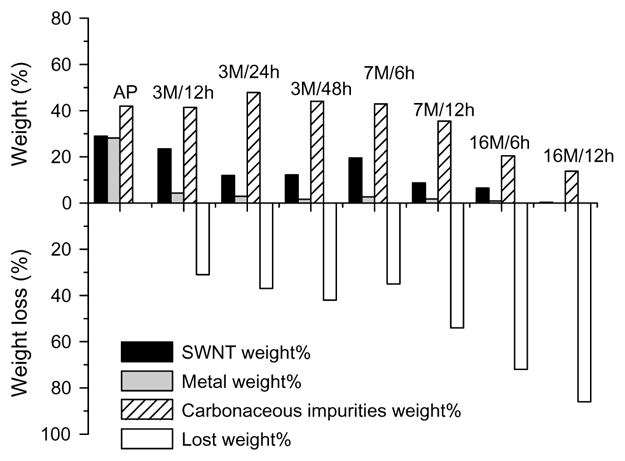
Mass balance of the normalized weight percent of all components including SWNT, metal, carbonaceous impurities, and weight loss of the SWNT samples under various nitric acid treatment conditions.36 The treatment conditions are given above the columns as xM/yh, where xM denotes x molar HNO3 and yh indicates the time of treatment in hours. AP is as-prepared SWNTs.
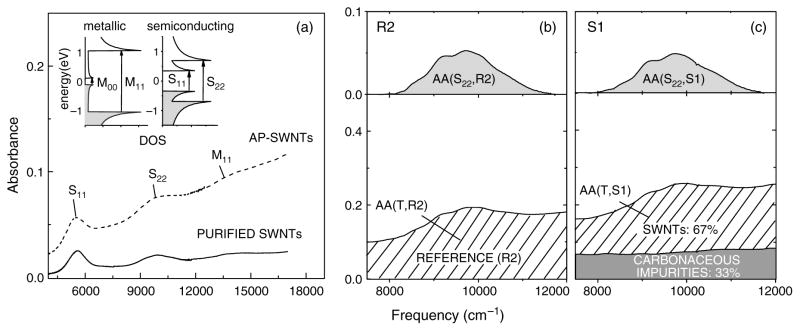
Significant efforts have been devoted to the development of analytical tools for the evaluation of the quality of SWNT materials based on techniques such as Raman spectroscopy,42–45 thin film near-infrared (NIR) spectroscopy,25, 46–49 thermogravimetric analysis (TGA),42, 43, 50 and combinations of these analytical methods.51 Recently a method based on solution-phase NIR spectroscopy has been proposed as an unambiguous measure of the quantitative purity of SWNTs.48 Because semiconducting (S) and metallic (M) CNTs exhibit characteristic interband transitions in the NIR region of the electromagnetic spectrum (Fig. 4), it is possible to relate this spectral signature to the relative carbonaceous SWNT purity (for details see48). In combination with a determination of metal content by TGA, this allows a full analysis of the composition of SWNT samples.
Fig. 4.
(a) Absorption spectra of as-prepared (AP-SWNTs) and purified SWNTs after controlled heating in oxygen.52 The insets illustrate the density of state (DOS) in SWNTs contributing to the near-IR absorption: M11 is the first metallic transition in metallic SWNTs; S11 and S22 are the first and second interband transitions in semiconducting SWNTs. M00 is the curvature induced energy gap in metallic SWNTs. (b and c) Absorption spectrum of (b) reference, R2, and (c) arc-produced SWNTs, S1, in the range of the S22 interband transition before (bottom frame) and after (top frame) baseline subtraction. The relative carbonaceous purity of a SWNT sample is estimated from the ratio of the integral absorptions under the curves in the top and bottom frames, AA(S22, S1)/AA(T, S1) related to the same ratio of integral absorbances for the reference sample AA(S22, R2)/AA(T, R2). Thus, the relative purity RP(X) =[AA(S, S1)/AA(T, S1)]/[AA(S, R)/AA(T, R)].48
3. CHEMICAL MODIFICATION OF CARBON NANOTUBES
The chemical inertness of CNTs hampers their processability and this has impeded the full realization of their potential. Chemical functionalization of CNTs has been shown to impart solubility in a variety of solvents, to modify their electronic properties and to cause significant de-bundling.
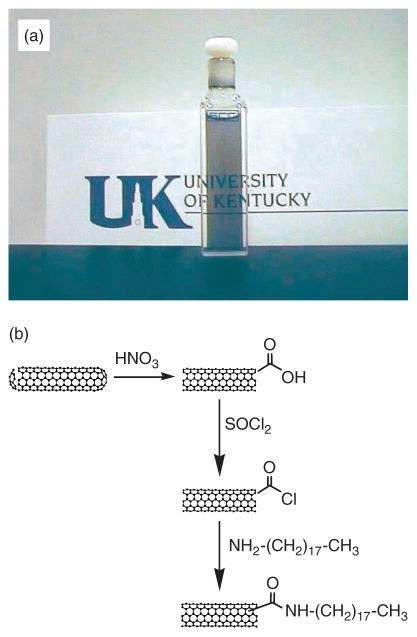
The chemical reactivity of CNTs arises from the curvature induced strain due to misalignment of the π-orbitals of adjacent conjugated carbon atoms and an increase in the pyramidalization angle.53 The induced strain is higher at the carbon atoms that comprise the nanotube caps because they are curved in two-dimensions, and therefore the caps are more reactive than the sidewalls. Hence, treatment in strong oxidizing agents such as HNO3 or H2SO4 preferentially disrupts the aromatic ring structure at the caps of CNTs and introduces carboxylic acid groups which can undergo further chemical reactions.36, 53, 54 Thus, refluxing arc discharge produces SWNTs in nitric acid typically leaving 3–6% carboxylic acid functionalities as determined by acid-base titrations;40, 41 such acid functionalized SWNTs are often denoted as SWNT-COOH. An important step in the chemistry of SWNTs is the solubilization of CNTs, achieved by covalently linking long-chain hydrocarbons to the open ends of SWNTs (Fig. 5).26 When SWNTs-COOH are treated with thionyl chloride (SOCl2) to form the acyl chloride (SWNT-COCl),38 and then reacted with octadecylamine (ODA) to give SWNT-CONH(CH2)17CH3, the material proves to be soluble in a number of common organic solvents,26 allowing the study of SWNT chemistry in the solution phase, and the characterization of the SWNTs by solution spectroscopy.26, 29, 30, 55
Fig. 5.
(a) A solution of SWNTs, chemically functionalized with octadecylamine (ODA) by a general functionalization scheme illustrated in (b).
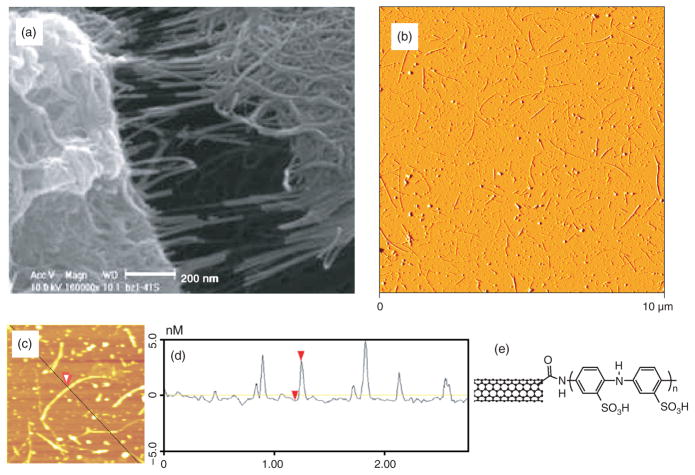
Numerous amidation and esterification reactions of oxidized SWNTs have now been reported.56–60 However, the use of CNTs in biological applications has been severely hampered by the insolubility of SWNTs in water; in order to study CNTs in the presence of live cells the use of an aqueous medium is essential. It has been shown that it is possible to solubilize carbon nanotubes in aqueous solutions by covalently attaching water soluble linear polymers.59 By applying the preceding functionalization scheme, poly-m-aminobenzene sulphonic acid (PABS), has been covalently linked to SWNTs to form a water soluble nanotube-graft copolymer (Fig. 6),61 which could be used for future biological applications.
Fig. 6.
(a) SEM image of SWNT-PABS showing fibrous morphology61 and (b) Atomic force microscopy (AFM) image illustrating good dispersion of small bundles of SWNT-PABS. (c) A bundle of functionalized SWNTs, red arrowhead. (d) The cross-section of the bundle imaged in (c), has a height of 3.5 nm (red arrowheads) corresponding to the bundle diameter. (e) Scheme of the SWNT-PABS chemical structure.
In addition to the chemistry that occurs at the oxidized open ends of SWNTs, it is also possible to react the sidewall carbon atoms with highly reactive reagents, such as carbenes,26, 31, 62 fluorine,63, 64 aryl radicals65, 66 and azomethine ylides.67, 68 Furthermore, the surface chemistry developed for SWNTs has been applied to MWNTs in specific cases. For example, MWNTs chemically modified by the covalent attachment of PABS have been studied as a substrate for neuronal growth (discussed below).
4. MODIFICATION OF CARBON NANOTUBES WITH BIOLOGICAL MOLECULES
Both non-covalent and covalent strategies have been explored to engineer the interface between biological molecules and SWNTs with the goal of preserving the functional properties of the biomolecules. A non-covalent approach has been developed which involves the bi-functional molecule 1-pyrenebutanoic acid succinimidyl ester, which irreversibly adsorbs on the hydrophobic graphene surface of the SWNTs via a π-stacking interaction.69 This approach allows subsequent protein attachment through a nucleophilic substitution reaction of succinimidyl by an amine group of the protein, and imparts specific biological recognition properties to the carbon nanotubes.
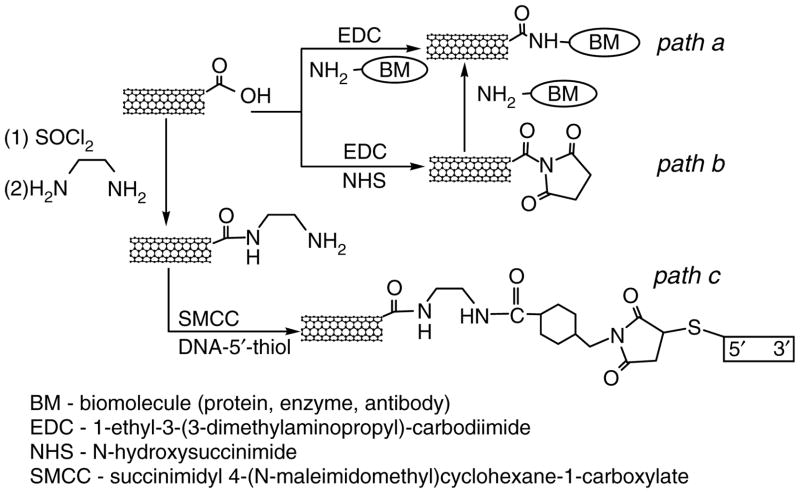
The covalent linking of CNTs to proteins70 and DNA71, 72 has been achieved with carbodiimide chemistry. Typically, the covalent binding of proteins, including enzymes and antibodies, utilizes the diimide-activated amidation of carboxylic acid-functionalized carbon nanotubes as illustrated in Scheme 1.70–73 A fiber composed of SWNTs was functionalized with bovine serum albumin (BSA) and the functionalization was confirmed by indirect immunochemistry as follows. The assembled BSA-functionalized SWNT fiber was incubated with an antibody raised against BSA in order to provide a specific antibody-antigen interaction. After washing, the primary antibody was tagged with a secondary, fluorescently (FITC)-conjugated, antibody. This complex allowed visualization of the fiber by fluorescent microscopy (Fig. 7). Similarly, it is possible to link amine-terminated DNA to the carboxylic acid functional groups of CNTs using amidation reactions (Scheme 1; paths a–b). Alternatively, DNA can be covalently linked to amine-terminated SWNTs by using cross linking with succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) to produce maleimide groups which are then further reacted with thiol-terminated DNA (Scheme 1; path c).75
Scheme 1.
Schematic illustration of procedures for covalent functionalization of SWNTs with biological molecules.
Fig. 7.
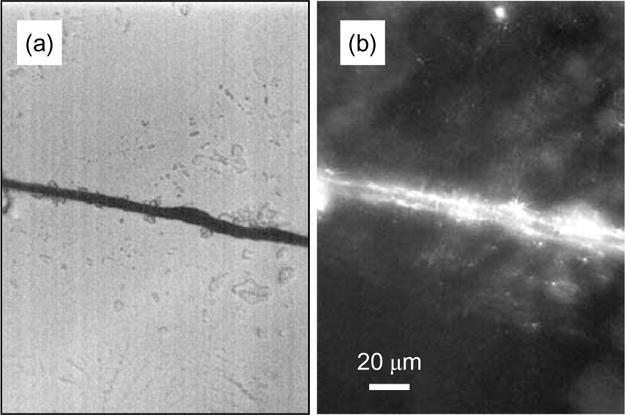
(a) Differential interference contrast and (b) immunofluorescent images of a SWNT-fiber functionalized with bovine serum albumin.
The high specificity of the biotin-avidin/streptavidin interaction (Ka = 1015 M−1) and the numerous applications of the biotin-streptavidin complex in biological sciences have lead to widespread interest in the use of these biomolecules with CNTs. Indeed, both biotin and streptavidin have been immobilized on SWNTs;76–78 the biotin-streptavidin binding was detected electronically in a SWNT field-effect transistor (FET)79 and by an electrochemiluminescent technique.77 Exploitation of the specificity of biotin-streptavidin binding for the assembly of functionalized CNTs into novel architectures holds promise for the development of advanced devices and molecular electronics (discussed below).
5. DISPERSION AND SEPARATION OF SWNTs USING DNA
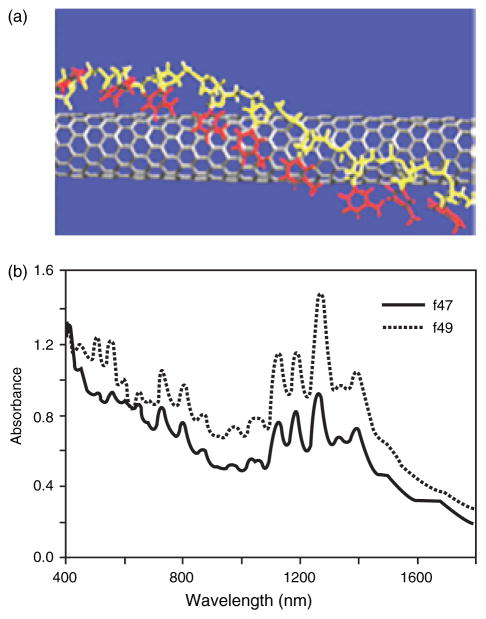
All known synthetic methods produce SWNTs of varying length and diameter, and as a mixture of metals and semiconductors that are usually hexagonally packed in bundles. The current inability to separate individual SWNTs by length, diameter or electronic properties represents the main obstacle to their application in electronics,80 field emission displays81 and nanosensors.82, 83 The chemical modifications discussed earlier can assist in dissolving and de-bundling SWNTs. Recent reports suggest that DNA complexation could offer a solution to the dispersion and de-bundling of SWNTs and their separation by electronic structure.76, 84, 85 Sonication of bundled SWNTs in the presence of an aqueous solution of single stranded DNA resulted in a stable dispersion.76, 84 Molecular modeling studies suggested that the DNA helically wraps individual SWNTs and effectively competes with the binding of SWNTs to neighboring tubes thereby dispersing individual SWNTs. It is proposed that a π-stacking interaction between the bases within the DNA and the sidewall of the SWNT is responsible for the binding. This interaction results in the helical wrapping of DNA so that its hydrophilic sugar-phosphate groups are exposed to the solution (Fig. 8a). The phosphate groups on a SWNT-DNA hybrid provide a negative charge density on the surface of the CNT leading to separation and solubilization of the individual nanotubes in water. This dissolution process enables the fractional separation of DNA-metallic SWNTs from DNA-semiconducting SWNTs using ion-exchange liquid chromatography (Fig. 8b). Thus, this process relies on DNA to disperse individual SWNTs and to provide differentiation of these macromolecules based on their electronic structure.
Fig. 8.
(a) Schematic of a carbon nanotube wrapped with single stranded DNA.76 (b) Absorption spectra of two different fractions (f47 and f49) of SWNTs wrapped with DNA after chromatographic ion exchange separation. Fraction 47 has a more pronounced absorption in the metallic interband transition, M11 (400 to 600 nm), with weaker absorption in the semiconducting interband transition, S11 (900 to 1600 nm) indicating that f47 is enriched in metallic nanotubes.76 Data courtesy of Ming Zheng, DuPont. Reproduced with permission of Nature Publishing Group.
6. MODIFIED CARBON NANOTUBES FOR BIOSENSORS
While current biosensing techniques are highly sensitive and specific, they are difficult to miniaturize. SWNT-based biosensors lend themselves to miniaturization because of the small size and structural properties of carbon nanotubes. With SWNTs every carbon atom lies on the surface, thereby achieving a large area to volume ratio for interactions with molecules of interest. Additionally, due to their unique electronic structure and ballistic conductance, SWNTs can be used for the electrical detection of binding events thereby providing a very straightforward means of biosensing. Several groups have already demonstrated the use of SWNTs as versatile biosensors.79, 86–88 SWNTs have shown to exhibit a significant conductance change in response to the presence of small biomolecules and proteins.79, 86–88 The adsorption of cytochrome c, a redox catalyst in the respiratory chain of mitochondria, has been detected in situ using a SWNT device.88 Biotin-modified SWNTs have been used to electronically detect biotin-streptavidin binding.79 It has been demonstrated that the binding of streptavidin to biotin-functionalized SWNTs results in a reduced conductance of the carbon nanotubes. Although the mechanism of chemical sensing exhibited by SWNTs has not been unequivocally identified, it seems probable that the resistance changes experienced by these devices originate from the doping of the carbon nanotubes as a result of charge transfer processes that are associated with interactions between the SWNTs and the analyte. Nevertheless, the interpretation of the electrical responses in thin film devices is complicated by the nature of carbon nanotube networks which are a mixture of bundled semiconducting and metallic SWNTs. In some cases the conductance change originates from electronic effects occurring at the metal-nanotube contacts during adsorption.89 Despite the absence of a definitive understanding of the sensing mechanism, remarkable achievements in electrical biosensing have been reported. Covalent coupling of the alkaline phosphatase (ALP) enzyme to CNTs has lead to the highest sensitivity (detection limit of 1 pg L−1) reported thus far for electrical detection of DNA (Fig. 9).90 This CNT-ALP-linked assay can be modified for antigen detection by using specific antibody-antigen recognition. Thus, it could provide a fast and simple solution for molecular diagnosis in pathologies where molecular markers exist, such as DNA or protein.
Fig. 9.
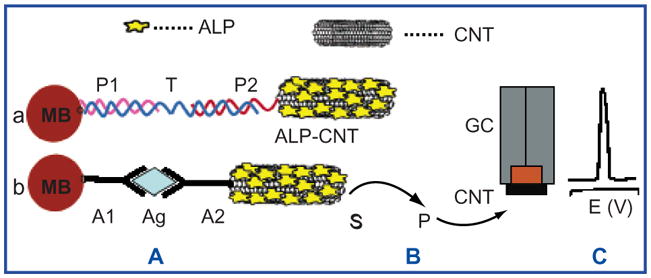
Schematic of biosensing with modified carbon nanotubes. (A) Binding of the alkaline phosphatase (ALP)-loaded CNTs to streptavidin-modified magnetic beads, MB, by (a) sandwich DNA hybridization or (b) antibody-antigen-antibody interaction. (B) Enzymatic reaction. (C) Electrochemical detection of the product of the enzymatic reaction. P—DNA probe 1; T—DNA target; P2—DNA probe 2; A1—antibody attached to the magnetic bead; Ag—antigen; A2—antibody conjugated to ALP-CNT; S and P—substrate and product, respectively, of the enzymatic reaction; GC—glassy carbon electrode; CNT—carbon nanotube layer. Data reproduced with permission from Ref. [90], J. Am. Chem Soc.
Demand for the reliable monitoring of blood glucose has stimulated research on the development of biosensors based on CNTs. The voltametric behavior of oxidized SWNTs with physically adsorbed glucose oxidase has been investigated.73 The magnitude of the catalytic response to the addition of β-D-glucose was 10-fold greater than that observed with a glassy carbon electrode. Further improvements in sensitivity and temporal resolution were made by using glucose oxidase-functionalized individual SWNTs in a FET configuration which allowed for the measurement of enzymatic activity at the level of a single molecule.86 The individual SWNT-FET device involves a complex CVD production technique and may prove more useful if FET devices could be fabricated from thin films of SWNTs.91 The preparation of optically transparent conductive SWNT films92 provides an opportunity for optical, in addition to electrical, studies of biochemical compounds and processes using SWNTs. Meanwhile, selective and sensitive detection of glucose has also been achieved by CNT nanoelectrode assemblies.93 The high performance of this biosensor is due to the specific configuration of the nanoelectrode assemblies. Overlap of the diffusion layers between adjacent electrodes is prevented because the spacing between the nanoelectrodes is many times greater than diameter of the nanotubes. This brings about a linear response of the biosensor to concentrations of glucose up to 30 mM with a detection limit as low as 0.08 mM. In addition, the electrochemical analysis for glucose remains effective even in the presence of acetaminophen, uric acid and ascorbic acid which often interfere with the detection of glucose. Thus, such devices are expected to find application for the detection of glucose in a variety of biological fluids (saliva, sweat, urine and serum).
The ability of SWNTs to interact with and therefore sense biological molecules was used in studies of the electrochemical properties of biologically relevant molecules, such as hydrogen peroxide,94, 95 reduced β-nicotinamide adenine dinucleotide (NADH),94 and dopamine,96 where the CNTs showed superior performance in comparison with conventional carbon electrodes. Their electrocatalytic properties and small size make them ideal for achieving direct electron transfer to proteins, as demonstrated with microperoxidase MP-11.97
In order to extend the usefulness of CNT-based devices for biosensing, various strategies for the fabrication of nanoelectrode arrays have been proposed. It has been possible to mechanically strengthen a MWNT array grown by CVD with a spin-on-glass film.98 Acid oxidation of the nanotubes allows further functionalization of the open ends with nucleic acids by using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and sulfo-N-hydroxysuccinimide (NHS; Scheme 1). In a related study, a MWNT nanoelectrode array has been prepared by a bottom-up approach99 in which aligned MWNTs have been encapsulated with SiO2 using a tetraethoxy silane CVD process, followed by chemical polishing and covalent linking of primary amine terminated oligonucleotides.99 The combination of such electrodes with the mediated glucose oxidation method allows the hybridization of less than a few attomoles of oligonucleotide to be detected.
Carbon fiber microelectrodes combined with amperometric measurements were used to detect oxidizable secretory products or transmitters100, 101 such as dopamine and serotonin, and also non-oxidizable transmitters, such as glutamate, when the carbon fibers were enzymatically functionalized.102, 103 Such developments suggest future directions for carbon nanotube-based devices. Recently, a nanofabricated carbon-based detector (NACAD) has been constructed, capable of measuring the secretion from a single secretory vesicle (the spherical structure that stores transmitters).104 The future applications of this device are not confined to the investigation of intracellular organelles, but could provide similar measurements of whole cells or specific regions of a cell. One possibility would be to build individually addressable arrays of miniaturized NACADs, where each detector would be an individual SWNT which is wired to allow the continuous monitoring of the electrical status of every SWNT in the array (Fig. 10). Since the growth of neurons on CNTs has already been demonstrated (see below),105, 106 it would be possible to position a neuronal cell on top of such an array and as these cells release transmitters in specific regions, namely the presynaptic terminals, create a two-dimensional map of transmitter release at a single synaptic terminal by sensing and measuring transmitter release through a dense array of SWNTs. This experiment provides the logical extension of the original NACAD experiments by application of nano-materials and technologies.
Fig. 10.
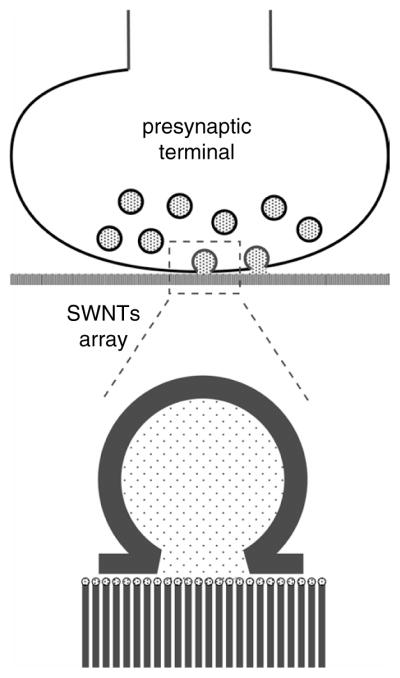
Proposed SWNT array for detection of neurotransmitter release from individual presynaptic terminals.
7. CARBON NANOTUBES AS MOLECULAR CARRIERS
The diversity of available chemistries and cell-penetrating structures107, 108 makes CNTs viable candidates as carriers for the delivery of drugs, DNA, proteins and other molecular probes into mammalian cells. One of the prerequisites for such a task is the ability of the carrier to bind to biologically relevant molecules. Early experimental studies regarding interactions between MWNTs and proteins revealed the self-organization of streptavidin molecules and the growth of its helical crystals on the nanotube surface.109 Similarly, DNA molecules may be adsorbed on MWNTs,109–111 and small protein molecules, such as cytochrome c and β-Lactase I, can be inserted within the interior cavity of open carbon nanotubes.112 As an alternative to the binding of molecules to the outside of the CNTs, it would be convenient to fill the interior cavity of tubes, whose open ends might be capped to generate a nanopill containing a drug for delivery to the cell. Towards that end, a template method has been used to synthesize nano test tubes, which are nanotubes with one end closed and the other open.113 Such an approach might be construed as a first step toward the development of a nanopill, in which the substance to be delivered is introduced into the interior of the nanotube and then bottled by resealing the open end.
An important issue in intracellular drug delivery is the poor permeability of the plasma membrane to many drugs. Thus, various carriers, including polyethylene glycol, peptides and lipids, have been developed to facilitate the cellular entry of drugs.6 Recently, the feasibility of using SWNTs for intracellular drug delivery has been demonstrated.107, 108 Water soluble SWNTs were functionalized with a fluorescent probe, FITC, to allow tracking of SWNTs.107 When murine and human fibroblast cell lines were exposed to SWNT-FITC, the nanotubes could be shown to accumulate within the cells. Similarly, SWNTs, covalently functionalized with biotin and reacted with streptavidin, were internalized within human promyelocytic leukemia (H60) cells, human T cells, Chinese hamster ovary (CHO) and 3T3 fibroblast cell lines.108 While the mechanism of the CNT cell entry remains undefined, these experiments suggest the viability of CNTs as carriers for delivering relatively large molecules to mammalian cells.
8. ASSEMBLY OF FUNCTIONAL STRUCTURES AND DEVICES BASED ON CARBON NANOTUBES
A variety of organized nanotube architectures have been fabricated using CVD. Recently, freestanding monolithic macroscopic cylinders with radially aligned MWNTs have been demonstrated as membranes for filtration of bacterial contaminants such as Escherichia coli and nanometer sized poliovirus from water.114 The self-assembly principles of biology offer an alternative route for constructing nanoscale devices based on CNTs. The major obstacles to the biological assembly of CNTs arise from difficulties in their manipulation, dispersion and separation. Biomolecules, specifically DNA76, 84 peptides115, 116 and phospholipids,117 have been suggested to be superior in their ability to disperse CNTs in comparison with surfactants, which are currently widely used for this purpose.
The ability of peptides to assemble structures based on CNTs has been demonstrated.116, 118 Amphiphilic α-helical 29 residue peptides de-bundle and stabilize individual SWNTs, while the controlled assembly of the nanotubes into macromolecular structures can be achieved by making use of specific peptide-peptide interactions.116 About 25% of the dispersed SWNTs have been found to form X, Y and intraloop junctions.116 The self-assembling ability of this amphiphilic α-helix into more complex structures has been demonstrated,118 where head-to-tail stacking of the helices through H-bonding results in wrapping of the SWNTs along their entire length and leads to the formation of an extensive network of microfibrils. The size and morphology of the fibrils can be controlled by the environment; increasing the concentration of NaCl increases the size of the fibrils, whereas the solvent dimethylformamide has been found to produce ribbon-like structures.
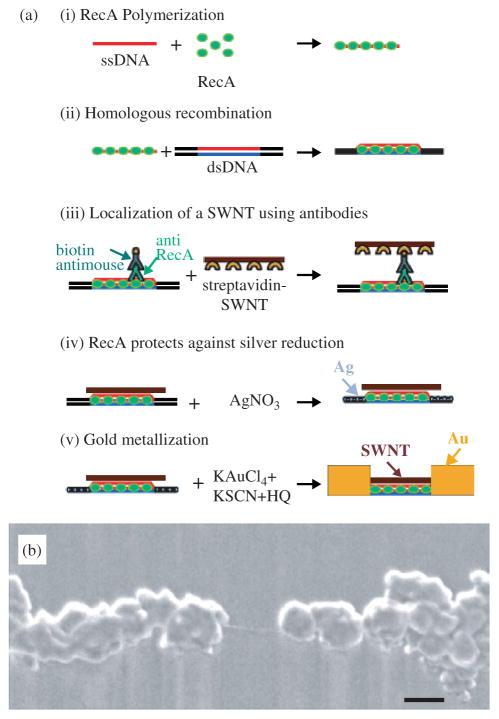
A novel bottom-up approach for the fabrication of FETs, based on individual SWNTs, that uses DNA mediated self assembly and homologous genetic recombination has been developed (Fig. 11).78 In this approach, a semiconducting SWNT was localized at a desired address on a DNA scaffold molecule, using homologous recombination by the RecA protein from Escherichia coli bacteria. DNA metallization led to the formation of wires that electrically contact the SWNTs. This approach could be generalized to form functional circuits on a scaffold DNA network and numerous molecular devices could be localized simultaneously at different addresses on the network, interconnected by DNA-templated wires. The realization of DNA-assisted assembly of SWNT-FETs highlights the power of self-assembly techniques for the construction of CNT-based electronics.
Fig. 11.
(a) Assembly of a DNA-templated FET. (i) Polymerization of RecA monomers on a ssDNA molecule. (ii) Homologous recombination reaction binding the nucleoprotein filament to an aldehyde-derivatized scaffold dsDNA molecule. (iii) Localization of the streptavidin-functionalized SWNT on the scaffold dsDNA using a primary antibody to the bound RecA and a biotin-conjugated secondary antibody. (iv) Formation of silver clusters on the segments unprotected by RecA by incubation in an AgNO3 solution. (v) Gold deposition to form two DNA-templated gold wires contacting the SWNT. (b) SEM image of an individual SWNT contacted by self-assembled DNA-templated gold wires.78 Scale bar, 100 nm. Data courtesy of Erez Braun, Technion-Israel Institute of Technology, Israel. Reproduced with permission from Science.
9. STRUCTURAL AND FUNCTIONAL IMAGING USING SWNT SCANNING PROBE TIPS
Atomic force microscopy (AFM) is commonly used to study surface topography at nanoscale resolution by monitoring the response of a cantilever, that senses the force between the tip and the sample, as the tip is scanned over the surface.119 As a result of their high aspect ratio, strength and chemical versatility, CNTs can be used to improve upon standard AFM tips in several ways, most obviously by improving the AFM resolution by decreasing the scanning tip size. The smallest standard AFM probes have tips with radial curvatures of about 5–10 nm, while the use of a SWNT can reduce the tip diameter to 1–2 nm. Several other properties of carbon nanotubes make them useful AFM probes:120 they are extremely stiff (Young’s modulus ~ 1 TPa), they undergo elastic deformation, and by using metal-catalyzed CVD techniques, individual SWNTs can be reliably grown on commercially available AFM tips.121–124
Such carbon nanotube probes can provide enhanced imaging in diverse areas, but are particularly suited to the field of biology. The use of SWNT probes improved the imaging resolution of small proteins, such as antibodies,121, 123 and in DNA analysis.120, 125 The logical final step in the development of SWNT AFM tips will be the extension of these techniques into medicine and the widespread availability of nanosurgery, which involves the manipulation of single cells or cell structures; as we show below, AFM has already been used in applications which are highly relevant to nanosurgery.
Macroscale manipulations are readily performed with standard AFM probes, such as the peeling or removing of an entire cell from a substrate.126, 127 Smaller scale manipulations such as the dissection of a hole in a cell membrane128 or removing specific organelles would benefit greatly from the much finer CNT-based probes. In Figure 12 we show nanosurgery performed on the processes (neurites) extending out of the body of a live neuron, that was acquired by AFM using a force of 12 nN with a standard silicon nitride probe: 200 μm wide type, tip curvature of ~40 nm, and opening angle of ~70° (provided by Digital Instruments, Santa Barbara, CA).127 Images (a) and (b) in Figure 12 show the effect of increasing the force exerted by the tip to 72 nN within the region of the neurite containing a varicosity. This resulted in the excision of a portion of the neurite, as revealed by a subsequent image which was acquired at 12 nN (Fig. 12b). By using a fine SWNT tip as a nanoscalpel, a much more delicate excision could be made which could make feasible operations that require the severance of a specific neurite branch from a neuron or the selective removal of a specific organelle from a cell. It may also turn out that the SWNT would bend, disabling such hypothetical procedures.
Fig. 12.
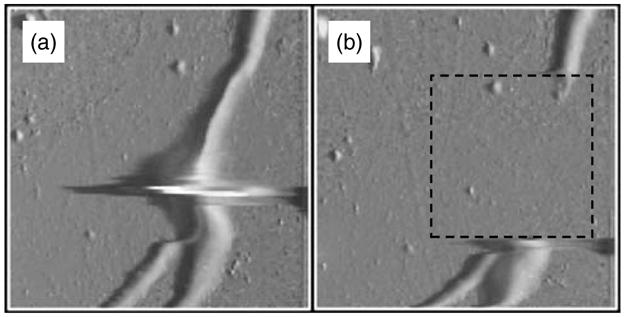
AFM images of neurites (~500 nm in diameter) containing a varicosity. (a) Deflection mode AFM image; imaging force, 12 nN. Artifact at neurite varicosity is due to delay in the feedback loop. The box in (b) indicates the area that was scanned at 72 nN after acquisition of (a) in order to remove a portion of the neurite. (b) Image of neurites after excision; imaging force, 12 nN.
Another advantage of CNT AFM probes is that they can be easily functionalized, and the range of chemical groups that can be added specifically to the tip of a CNT make them an ideal high resolution probe for mapping chemical domains by using chemical force microscopy.129 Thus probes can be developed to sense polarity, pH and many other chemical characteristics of the sample by adding different residues to the nanotube, as shown by studies using CNT AFM tips patterned with terminal carboxylic groups in which it was possible to chemically map the substrate by using tapping mode AFM (Fig. 13).130 An interesting extension of this work would be to apply CNTs to AFM probes to create a scanning biomolecule sensor capable of detecting the spatio-temporal location of a specific molecule. In this approach the temporal resolution would only be limited by the scanning speed and biosensor properties, while the spatial resolution would be limited by the diameter of the CNT. For example, nanotubes capable of sensing glucose131, 132 or neurotransmitters could be used to scan for the uptake and release of these compounds from specific cells under various stimuli. In fact SWNTs have been used for direct visualization of individual DNA molecules and this method has been implemented for determination of haplotypes on UGT1A7,130 a gene suggested to be a cancer risk factor. The use of SWNTs as a tip gives a resolution of approximately 10 bases, which enables direct reading of DNA sequences, and it is clear that the utilization of SWNT tips to haplotyping could be applied to the understanding and diagnosis of genetic diseases.
Fig. 13.
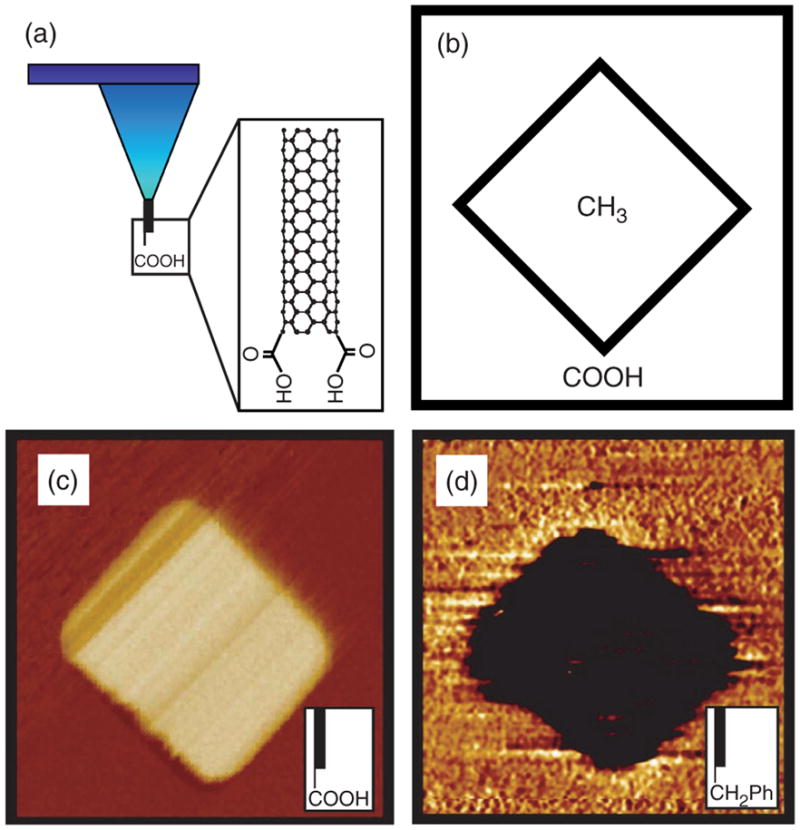
(a) Schematic of a SWNT tip with attached carboxylic acid groups. (b–d) Chemical mapping with functionalized SWNT tip. (b) Schematic of a patterned substrate consisting of a self-assembled monolayer (SAM) region terminated with methyl groups and surrounded by carboxylic acid-terminated SAM. (c) Tapping mode phase image of the patterned SAM in (b) imaged with a carboxylic acid-terminated SWNT tip, and (d) phase image of a similar substrate imaged with C6H5-terminated SWNT tip. Courtesy of Charles M. Lieber, Harvard University, USA.
10. MODIFICATION OF CARBON NANOTUBES FOR NEURONAL GROWTH
The first use of carbon nanotubes in contact with living cells made use of carbon nanotubes as a substrate for neuronal growth.105 In this work, cultured hippocampal neurons were grown on MWNTs deposited on polyethyleneimine-coated coverslips, and scanning electron microscopy was used to identify the morphological changes of neuron growth brought about by the presence of the MWNTs. The neuronal bodies were found to adhere to the surface of the MWNTs with their neurites extending through the bed of CNTs and elaborating into many small branches. The neurons remained alive on the nanotubes for at least 11 days, and it was shown that the physisorption of 4-hydroxynonenal on the MWNTs enhanced both neurite outgrowth and branching.
In a recent study using hippocampal neuronal cultures neuronal growth was systematically controlled by functionalizing MWNTs with different chemicals,106 using a methodology that was comparable to the original study.105 The work was further extended by characterizing the morphological features of live (rather than fixed) neurons (Fig. 14) by labeling the neurons with the fluorescent dye calcein, that stains only live cells (vital stain), and then visualizing the sample with a fluorescent microscope. The study covered three types of chemically modified MWNTs, prepared by covalently conjugating CNTs with functionalities designed to carry negative, neutral or positive charges at physiological pH. By using these CNTs as the scaffold for neuron growth, it was found that the neurons grown on positively charged MWNTs showed more numerous growth cones, longer neurite outgrowth and more successful neurite branching than the neurons grown on negatively charged CNTs (Fig. 14). Thus, by varying the electrostatic charge on the MWNTs it was possible to manipulate the growth pattern of the neurons.
Fig. 14.
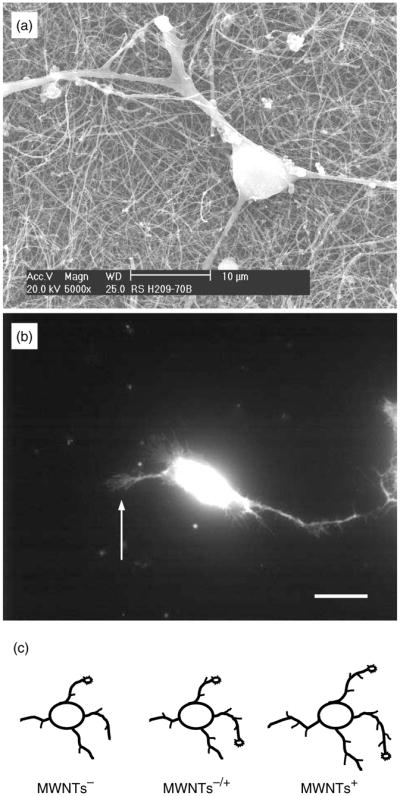
(A) SEM image of a neuron grown on as-prepared MWNTs. (b) Fluorescent image showing a live neuron on as-prepared MWNTs, which accumulated the vital stain, calcein. Arrow indicates a growth cone. Scale bar, 20 μm. (c) Drawing summarizing the effects of MWNT charges on growth cones, neurite outgrowth and branching. Modified from Ref. 106.
Taken together these studies demonstrate that CNTs can be used as a substrate for neuronal growth and that modifications of the CNTs can be employed to modulate the development of neurons. This suggests that in the future, it may be possible to employ suitably functionalized CNTs as neural prostheses in neurite regeneration.
11. TOXICITY
An important concern regarding the use of CNTs in biology and medicine is their toxicity.133, 134 At the present time CNTs are mainly under investigation in laboratories, but if there is widespread commercialization the exposure of the general populace to this material must not occur without adequate testing. Thus, toxicological studies will be needed, and there are several studies indicating that CNTs could have undesirable effects on human health. The exposure of cultured human skin cells to SWNTs caused oxidative stress and loss of cell viability,135 indicating that dermal exposure may lead to skin conditions. This is perhaps to be expected, since graphite and carbon materials have been associated with increased dermatitis and keratosis. Additional studies have investigated the pulmonary toxicity of SWNTs,136, 137 and it was shown that exposure to SWNTs lead to the development of granulomas in rodents. Since these studies used very high concentrations of SWNTs which were directly instilled into the lungs of the animals, further testing is required to establish their inhalation toxicity. Estimates of the airborne contamination and glove deposits of SWNTs during production indicated very low workplace exposure (perhaps one million times lower than the concentrations used in the toxicity studies).138 It is clear that future toxicological studies of CNTs should be conducted to determine safe exposure limits, and since similar measurements were performed on carbon soot and carbon black-based toners it seems likely that the appropriate measurements can be performed in a timely manner.
12. CONCLUDING REMARKS
As a result of their unique electrical, chemical, mechanical and thermal properties carbon nanotubes represent one of the most promising materials for application in the electronics, computer and aerospace industries. The present review shows that their immense potential for biotechnology and biomedicine are only just starting to be realized.
Acknowledgments
This work was supported by the National Institute of Mental Health (MH 069791) and by DOD/DARPA/DMEA under Award No. DMEA90-02-2-0216. VP is an Institute for Complex Adaptive Matter Senior Fellow.
Biography
![]() Vladimir Parpura (right) holds both an M.D. degree, awarded from the University of Zagreb in Croatia in 1989, and a Ph.D. degree, received in neuroscience and zoology from Iowa State University in 1993. He held a research post-doctoral fellowship at the Mayo Clinic. His academic honors include a 1988 Rector’s Award from the University of Zagreb, a 1993 Research Excellence Award from Iowa State University, and a 2004 Institute for Complex Adaptive Matter Senior Fellowship. For the past four years, he has been a faculty member at the University of California, Riverside. His research focuses on understanding the interactions between two principal cell types in the brain, neurons and glia. In a collaborative effort with the research group of Dr. Robert Haddon (middle), Parpura is interested in the development of carbon nanotube-based materials for neuronal and glial growth. Dr. Elena Bekyarova (left) is a postdoctoral fellow in Haddon’s research group, working on biosensors and electronic properties of carbon nanotubes.
Vladimir Parpura (right) holds both an M.D. degree, awarded from the University of Zagreb in Croatia in 1989, and a Ph.D. degree, received in neuroscience and zoology from Iowa State University in 1993. He held a research post-doctoral fellowship at the Mayo Clinic. His academic honors include a 1988 Rector’s Award from the University of Zagreb, a 1993 Research Excellence Award from Iowa State University, and a 2004 Institute for Complex Adaptive Matter Senior Fellowship. For the past four years, he has been a faculty member at the University of California, Riverside. His research focuses on understanding the interactions between two principal cell types in the brain, neurons and glia. In a collaborative effort with the research group of Dr. Robert Haddon (middle), Parpura is interested in the development of carbon nanotube-based materials for neuronal and glial growth. Dr. Elena Bekyarova (left) is a postdoctoral fellow in Haddon’s research group, working on biosensors and electronic properties of carbon nanotubes.
References and Notes
- 1.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 2.Chang TM, Prakash S. Procedures for microencapsulation of enzymes, cells and genetically engineered microorganisms. Mol Biotechnol. 2001;17:249. doi: 10.1385/MB:17:3:249. [DOI] [PubMed] [Google Scholar]
- 3.Chan WCW, Nei S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 4.Cao YWC, Jin RC, Mirkin CA. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science. 2002;297:1536. doi: 10.1126/science.297.5586.1536. [DOI] [PubMed] [Google Scholar]
- 5.Koltover I, Salditt T, Radler JO, Safinya CR. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 1998;281:78. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- 6.Ulrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem Rev. 1999;99:3181. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 7.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56. [Google Scholar]
- 8.Iijima S, Ichihashi T. Single-shell carbon nanotubes of 1-nm diameter. Nature. 1993;363:603. [Google Scholar]
- 9.Ebbesen TW, Ajayan PM. Large scale synthesis of carbon nanotubes. Nature. 1992;358:220. [Google Scholar]
- 10.Rao CNR, Govindaraj A. Carbon nanotubes from organometallic precursors. Acc Chem Res. 2002;35:998. doi: 10.1021/ar0101584. [DOI] [PubMed] [Google Scholar]
- 11.Ren ZF, Huang ZP, Xu JW, Wang JH, Bush P, Siegal MP, Provencio PN. Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science. 1998;282:1105. doi: 10.1126/science.282.5391.1105. [DOI] [PubMed] [Google Scholar]
- 12.Dresselhaus MS, Dresselhaus G, Eklund PC. Science of Fullerenes and Carbon Nanotubes. Academic; San Diego: 1996. [Google Scholar]
- 13.Qin LC, Zhao X, Hirahara K, Miyamoto Y, Ando Y, Iijima S. The smallest carbon nanotube. Nature. 2000;408:50. doi: 10.1038/35040699. [DOI] [PubMed] [Google Scholar]
- 14.Wang N, Tang ZK, Li GD, Chen JS. Single-walled 4 A carbon nanotube arrays. Nature. 2000;408:50. doi: 10.1038/35040702. [DOI] [PubMed] [Google Scholar]
- 15.Journet C, Maser WK, Bernier P, Loiseau A, Lamy de la Chappelle M, Lefrant S, Deniard P, Lee R, Fischer JE. Large scale production of single-walled carbon nanotubes by the electric-arc technique. Nature. 1997;388:756. [Google Scholar]
- 16.Rinzler AG, Liu J, Dai H, Nikolaev P, Huffman CB, Rodriguez-Macias FJ, Boul PJ, Lu AH, Heymann D, Colbert DT, Lee RS, Fischer JE, Rao AM, Eklund PC, Smalley RE. Large-scale purification of single-wall carbon nanotubes: Process, product and characterization. Appl Phys A. 1998;67:29. [Google Scholar]
- 17.Cassel AM, Raymakers JA, Kong J, Dai H. Large scale CVD synthesis of single-walled carbon nanotubes. J Phys Chem B. 1999;103:6484. [Google Scholar]
- 18.Yudasaka M, Kikuchi R, Matsui T, Ohki Y, Yoshimura S, Ota E. Specific conditions for Ni catalyzed carbon nanotube growth by chemical vapor deposition. Appl Phys Lett. 1995;67:2472. [Google Scholar]
- 19.Su M, Zheng B, Liu J. A scalable CVD method for the synthesis of single-walled carbon nanotubes with high catalyst productivity. Chem Phys Lett. 2000;322:321. [Google Scholar]
- 20.Nikolaev P, Bronikowski MJ, Bradley RK, Rohmund F, Colbert DT, Smith KA, Smalley RE. Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chem Phys Lett. 1999;313:91. [Google Scholar]
- 21.Mintmire JW, Dunlap BI, White CT. Are fullerene tubules metallic? Phys Rev Lett. 1992;68:631. doi: 10.1103/PhysRevLett.68.631. [DOI] [PubMed] [Google Scholar]
- 22.Hamada N, Sawada SI, Oshiyama A. New one-dimensional conductors: Graphitic microtubules. Phys Rev Lett. 1992;68:1579. doi: 10.1103/PhysRevLett.68.1579. [DOI] [PubMed] [Google Scholar]
- 23.Saito R, Fujita M, Dresselhaus G, Dresselhaus MS. Electronic structure of chiral graphene tubules. Appl Phys Lett. 1992;60:2204. doi: 10.1103/physrevb.46.1804. [DOI] [PubMed] [Google Scholar]
- 24.Saito R, Dresselhaus G, Dresselhaus MS. Physical Properties of Carbon Nanotubes. Imperial College Press; Singapore: 1998. [Google Scholar]
- 25.Kataura H, Kumazawa Y, Maniwa Y, Umezu I, Suzuki S, Ohtsuka Y, Achiba Y. Optical properties of single-wall carbon nanotubes. Synth Met. 1999;103:2555. [Google Scholar]
- 26.Chen J, Hamon MA, Hu H, Chen Y, Rao AM, Eklund PC, Haddon RC. Solution properties of single-walled carbon nanotubes. Science. 1998;282:95. doi: 10.1126/science.282.5386.95. [DOI] [PubMed] [Google Scholar]
- 27.Hu H, Zhao B, Hamon MA, Kamaras K, Itkis ME, Haddon RC. Sidewall functionalization of single-walled carbon nanotubes by addition of dichlorocarbene. J Am Chem Soc. 2003;125:14893. doi: 10.1021/ja0356737. [DOI] [PubMed] [Google Scholar]
- 28.O’Connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, Rialon KL, Boul PJ, Noon WH, Kittrell C, Ma J, Hauge RH, Weisman BR, Smalley RE. Band gap fluorescence from individual single-walled carbon nanotubes. Science. 2002;297:593. doi: 10.1126/science.1072631. [DOI] [PubMed] [Google Scholar]
- 29.Bachilo SM, Strano MS, Kittrell C, Hauge RH, Smalley RE, Weisman RB. Structure-assigned optical spectra of single-walled carbon nanotubes. Science. 2002;298:2361. doi: 10.1126/science.1078727. [DOI] [PubMed] [Google Scholar]
- 30.Strano MS, Dyke CA, Usrey ML, Barone PW, Allen MJ, Shan H, Kittrell C, Hauge RH, Tour JM, Smalley RE. Electronic structure control of single-walled carbon nanotube functionalization. Science. 2003;301:1519. doi: 10.1126/science.1087691. [DOI] [PubMed] [Google Scholar]
- 31.Kamaras K, Itkis ME, Hu H, Zhao B, Haddon RC. Covalent bond formation to a carbon nanotube metal. Science. 2003;301:1501. doi: 10.1126/science.1088083. [DOI] [PubMed] [Google Scholar]
- 32.Moon JM, An KH, Lee YH, Park YS, Bae DJ, Park GS. High-yield purification process of single walled carbon nanotubes. J Phys Chem B. 2001;105:5677. [Google Scholar]
- 33.Chiang IW, Brinson BE, Smalley RE, Margrave JL, Hauge RH. Purification and characterization of single-wall carbon nanotubes obtained from gas phase decomposition of CO (HiPco process) J Phys Chem B. 2001;105:1157. [Google Scholar]
- 34.Niyogi S, Hu H, Hamon MA, Bhowmik P, Zhao B, Rozenzhak SM, Chen J, Itkis ME, Meier MS, Haddon RC. Chromatographic purification of soluble single-walled carbon nanotubes (s-SWNTs) J Am Chem Soc. 2001;123:733. doi: 10.1021/ja0024439. [DOI] [PubMed] [Google Scholar]
- 35.Zhao B, Hu H, Niyogi S, Itkis ME, Hamon M, Bhowmik P, Meier MS, Haddon RC. Chromatographic purification and properties of soluble single walled carbon nanotubes. J Am Chem Soc. 2001;123:11673. doi: 10.1021/ja010488j. [DOI] [PubMed] [Google Scholar]
- 36.Hu H, Zhao B, Itkis ME, Haddon RC. Nitric acid purification of single-walled carbon nanotubes. J Phys Chem B. 2003;107:13838. doi: 10.1021/jp050781w. [DOI] [PubMed] [Google Scholar]
- 37.Haddon RC, Sippel J, Rinzler AG, Papadimitrakopoulos F. Purification and separation of carbon nanotubes. MRS Bulletin. 2004;29:252. [Google Scholar]
- 38.Liu J, Rinzler AG, Dai H, Hafner JH, Bradley RK, Boul PJ, Lu A, Iverson T, Shelimov K, Huffman CB, Rodriguez-Macias F, Shon Y-S, Lee TR, Colbert DT, Smalley RE. Fullerene pipes. Science. 1998;280:1253. doi: 10.1126/science.280.5367.1253. [DOI] [PubMed] [Google Scholar]
- 39.Mawhinney DB, Naumenko V, Kuznetsova A, Yates JTJ, Liu J, Smalley RE. Surface defect site density on single walled carbon nanotubes by titration. Chem Phys Lett. 2000;324:213. [Google Scholar]
- 40.Hamon MA, Hu H, Bhowmik P, Niyogi S, Zhao B, Itkis ME, Haddon RC. End-group and defect analysis of soluble single-walled carbon nanotubes. Chem Phys Lett. 2001;347:8. doi: 10.1021/ja010488j. [DOI] [PubMed] [Google Scholar]
- 41.Hu H, Bhowmik P, Zhao B, Hamon MA, Itkis ME, Haddon RC. Determination of the acidic sites of purified single-walled carbon nanotubes by acid-base titration. Chem Phys Lett. 2001;345:25. [Google Scholar]
- 42.Dillon AC, Gennett T, Jones KM, Alleman JL, Parilla PA, Heben MJ. A simple and complete purification of single-walled carbon nanotube materials. Adv Mater. 1999;11:1354. [Google Scholar]
- 43.Dillon AC, Gennett T, Parilla PA, Alleman JL, Jones KM, Heben MJ. Evaluating the purity of single-wall nanotube materials. Mat Res Soc Symp Proc. 2001;633:A5.2.1 . [Google Scholar]
- 44.Williams KA, Tachibana M, Allen JL, Grigorian L, Cheng SC, Fang SL, Sumanasekera GU, Loper AL, Williams JH, Eklund PC. Single-wall carbon nanotubes from coal. Chem Phys Lett. 1999;310:31. [Google Scholar]
- 45.Dresselhaus MS, Dresselhaus G, Jorio A, Souza Filsho AG, Pimenta MA, Saito R. Single nanotube raman spectroscopy. Acc Chem Res. 2002;35:1070. doi: 10.1021/ar0101537. [DOI] [PubMed] [Google Scholar]
- 46.Jost O, Gorbunov AA, Pompe W, Pichler T, Friedlein R, Knupfer M, Reibold M, Bauer HD, Dunsch L, Golden MS, Fink J. Diameter grouping in bulk samples of single-walled carbon nanotubes from optical absorption spectroscopy. App Phys Lett. 1999;75:2217. [Google Scholar]
- 47.Itkis ME, Niyogi S, Meng M, Hamon M, Hu H, Haddon RC. Spectroscopic study of the Fermi level electronic structure of single walled carbon nanotubes. NanoLett. 2002;2:155. [Google Scholar]
- 48.Itkis ME, Perea D, Niyogi S, Rickard S, Hamon M, Hu H, Zhao B, Haddon RC. Purity evaluation of as-prepared single-walled carbon nanotube soot by use of solution phase near-IR spectroscopy. Nano Lett. 2003;3:309. [Google Scholar]
- 49.Nishide D, Kataura H, Suzuki S, Tsukagoshi K, Aoyagi Y, Achiba Y. High-yield production of single-wall carbon nanotubes in nitrogen gas. Chem Phys Lett. 2003;372:45. [Google Scholar]
- 50.Zhang M, Yudasaka M, Koshio A, Iijima S. Thermogravimetric analysis of single-wall carbon nanotubes ultrasonicated in monochlorobenzene. Chem Phys Lett. 2002;364:420. [Google Scholar]
- 51.Arepalli S, Nikolaev P, Gorelik OP, Nadjiev VG, Holmes W, Files B, Yowell L. Protocol for the characterization of single-wall carbon nanotube material quality. Carbon. 2004;42:1783. [Google Scholar]
- 52.Sen R, Rickard SM, Itkis ME, Haddon RC. Controlled purification of single-walled carbon nanotube films by use of selective oxidation and near-IR spectroscopy. Chem Mater. 2003;15:4273. [Google Scholar]
- 53.Niyogi S, Hamon MA, Hu H, Zhao B, Bhowmik P, Sen R, Itkis ME, Haddon RC. Chemistry of single-walled carbon nanotubes. Acc Chem Res. 2002;35:1105. doi: 10.1021/ar010155r. [DOI] [PubMed] [Google Scholar]
- 54.Zhao B, Hu H, Bekyarova E, Itkis ME, Niyogi S, Haddon RC. Carbon nanotubes, chemistry. Dekker . Encyclopedia of Nanoscience and Nanotechnology. 2004:493. [Google Scholar]
- 55.Strano MS, Huffman CB, Moore VC, O’Connell MJ, Haroz EH, Hubbard J, Miller M, Rialon K, Kittrell C, Ramesh S, Hauge RH, Smalley RE. Reversible, band-gap-selective protonation of single-walled carbon nanotubes in solution. J Phys Chem B. 2003;107:6979. [Google Scholar]
- 56.Hamon MA, Chen J, Hu H, Chen Y, Itkis ME, Rao AM, Eklund PC, Haddon RC. Dissolution of single-walled carbon nanotubes. Adv Mater. 1999;11:834. [Google Scholar]
- 57.Hamon MA, Hu H, Bhowmik P, Itkis ME, Haddon RC. Ester-functionalized soluble single-walled carbon nanotubes. Appl Phys A. 2002;74:333. [Google Scholar]
- 58.Pompeo F, Resasco DE. Water solubilization of single-walled carbon nanotubes by functionalization with glucosamine. Nano Lett. 2002;2:369. [Google Scholar]
- 59.Riggs JE, Guo ZX, Carroll DL, Sun YP. Strong luminescence of solubilized carbon nanotubes. J Am Chem Soc. 2000;122:5879. [Google Scholar]
- 60.Kahn MGC, Banerjee S, Wong SS. Solubilization of oxidized single-walled carbon nanotubes in organic and aqueous solvents through organic derivatization. Nano Lett. 2002;2:1215. [Google Scholar]
- 61.Zhao B, Hu H, Haddon RC. Synthesis and properties of a water soluble single-walled carbon nanotube-poly (m-aminobenzene sulphonic acid) graft copolymer. Adv Func Mater. 2004;14:71. [Google Scholar]
- 62.Chen Y, Haddon RC, Fang S, Rao AM, Eklund PC, Lee WH, Dickey EC, Grulke EA, Pendergrass JC, Chavan A, Haley BE, Smalley RE. Chemical attachment of organic functional groups to single-walled carbon nanotube material. J Mater Res. 1998;13:2423. [Google Scholar]
- 63.Mickelson ET, Huffman CB, Rinzler AG, Smalley RE, Hauge RH, Margrave JL. Fluorination of single-wall carbon nanotubes. Chem Phys Lett. 1998;296:188. [Google Scholar]
- 64.Mickelson ET, Chiang IW, Zimmerman JL, Boul PJ, Lozano J, Liu J, Smalley RE, Hauge RH, Margrave JL. Solvation of fluorinated single-wall carbon nanotubes in alcohol solvents. J Phys Chem B. 1999;103:4318. [Google Scholar]
- 65.Bahr JL, Yang J, Kosynkin DV, Bronikowski MJ, Smalley RE, Tour JM. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: A bucky paper electrode. J Am Chem Soc. 2001;123:6536. doi: 10.1021/ja010462s. [DOI] [PubMed] [Google Scholar]
- 66.Bahr JL, Tour JL. Highly functionalized carbon nanotubes using in situ generated diazonium compounds. Chem Mater. 2001;13:3823. [Google Scholar]
- 67.Georgakilas V, Kordatos K, Prato M, Guldi DM, Holzinger M, Hirsch A. Organic functionalization of carbon nanotubes. J Am Chem Soc. 2002;124:760. doi: 10.1021/ja016954m. [DOI] [PubMed] [Google Scholar]
- 68.Georgakilas V, Tagmatarchis N, Pantarotto D, Bianco A, Briand JP, Prato M. Amino acid functionalization of water soluble carbon nanotubes. Chem Commun. 2002:3050. doi: 10.1039/b209843a. [DOI] [PubMed] [Google Scholar]
- 69.Chen RJ, Zhang Y, Wang D, Dai H. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J Am Chem Soc. 2001;123:3838. doi: 10.1021/ja010172b. [DOI] [PubMed] [Google Scholar]
- 70.Huang W, Taylor S, Fu K, Lin Y, Zhang D, Hanks TW, Rao AM, Sun Y-P. Attaching proteins to carbon nanotubes via diimide-activated amidation. Nano Lett. 2002;2:311. [Google Scholar]
- 71.Dwyer C, Guthold M, Falvo M, Washburn S, Superfine R, Erie D. DNA-functionalized single-walled carbon nanotubes. Nanotechnology. 2002;13:601. [Google Scholar]
- 72.Williams KA, Veenhuizen PTM, de la Torre BG, Eritja R, Dekker C. Nanotechnology: Carbon nanotubes with DNA recognition. Nature. 2002;420:761. doi: 10.1038/420761a. [DOI] [PubMed] [Google Scholar]
- 73.Azamian BR, Davis JJ, Coleman KS, Bagshaw CB, Green MLH. Bioelectrochemical single-walled carbon nanotubes. J Am Chem Soc. 2002;124:12664. doi: 10.1021/ja0272989. [DOI] [PubMed] [Google Scholar]
- 74.Gommans HH, Alldredge JW, Tashiro H, Park J, Magnuson J, Rinzler AG. Fibers of aligned single-walled carbon nanotubes: Polarized Raman spectroscopy. J Appl Phys. 2000;88:2509. [Google Scholar]
- 75.Baker SE, Cai W, Lasseter TL, Weidkamp KP, Hamers RJ. Covalently bonded adducts of deoxyribonucleic acid (DNA) oligonucleotides with single-wall carbon nanotubes: Synthesis and hybridization. Nano Lett. 2002;2:1413. [Google Scholar]
- 76.Zheng M, Jagota A, Semke ED, Diner BA, McLean RS, Lustig SR, Richardson RE, Tassi NG. DNA-assisted dispersion and separation of carbon nanotubes. Nature Mater. 2003;2:338. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- 77.Wohlstadter JN, Wilbur JL, Sigal GB, Biebuyck HA, Billadeau MA, Dong L, Fischer AB, Gudibande SR, Jameison SH, Kenten JH, Leginus J, Leland JK, Massey RJ, Wohlstadter SJ. Carbon nanotube-based biosensor. Adv Mater. 2003;15:1184. [Google Scholar]
- 78.Keren K, Berman RS, Buchstab E, Sivan U, Braun E. DNA-templated carbon nanotube field-effect transistor. Science. 2003;302:1380. doi: 10.1126/science.1091022. [DOI] [PubMed] [Google Scholar]
- 79.Star A, Gabriel JCP, Bradley K, Gruner G. Electronic detection of specific protein binding using nanotube FET devices. Nano Lett. 2003;3:459. [Google Scholar]
- 80.Avouris P. Molecular electronics with carbon nanotubes. Acc Chem Res. 2002;35:1026. doi: 10.1021/ar010152e. [DOI] [PubMed] [Google Scholar]
- 81.Fan S, Chapline MG, Franklin NR, Tomber TW, Cassell AM, Dai H. Self-oriented regular arrays of carbon nanotubes and their field emission properties. Science. 1999;283:512. doi: 10.1126/science.283.5401.512. [DOI] [PubMed] [Google Scholar]
- 82.Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H. Nanotube molecular wires as chemical sensors. Science. 2000;287:622. doi: 10.1126/science.287.5453.622. [DOI] [PubMed] [Google Scholar]
- 83.Collins PG, Bradley K, Ishigami M, Zettl A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science. 2000;287:1801. doi: 10.1126/science.287.5459.1801. [DOI] [PubMed] [Google Scholar]
- 84.Zheng M, Jagota A, Strano MS, Santos AP, Barone P, Chou SG, Diner BA, Dresselhaus MS, Mclean RS, Onoa GB, Samsonidze GG, Semke ED, Usrey ML, Walls DJ. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science. 2003;302:1545. doi: 10.1126/science.1091911. [DOI] [PubMed] [Google Scholar]
- 85.Strano MS, Zheng M, Jagota A, Onoa GB, Heller DA, Barone PW, Usrey ML. Understanding the nature of the DNA-assisted separation of single-walled carbon nanotubes using fluorescence and Raman spectroscopy. Nano Lett. 2004;4:543. [Google Scholar]
- 86.Besteman K, Lee JO, Wiertz FG, Heering HA, Dekker C. Enzyme-coated carbon nanotubes as single-molecule biosensors. Nano Lett. 2003;3:727. [Google Scholar]
- 87.Chen RJ, Bangsaruntip S, Drouvalakis KA, Kam NWS, Shim M, Li Y, Kim W, Utz PJ, Dai H. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc Nat Acad Sci USA. 2003;100:4984. doi: 10.1073/pnas.0837064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boussaad S, Tao NJ, Zhang R, Hopson T, Nagahara LA. In situ detection of cytochrome c adsorption with single walled carbon nanotube device. Chem Commun. 2003:1502. [Google Scholar]
- 89.Chen RJ, Choi HC, Bangsaruntip S, Yenilmez E, Tang X, Wang Q, Chang Y-L, Dai H. An investigation of the mechanism of electronic sensing of protein adsorption on carbon nanotube devices. J Am Chem Soc. 2004;126:1563. doi: 10.1021/ja038702m. [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Liu G, Jan MR. Ultrasensitive electrical biosensing of proteins and DNA: Carbon-nanotube derived amplification of the recognition and transduction events. J Am Chem Soc. 2004;126:3010. doi: 10.1021/ja031723w. [DOI] [PubMed] [Google Scholar]
- 91.Bekyarova E, Davis M, Burch T, Itkis ME, Zhao B, Sunshine S, Haddon RC. Chemically functionalized single-walled carbon nanotubes as ammonia sensors. J Phys Chem B. 2005;108:19717. [Google Scholar]
- 92.Wu Z, Chen Z, Du X, Logan JM, Sippel J, Nikolou M, Kamaras K, Reynolds JR, Tanner DB, Hebard AF, Rinzler AG. Transparent, conductive carbon nanotube films. Science. 2004;305:1273. doi: 10.1126/science.1101243. [DOI] [PubMed] [Google Scholar]
- 93.Lin Y, Lu F, Tu Y, Ren Z. Glucose biosensors based on carbon nanotube nanoelectrode ensemblies. Nano Lett. 2004;4:191. [Google Scholar]
- 94.Wang J, Musameh M. Carbon nanotube/teflon composite electrochemical sensors and biosensors. Anal Chem. 2003;75:2075. doi: 10.1021/ac030007+. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, Musameh M, Lin Y. Solubilization of carbon nanotubes by Nafion toward the preparation of amperometric biosensors. J Am Chem Soc. 2003;125:2408. doi: 10.1021/ja028951v. [DOI] [PubMed] [Google Scholar]
- 96.Britto PJ, Santhaman SKV, Ajayan PM. Carbon nanotube electrode for oxidation of dopamine. Bioelectrochem Bioelectronics. 1996;41:121. [Google Scholar]
- 97.Gooding JJ, Wibowo R, Liu J, Yang W, Losic D, Orbons S, Mearns FJ, Shapter JG, Hibbert DB. Protein electrochemistry using aligned carbon nanotube arrays. J Am Chem Soc. 2003;125:9006. doi: 10.1021/ja035722f. [DOI] [PubMed] [Google Scholar]
- 98.Nguyen CV, Delzeit L, Cassell AM, Li J, Han J, Meyyappan M. Preparation of nucleic acid functionalized carbon nanotubes arrays. Nano Lett. 2002;2:1079. [Google Scholar]
- 99.Li J, Ng HT, Cassell A, Fan W, Chen HY, Ye Q, Koehne J, Han J, Meyyappan M. Carbon nanotube nanoelectrode array for ultrasensitive DNA detection. Nano Lett. 2003;3:597. [Google Scholar]
- 100.Alvarez de Toledo G, Fernandez-Chacon R, Fernandez JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- 101.Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci USA. 1991;88:10754. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuhr WG, Barrett VL, Gagnon MR, Hopper P, Pantano P. Dehydrogenase-modified carbon fiber microelectrodes for the measurement of neurotransmitter dynamics. 1. NADH voltametry. Anal Chem. 1993;65:617. doi: 10.1021/ac00053a022. [DOI] [PubMed] [Google Scholar]
- 103.Pantano P, Kuhr WG. Enzyme modified microelectrodes for in-vivo neurochemical measurements. Electroanalysis. 1995;7:405. [Google Scholar]
- 104.Parpura V. Nanofabricated carbon-based detector. Anal Chem. 2005;77:681. doi: 10.1021/ac0487404. [DOI] [PubMed] [Google Scholar]
- 105.Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci. 2000;14:175. doi: 10.1385/JMN:14:3:175. [DOI] [PubMed] [Google Scholar]
- 106.Hu H, Ni Y, Montana V, Haddon RC, Parpura V. Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano Lett. 2004;4:507. doi: 10.1021/nl035193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pantarotto D, Briand J-P, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun. 2004;16 doi: 10.1039/b311254c. [DOI] [PubMed] [Google Scholar]
- 108.Kam NWS, Jessop TC, Wender PA, Dai H. Nanotube molecular transporters: Internalization of carbon nanotube-protein conjugates into mammalian cells. J Am Chem Soc. 2004;126:6850. doi: 10.1021/ja0486059. [DOI] [PubMed] [Google Scholar]
- 109.Balavoine F, Schultz P, Richard C, Mallouh V, Ebbesen TW, Mioskowski C. Helical crystallization of proteins on carbon nanotubes: A first step towards the development of new biosensors. Angew Chem Int Ed. 1999;38:1912. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1912::AID-ANIE1912>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 110.Tsang SC, Guo Z, Chen YK, Green MLH, Hill HAO, Hambley TW, Sadler PJ. Immobilization of platinated and iodinated oligonucleotides on carbon nanotubes. Angew Chem Int Ed. 1997;36:2198. [Google Scholar]
- 111.Guo Z, Sadler PJ, Tsang SC. Immobilization and visualization of DNA and proteins on carbon nanotubes. Adv Mater. 1998;10:701. [Google Scholar]
- 112.Davis JJ, Green MLH, Hill HAO, Leung YC, Sadler PJ, Sloan J, Xavier AV, Tsang SC. The immobilization of proteins in carbon nanotubes. Inorg Chem Acta. 1998;272:261. [Google Scholar]
- 113.Gasparac R, Kohli P, Mota MO, Trofin L, Martin CR. Template synthesis of nano test tubes. Nano Lett. 2004;4:513. [Google Scholar]
- 114.Srivastava A, Srivastava ON, Talapatra S, Vajtai R, Ajayan PM. Carbon nanotube filters. Nature Mater. 2004;3:610. doi: 10.1038/nmat1192. [DOI] [PubMed] [Google Scholar]
- 115.Wang S, Humphreys ES, Chung SY, Delduco DF, Lusting SR, Wang H, Parker KN, Rizzo NW, Subramoney S, Chiang YM, Jagota A. Peptides with selective affinity for carbon nanotubes. Nature Mater. 2003;2:196. doi: 10.1038/nmat833. [DOI] [PubMed] [Google Scholar]
- 116.Zorbas V, Ortiz-Acevedo A, Dalton AB, Yoshida MM, Dieckmann GR, Draper RK, Baughman RH, Jose-Yacaman M, Musselman IH. Preparation and characterization of individual peptide-wrapped single-walled carbon nanotubes. J Am Chem Soc. 2004;126:7222. doi: 10.1021/ja049202b. [DOI] [PubMed] [Google Scholar]
- 117.Richard C, Balavoine F, Schultz P, Ebbesen TW, Mioskowski C. Supramolecular self-assembly of lipid derivatives on carbon nanotubes. Science. 2003;300:775. doi: 10.1126/science.1080848. [DOI] [PubMed] [Google Scholar]
- 118.Dieckmann GR, Dalton AB, Johnson PA, Razal J, Chen J, Giordano GM, Munoz E, Musselman IH, Baughman RH, Draper RK. Controlled assembly of carbon nanotubes by designed amphiphilic peptide helices. J Am Chem Soc. 2003;125:1770. doi: 10.1021/ja029084x. [DOI] [PubMed] [Google Scholar]
- 119.Kasas S, Thomson NH, Smith BL, Hansma PK, Miklossy J, Hansma HG. Biological application of the AFM: From single molecules to organs. Int J Imaging Syst Technol. 1997;8:151. [Google Scholar]
- 120.Wong SS, Joselevich E, Wooley AT, Cheung CL, Lieber SL. Covalently functionalized nanotubes as nanometer-sized probes in chemistry and biology. Nature. 1998;394:52. doi: 10.1038/27873. [DOI] [PubMed] [Google Scholar]
- 121.Hafner J, Cheung CL, Lieber CM. Growth of nanotubes for probe microscopy tips. Nature. 1999;398:761. [Google Scholar]
- 122.Hafner J, Cheung CL, Lieber CM. Direct growth of single-walled carbon nanotube scanning probe microscopy tips. J Am Chem Soc. 1999;121:9750. [Google Scholar]
- 123.Cheung CL, Hafner J, Lieber CM. Carbon nanotube atomic force microscopy tips: Direct growth by chemical vapor deposition and application to high resolution imaging. Proc Nat Acad Sci USA. 2000;97:3809. doi: 10.1073/pnas.050498597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cheung CL, Hafner J, Odom TW, Kim K, Lieber CM. Growth and fabrication with single-walled carbon nanotube probe microscopy tips. Appl Phys Lett. 2000;76:3136. [Google Scholar]
- 125.Chen L, Cheung CL, Ashby PD, Lieber CM. Single-walled carbon nanotube AFM probes: Optimal imaging resolution of nanoclusters and biomolecules in ambient and fluid environments. Nano Lett. 2004;4:1725. [Google Scholar]
- 126.Parpura V, Haydon PG, Sakaguchi DS, Henderson E. Atomic force microscopy and manipulation of living cells. J Vac Sci Technol A. 1992;11:773. [Google Scholar]
- 127.Parpura V, Haydon PG, Henderson E. Three-dimensional imaging of living neurons and glia with the atomic force microscopy. J Cell Sci. 1993;105:427. doi: 10.1242/jcs.104.2.427. [DOI] [PubMed] [Google Scholar]
- 128.Henderson E, Haydon PG, Sakaguchi DS. Actin filament dynamics in living glial cells imaged by atomic force microscopy. Science. 1992;257:1944. doi: 10.1126/science.1411511. [DOI] [PubMed] [Google Scholar]
- 129.Wong SS, Harper JD, Lansbury PT, Lieber CM. Carbon nanotube tips: High-resolution probes for imaging biological systems. J Am Chem Soc. 1998;120:603. [Google Scholar]
- 130.Woolley AT, Cheung CL, Hafner J, Lieber CM. Structural biology with carbon nanotube AFM probes. Chem Biol. 2000;7:R193. doi: 10.1016/s1074-5521(00)00037-5. [DOI] [PubMed] [Google Scholar]
- 131.Wang SG, Zhang Q, Wang R, Yoon SF. A novel multi-walled carbon nanotube based biosensor for glucose detection. Biochem Biophys Res Commun. 2003;311:572. doi: 10.1016/j.bbrc.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 132.Tang H, Chen J, Yao S, Nie L, Deng G, Kuang Y. Amperometric glucose biosensor based on adsorption of glucose oxidase at platinum nanoparticle modified carbon nanotube electrode. Anal Biochem. 2004;331:89. doi: 10.1016/j.ab.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 133.Service RF. Nanomaterials show signs of toxicity. Science. 2003;300:243. doi: 10.1126/science.300.5617.243a. [DOI] [PubMed] [Google Scholar]
- 134.Service RF. Nanotoxicology. Nanotechnology grows up. Science. 2004;304:1732. doi: 10.1126/science.304.5678.1732. [DOI] [PubMed] [Google Scholar]
- 135.Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, Gandelsman VZ, Maynard A, Baron P. Exposure to carbon nanotube material: Assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66:1909. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 136.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 137.Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77:117. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 138.Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: Aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health A. 2004;67:87. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]