Abstract
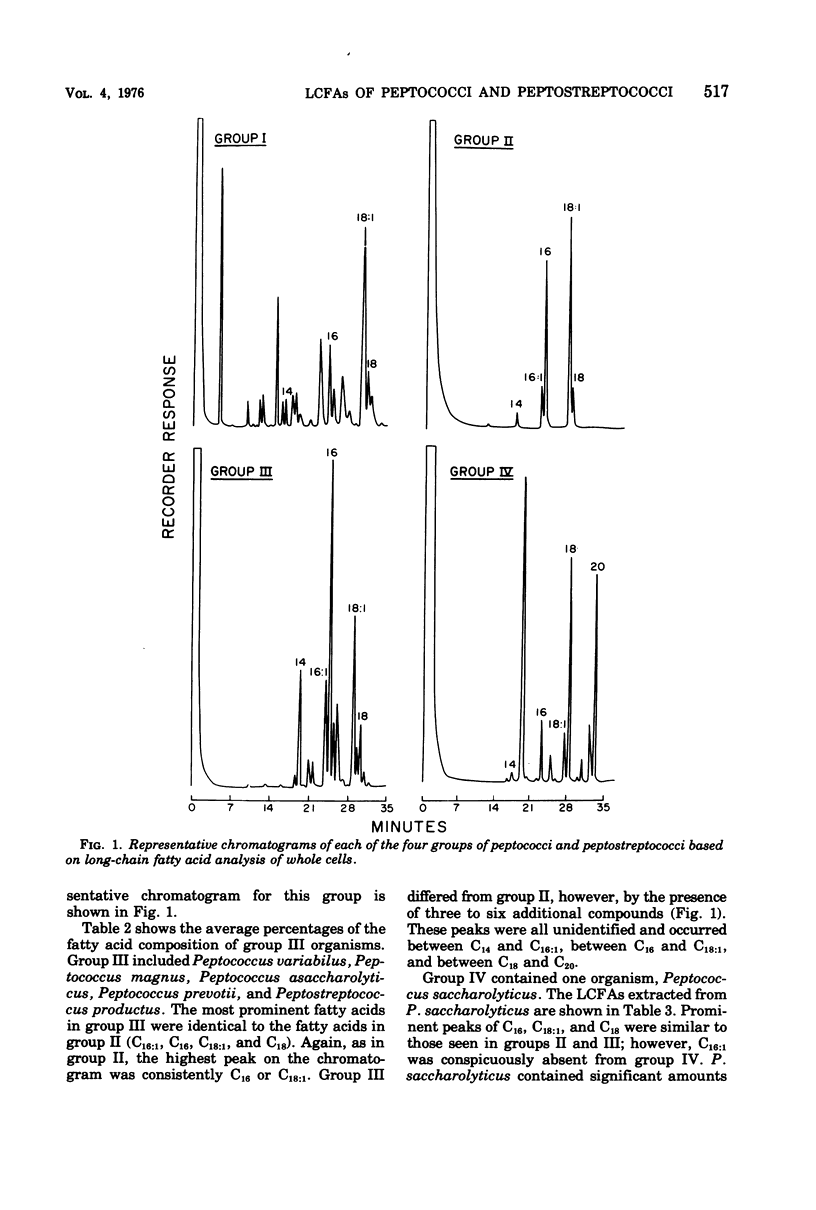
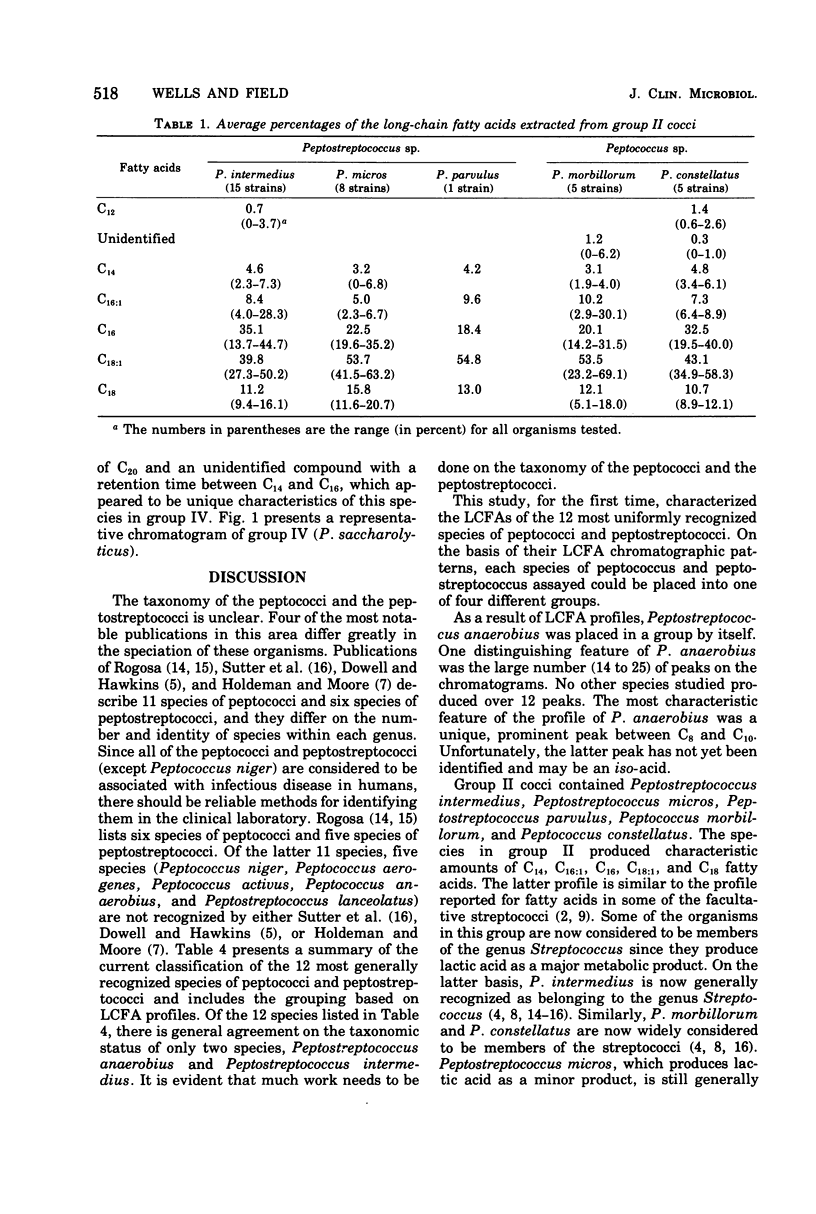
The long-chain fatty acids extracted from the whole cells of 12 clinically significant species of peptococci and peptostreptococci were characterized by gas-liquid chromatography. The resulting methylated fatty acid profiles (and some unidentified compounds) of 82 strains allowed the 12 species to be separated into four groups. Fifteen strains of Peptostreptococcus anaerobius were placed in group I because they had a unique, prominent compound that occurred in the area where a C8 to C10 fatty acid would be expected. Group II, consisting of Peptostreptococcus intermedius, Peptostreptococcus micros, Peptostreptococcus parvulus, Peptococcus morbillorum, and Peptococcus constellatus, produced C14, C16:1, C18:1, and C18 fatty acids. Peptococcus prevotii, Peptococcus variabilus, Peptococcus magnus, Peptococcus asaccharolyticus, and Peptostreptococcus productus were placed in group III because they contained three to six additional, unidentified compounds that strikingly differentiated them from group II. Peptococcus saccharolyticus was the single species assigned to group IV because it yielded C14, C16, C18:1, C18, and C20 fatty acids and a prominent unidentified peak that occurred between C14 and C16 fatty acids. This study indicated that cellular long-chain fatty acids may be an important tool in clarifying the taxonomy of the peptococci and peptostreptococci.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P., KATZ I., KEENEY M. Metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. II. Biosynthesis of higher branched-chain fatty acids and aldehydes. J Bacteriol. 1962 May;83:1084–1093. doi: 10.1128/jb.83.5.1084-1093.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstein C. F., Hartman P. A. Differentiation of some enterococci by gas chromatography. J Bacteriol. 1973 Jan;113(1):38–41. doi: 10.1128/jb.113.1.38-41.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M. A., Moss C. W. Cellular fatty acid composition of Streptococcus mutans and related streptococci. J Dent Res. 1976 Jan;55:A96–102. doi: 10.1177/002203457605500128011. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Kellogg D. S., Jr, Farshy D. C., Lambert M. A., Thayer J. D. Cellular fatty acids of pathogenic Neisseria. J Bacteriol. 1970 Oct;104(1):63–68. doi: 10.1128/jb.104.1.63-68.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Lambert M. A., Merwin W. H. Comparison of rapid methods for analysis of bacterial fatty acids. Appl Microbiol. 1974 Jul;28(1):80–85. doi: 10.1128/am.28.1.80-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Samuels S. B., Weaver R. E. Cellular fatty acid composition of selected Pseudomonas species. Appl Microbiol. 1972 Oct;24(4):596–598. doi: 10.1128/am.24.4.596-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pien F. D., Thompson R. L., Martin W. J. Clinical and bacteriologic studies of anaerobic gram-positive cocci. Mayo Clin Proc. 1972 Apr;47(4):251–257. [PubMed] [Google Scholar]
- Zabransky R. J. Isolation of anaerobic bacteria from clinical specimens. Mayo Clin Proc. 1970 Apr;45(4):256–264. [PubMed] [Google Scholar]



