Abstract
Neutrophil counts in blood are determined by the differentiation and proliferation of precursor cells in the bone marrow, release of mature neutrophils into the blood, margination in organs like the lung and spleen, and transmigration through the endothelial lining followed by neutrophil apoptosis and uptake by phagocytes. This brief review summarizes how the regulation of neutrophil production by G-CSF is in part controlled by IL-17 and IL-23. Neutrophils are retained in the bone marrow through interaction of CXCL12 with its receptor CXCR4. The relevance of this mechanism is illustrated by rare diseases in which disrupting the desensitization of CXCR4 results in neutrophil accumulation in the bone marrow. Although blood neutrophil numbers in inbred mouse strains and individual human subjects are tightly controlled, the large variation of blood neutrophil counts among outbred populations suggests genetic control. One example is benign ethnic neutropenia, which is found in about 5% of African Americans. Reduced and elevated neutrophil counts, even within the normal range, are associated with excess all-cause mortality.
Introduction
Neutrophilic granulocytes (neutrophils), the most abundant, but also very short-lived human white blood cells, act as fast primary defense against infections (1). Neutrophil turnover is rapid - around 109 cells/kg body weight leave the bone marrow per day in healthy humans (2, 3). Bone marrow postmitotic transit time determined by maximal blood neutrophil radioactivity after a pulse of 3H-thymidine was 7 days (2, 3). The transit time in rabbits and mice was somewhat shorter: the peak of cell mobilization into peripheral blood occured about 95 hours after leaving the mitotic pool, where progenitors remained about 50 hours (4, 5). Within the circulation, the half-life of infused, radiolabeled neutrophils was 7–10 hours in humans (3, 6) and 11.4 hours in mice (4). In rabbits, a shorter half-life of 3.2 hours was reported (7, 8).
Neutrophil progenitor proliferation and differentiation
Hematopoietic cytokines promote neutrophil progenitor proliferation and differentiation acting in a complex network (9). The major cytokine for neutrophil proliferation and survival is G-CSF. Mice and humans deficient in either G-CSF or its receptor suffer from profound neutropenia (10–12). G-CSF currently is the major therapeutic agent for neutropenia of iatrogenic as well as genetic and various other origins (13–15). Extensive basic science and clinical data exist on the role of other granulopoietic cytokines such as M-CSF, GM-CSF, interleukin (IL)-6, IL-3, IL-17 and, most recently, IL-22 (10, 16–22) that have been reviewed elsewhere in detail (23). Genetic modification of intracellular messengers downstream of G-CSF in mice elucidated their stage-specific roles (24). For example, both STAT3 and SOCS3 deficiency resulted in neutrophilia and an increased pool of late stage progenitors in the bone marrow thus implicating an inhibitory role (25–29). The role of transcription factors and microRNA in neutrophilic differentiation has recently been reviewed (30, 31). A number of monogenic defects associated with rare forms of congenital neutropenia in humans are known. Maturation arrest and increased cell death of neutrophil progenitor proliferation have been observed in humans with elastase gene mutations, but also in genes encoding a number of transcription factors such as Growth factor independent 1 (GFI 1), HCLS1 associatied protein X-1 (HAX1), and lymphoid enhancer factor-1 (LEF-1) (32).
Neutrophil mobilization from the bone marrow
Release mechanisms of hematopoietic stem cells, myeloid progenitors and granulocytes from the bone marrow have been studied extensively under normal and emergency conditions (33–35). G-CSF is a most active soluble physiological factor. In regard to neutrophils, the interaction of stromal derived factor-1 (SDF1, CXCL12) with the chemokine receptor CXC-receptor 4 (CXCR4) is important for retention in the bone marrow. CXCR4 deficiency resulted in decreased bone marrow but increased peripheral neutrophils as identified by the marker Gr-1 (36). CXCR4 and CXCL12 are down-regulated by G-CSF (37, 38). Neutrophil mobilization can be induced by anti-CXR4 antibodies and a number of peptide antagonists (39, 40). Conversely, activating mutations of CXCR4 in humans cause neutrophil accumulation in the bone marrow together with peripheral neutropenia together with a complex immunological phenotype (WHIM syndrome; warts, hypogammaglobulinemia, immunodeficiency, myelokathexis) (41–43). This year, specific patients’ mutations have given further insights into downstream signaling: in one WHIM patient decreased expression of the G-protein coupled receptor kinase (GRK) 3 was observed. GRKs are essential for desensitization of CXCR4 and subsequent neutrophil release from the bone marrow (44). Another mutation inhibited internalization of phosphorylated CXCR4 (45).
Neutrophil serine protease expression correlates with neutrophil release from the bone marrow. Cathepsin G and neutrophil elastase, but also matrix metalloproteinase 9 were increased by G-CSF treatment. Inhibition by alpha-1-antitrypsin inhibited neutrophil release from the bone marrow (46–48). However, neither deficiency in both cathepsin G and neutrophil elastase nor a mouse model lacking the serine proteinase activator dipeptidylpeptidase I showed altered neutrophil mobilization, thus challenging the role of serine proteases in neutrophil liberation (38).
Margination, adhesion and migration into tissues
In mice, the circulating pool of neutrophils amounts to only 1–2% of the morphologically mature neutrophils in the bone marrow (49). Neutrophil homing studies have mainly depended on extracorporally labeled cells. Data have to be interpreted cautiously as partial cell activation as occurs during isolation alters homing properties (50). In one study, approximately a third of reinfused neutrophils was found in liver and bone marrow and about 15% in the spleen, but the target depended on the collection method. Neutrophils from thioglycollate-induced peritonitis preferentially homed to the liver and bone-marrow neutrophils to the bone marrow when assessed after 4 hours (51). Endotoxin- or cobra venom factor-mobilized neutrophils infused into rats were found at 21% in spleen, 22% in liver and 14% in lungs after 4.5 hours (52). The vasculature of the lung harbours a considerable neutrophil pool. In rabbits, about 20% of 51Cr labeled neutrophils stayed in the healthy lung, of those around 90% in capillaries (53). Catecholamines can mobilize marignated neutrophils. Interestingly, altered mobilization of marginated neutrophils may be a factor in ethnic neutropenia in humans: in addition to low baseline counts, affected subjects mobilized fewer neutrophils during marathon running or other strenuous exercise (54).
Integrins and selectins are essential initiating neutrophil exit from the blood pool (55, 56). Specific adhesion molecule deficiencies increase circulating neutrophil numbers. Mice deficient in leukocyte function associated antigen (CD11a, Itgal−/−) or the common chain of all β2-integrins (CD18, Itgal−/−) show marked leukocytosis (57, 58). Neutrophil migration to various tissues was reduced in Itga−/−-deficient mice (58, 59). Itgal-silencing by neutrophil specific microRNA recently confirmed this phenotype (60). Mild neutrophilia was also found in mice deficient for P-selectin (Selp−/−) (61, 62) and more marked if both E- and P-selectin (Selp−/− Sele−/−) or all selectins (Selp−/− Sele−/− Sell−/−), were absent (62, 63). Absence of and enzyme required for selectin glycosylation, core 2 beta-1,6-N-acetylglucosaminyltransferase (Core2−/−), resulted in neutrophilia (64).
Neutrophilia in adhesion molecule-deficient mouse strains was initially thought to be caused by passive neutrophil accumulation in blood vessels. To test whether adhesion-molecule-deficient neutrophils accumulated more than wild-type cells, several groups used mixed Itgal−/− and wild-type bone marrow transplants into wild-type mice. Surprisingly, the percentage of wild-type and Itgal−/− neutrophils in peripheral blood and in bone marrow was very similar (65–67). Even a small proportion of wild-type cells was sufficient to normalize blood neutrophil levels. Proliferation measured by BrdU incorporation of Gr1 positive bone-marrow cells did not differ between wildtype and Itgal−/− cells six months after transplantation. This argues against intravascular accumulation or autonomous proliferation as reasons for neutrophilia in Itgal−/− mice.
In humans, leukocyte adhesion deficiencies (LAD), rare diseases caused by either deficiency or signaling dysfunction of β2-integrins (LAD I), selectin ligands (LAD II), or downstream signaling molecules (LAD III) replicate the neutrophilic phenotype of the respective gene-deficient mice (68–70).
Apoptosis, clearance and feedback
Neutrophils rapidly die by apoptosis in the absence of external stimuli. When apoptosis was induced in vivo, neutrophils were mainly found in the liver, where they were phagocytosed by Kupffer cells (71). Apoptotic neutrophil phagocytosis has an anti-inflammatory role (72). The proinflammatory cytokine IL-23 consists of a p40 and a specific p19 subunit. It is induced in macrophages by a variety of transcription factors, including NFkappaB, that can be downregulated by neutrophil phagocytosis (73, 74). Transgenic overexpression of the IL-23 specific subunit p19 in mice induced neutrophilia (75). Conversely, IL-23 deficiency or blockade with an antibody decreased neutrophil counts in normal and neutrophilic mice (76).
IL-23 is a potent inducer of IL-17, the most prominent member of a cytokine family defining the TH17-CD4-subpopulation (77). In all strains of severely neutrophilic, adhesion molecule-deficient mice, elevated IL-17 levels were found, and IL-17 blockade by soluble IL-17 receptor demonstrated that their neutrophilia was indeed caused by IL-17 (65). Mice deficient in the IL-17 receptor (Il17ra−/−) had decreased neutrophil counts (19, 78). IL-17 stimulates G-CSF secretion (79), and G-CSF levels were elevated in all neutrophilic mouse strains, where blockade of G-CSF normalized neutrophil counts (65). Closing this feedback loop, IL-23 expression in peripheral tissues was reduced by apoptotic neutrophils (59). These data suggest a model where granulopoiesis is driven by a cytokine cascade starting with macrophage and dendritic cell IL-23 secretion. The resulting in T-cell IL-17 secretion increases G-CSF levels. When neutrophils arrive in peripheral tissues, their phagocytosis downregulates macrophage IL23 secretion and - via decreased IL17 and G-CSF- curbs granulopoiesis (80).
“Normal” neutrophil count
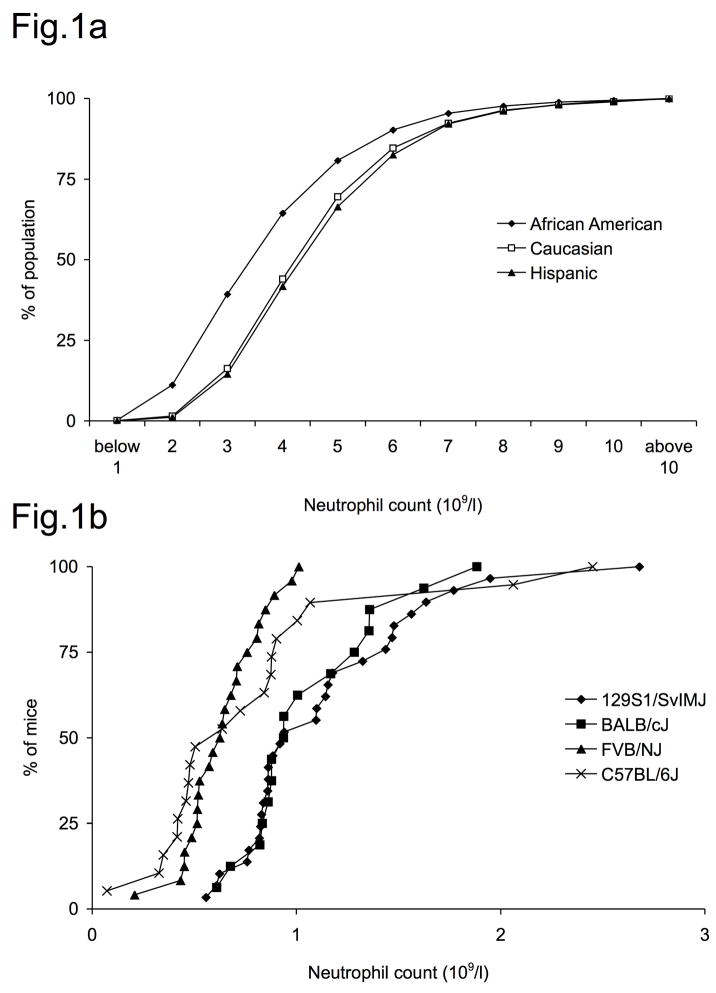
Baseline neutrophil counts are relatively stable in individuals but have a considerable normal range in healthy humans. A survey of more than 25,000 Americans found a mean neutrophil count of 4.3×109/l in adult males and 4.5×109/l in females for Caucasian participants (81). In addition to environmental factors, whose influence was highlighted by a recent study showing a global decrease of neutrophil counts in an US-Amercian population from 1958 to 2002 (82), the genetic background is important. Mean neutrophil counts are lower in African Americans, in one study 3.5×109/l in males and 3.8×109/l in females (Fig. 1a)(81). “Benign ethnic neutropenia” is a condition found in up to 5% of African Americans and is defined as a neutrophil count below 1.5× 109/l without overt cause or complication (81, 83). Little is known about the genetic factors that influence this difference or human steady state granulopoiesis within the normal range. Variation was also seen between different inbred mouse strains. Neutrophil counts from four commonly used mouse strains are given in figure 1b. (84, 85). Whole genome association studies in F2 intercrosses in mice and swine revealed chromosomal regions associated with blood neutrophil counts (86–88), some of them harbouring coding regions for cytokines such as IL-2, IL-15, IL-12 and chemokines such as CXCL8. However, specific mutations leading to functional alterations of these cytokines remain to be determined.
Figure 1. Normal range of neutrophil counts in humans and mice.
A) Neutrophil counts from 25,000 US Americans from (81) modified to show cumulative incidence. Mean counts in African Americans were significantly lower than in Caucasian or Hispanic individuals.
B) Neutrophil counts in inbred mouse strains. Neutrophil counts calculated from white blood counts and relative neutrophils counts from 129S1/SvlMJ (n=29), BALB/cJ (n=16), FVB/NJ (n=24) and C57BL6/6J (n=19) from the Jackson laboratory phenome database (84, 85).
Clinical relevance of baseline neutrophil counts
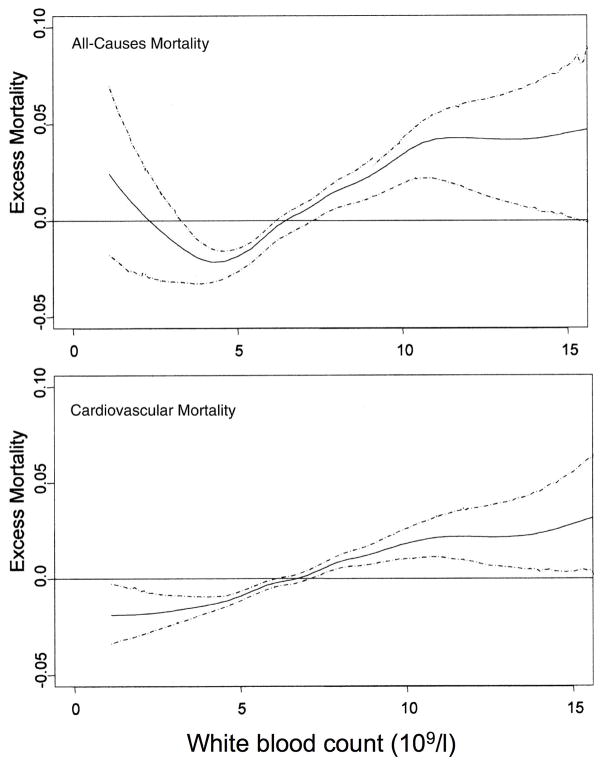
Neutrophilia is a classical indicator of acute inflammation, while idiopathic and acquired forms of neutropenia predispose to infections (89). However, total white blood counts (WBC), that are mainly determined by neutrophil counts in healthy humans, are also relevant in the absence of acute events. Increased WBCs have long been associated with increased all-cause mortality (90–94). A prospective study over 44 years revealed a j-shaped association curve of neutrophil, but not lymphocyte count and all cause mortality (82) (Fig. 2)
Figure 2. Relationship between excess mortality and white blood count.
Nearly 4000 individuals from the Baltimore/Washington area were observed from to 1958–2002. Excess mortality as difference between observed and expected mortality hazard over time is plotted against white blood cell count. The dashed lines represent the 95% confidence intervals (with permission from (82).
Neutrophils are the first defense against invading microorganisms. Increased susceptibility to common pathogens has usually been attributed to extremely low counts (below 0.5×109/l) (13) and individuals with “low normal” counts or ethnic neutropenia have not been reported to be at increased risk as long as counts are not further decreased. However, the probability to contract tuberculosis from patients with open pulmonary disease was inversely correlated with baseline neutrophil counts in a recent study (90). In contrast, an increased total WBC and neutrophil count has been shown to be an independent risk factor for cardiovascular mortality in a number of studies and subsequent metanalyses (82, 92, 95–99). Less data exist on neutrophil counts and cancer mortality, but data from the NHANES population show a higher total WBC as an independent risk factor for total cancer mortality (100). However, it is at present unclear if the elevated numbers of circulating neutrophils are causative of the observed increase in mortality or rather a measure of ongoing subclinical inflammation (101).
Summary
Stable neutrophil blood counts are the result of a highly dynamic feedback system. The study of genetically altered mice and monogenic diseases in humans has given insight into some of the involved mechanisms. However, neutrophil counts in healthy humans are regulated by a variety of environmental and genetic factors, most of which remain currently unknown. As elevated counts within the normal range are associated with excess mortality, elucidation of factors involved in steady state neutrophil regulation might have clinical relevance.
References
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest. 1976;58:705–715. doi: 10.1172/JCI108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price TH, Chatta GS, Dale DC. Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood. 1996;88:335–340. [PubMed] [Google Scholar]
- 4.Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–861. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- 5.Terashima T, Wiggs B, English D, Hogg JC, van Eeden SF. Polymorphonuclear leukocyte transit times in bone marrow during streptococcal pneumonia. Am J Physiol. 1996;271:L587–592. doi: 10.1152/ajplung.1996.271.4.L587. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright GE, Athens JW, Wintrobe MM. The Kinetics Of Granulopoiesis In Normal Man. Blood. 1964;24:780–803. [PubMed] [Google Scholar]
- 7.Price TH, Dale DC. Neutrophil preservation: the effect of short-term storage on in vivo kinetics. J Clin Invest. 1977;59:475–480. doi: 10.1172/JCI108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenshein MS, Price TH, Dale DC. Neutropenia, inflammation, and the kinetics of transfused neutrophils in rabbits. J Clin Invest. 1979;64:580–585. doi: 10.1172/JCI109496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. [PubMed] [Google Scholar]
- 11.Liu F, Wu HY, Wesselschmidt R, Kornaga T, Link DC. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity. 1996;5:491–501. doi: 10.1016/s1074-7613(00)80504-x. [DOI] [PubMed] [Google Scholar]
- 12.Dong F, Hoefsloot LH, Schelen AM, Broeders CA, Meijer Y, Veerman AJ, Touw IP, Lowenberg B. Identification of a nonsense mutation in the granulocyte-colony-stimulating factor receptor in severe congenital neutropenia. Proc Natl Acad Sci U S A. 1994;91:4480–4484. doi: 10.1073/pnas.91.10.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andres E, Maloisel F. Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr Opin Hematol. 2008;15:15–21. doi: 10.1097/MOH.0b013e3282f15fb9. [DOI] [PubMed] [Google Scholar]
- 14.Bhana N. Granulocyte colony-stimulating factors in the management of chemotherapy-induced neutropenia: evidence based review. Curr Opin Oncol. 2007;19:328–335. doi: 10.1097/01.cco.0000275309.58868.11. [DOI] [PubMed] [Google Scholar]
- 15.Marsh JC, Ganser A, Stadler M. Hematopoietic growth factors in the treatment of acquired bone marrow failure states. Semin Hematol. 2007;44:138–147. doi: 10.1053/j.seminhematol.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu S, Hodgson G, Zhang HH, Katz M, Quilici C, Dunn AR. “Emergency” granulopoiesis in G-CSF-deficient mice in response to Candida albicans infection. Blood. 2000;95:3725–3733. [PubMed] [Google Scholar]
- 18.Hibbs ML, Quilici C, Kountouri N, Seymour JF, Armes JE, Burgess AW, Dunn AR. Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol. 2007;178:6435–6443. doi: 10.4049/jimmunol.178.10.6435. [DOI] [PubMed] [Google Scholar]
- 19.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 21.Seymour JF, Lieschke GJ, Grail D, Quilici C, Hodgson G, Dunn AR. Mice lacking both granulocyte colony-stimulating factor (CSF) and granulocyte-macrophage CSF have impaired reproductive capacity, perturbed neonatal granulopoiesis, lung disease, amyloidosis, and reduced long-term survival. Blood. 1997;90:3037–3049. [PubMed] [Google Scholar]
- 22.Walker F, Zhang HH, Matthews V, Weinstock J, Nice EC, Ernst M, Rose-John S, Burgess AW. IL6/sIL6R complex contributes to emergency granulopoietic responses in G-CSF- and GM-CSF-deficient mice. Blood. 2008;111:3978–3985. doi: 10.1182/blood-2007-10-119636. [DOI] [PubMed] [Google Scholar]
- 23.Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28:509–554. doi: 10.1016/j.dci.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Panopoulos AD, Watowich SS. Granulocyte colony-stimulating factor: Molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine, advance online publication. 2008 doi: 10.1016/j.cyto.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, DePinho RA, Levy DE. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- 26.McLemore ML, Grewal S, Liu F, Archambault A, Poursine-Laurent J, Haug J, Link DC. STAT-3 activation is required for normal G-CSF-dependent proliferation and granulocytic differentiation. Immunity. 2001;14:193–204. doi: 10.1016/s1074-7613(01)00101-7. [DOI] [PubMed] [Google Scholar]
- 27.Panopoulos AD, Zhang L, Snow JW, Jones DM, Smith AM, El Kasmi KC, Liu F, Goldsmith MA, Link DC, Murray PJ, Watowich SS. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood. 2006;108:3682–3690. doi: 10.1182/blood-2006-02-003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L, Cluse LA, Sutherland KD, Hartley L, Williams E, Zhang JG, Hilton DJ, Nicola NA, Alexander WS, Roberts AW. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- 29.Kimura A, Kinjyo I, Matsumura Y, Mori H, Mashima R, Harada M, Chien KR, Yasukawa H, Yoshimura A. SOCS3 is a physiological negative regulator for granulopoiesis and granulocyte colony-stimulating factor receptor signaling. J Biol Chem. 2004;279:6905–6910. doi: 10.1074/jbc.C300496200. [DOI] [PubMed] [Google Scholar]
- 30.Rosmarin AG, Yang Z, Resendes KK. Transcriptional regulation in myelopoiesis: Hematopoietic fate choice, myeloid differentiation, and leukemogenesis. Exp Hematol. 2005;33:131–143. doi: 10.1016/j.exphem.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Lindsay MA. microRNAs and the immune response. Trends Immunol. 2008 doi: 10.1016/j.it.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Bohn G, Welte K, Klein C. Severe congenital neutropenia: new genes explain an old disease. Curr Opin Rheumatol. 2007;19:644–650. doi: 10.1097/BOR.0b013e3282f05cc2. [DOI] [PubMed] [Google Scholar]
- 33.Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14:3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Winkler IG, Levesque JP. Mechanisms of hematopoietic stem cell mobilization: when innate immunity assails the cells that make blood and bone. Exp Hematol. 2006;34:996–1009. doi: 10.1016/j.exphem.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 37.Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108:812–820. doi: 10.1182/blood-2005-10-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, Link DC. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104:65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 39.Iyer CV, Evans RJ, Lou Q, Lin D, Wang J, Kohn W, Yan LZ, Pulley S, Peng SB. Rapid and recurrent neutrophil mobilization regulated by T134, a CXCR4 peptide antagonist. Exp Hematol. 2008 doi: 10.1016/j.exphem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–571. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- 41.Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, Lebbe C, Kerob D, Dupuy A, Hermine O, Nicolas JF, Latger-Cannard V, Bensoussan D, Bordigoni P, Baleux F, Le Deist F, Virelizier JL, Arenzana-Seisdedos F, Bachelerie F. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449–2457. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 43.Kawai T, Choi U, Cardwell L, DeRavin SS, Naumann N, Whiting-Theobald NL, Linton GF, Moon J, Murphy PM, Malech HL. WHIM syndrome myelokathexis reproduced in the NOD/SCID mouse xenotransplant model engrafted with healthy human stem cells transduced with C-terminus-truncated CXCR4. Blood. 2007;109:78–84. doi: 10.1182/blood-2006-05-025296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balabanian K, Levoye A, Klemm L, Lagane B, Hermine O, Harriague J, Baleux F, Arenzana-Seisdedos F, Bachelerie F. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J Clin Invest. 2008;118:1074–1084. doi: 10.1172/JCI33187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lagane B, Chow KY, Balabanian K, Levoye A, Harriague J, Planchenault T, Baleux F, Gunera-Saad N, Arenzana-Seisdedos F, Bachelerie F. CXCR4 dimerization and {beta}-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood. 2008 doi: 10.1182/blood-2007-07-102103. [DOI] [PubMed] [Google Scholar]
- 46.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 47.van Pel M, van Os R, Velders GA, Hagoort H, Heegaard PM, Lindley IJ, Willemze R, Fibbe WE. Serpina1 is a potent inhibitor of IL-8-induced hematopoietic stem cell mobilization. Proc Natl Acad Sci U S A. 2006;103:1469–1474. doi: 10.1073/pnas.0510192103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winkler IG, Hendy J, Coughlin P, Horvath A, Levesque JP. Serine protease inhibitors serpina1 and serpina3 are down-regulated in bone marrow during hematopoietic progenitor mobilization. J Exp Med. 2005;201:1077–1088. doi: 10.1084/jem.20042299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 50.Hogg JC, Doerschuk CM. Leukocyte traffic in the lung. Annu Rev Physiol. 1995;57:97–114. doi: 10.1146/annurev.ph.57.030195.000525. [DOI] [PubMed] [Google Scholar]
- 51.Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS. Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L913–921. doi: 10.1152/ajplung.2001.281.4.L913. [DOI] [PubMed] [Google Scholar]
- 52.Savige JA, Saverymuttu SH, Pinching AJ. A functional comparison of IIIindium-labelled elicited peripheral blood neutrophils and peritoneal neutrophils in the rat. Clin Exp Immunol. 1984;58:737–744. [PMC free article] [PubMed] [Google Scholar]
- 53.Doerschuk CM, Markos J, Coxson HO, English D, Hogg JC. Quantitation of neutrophil migration in acute bacterial pneumonia in rabbits. J Appl Physiol. 1994;77:2593–2599. doi: 10.1152/jappl.1994.77.6.2593. [DOI] [PubMed] [Google Scholar]
- 54.Bain BJ, Phillips D, Thomson K, Richardson D, Gabriel I. Investigation of the effect of marathon running on leucocyte counts of subjects of different ethnic origins: relevance to the aetiology of ethnic neutropenia. Br J Haematol. 2000;108:483–487. doi: 10.1046/j.1365-2141.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- 55.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 56.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 57.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 58.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Smith CW, Montgomery CA, Rich S, Beaudet AL. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Cullere X, Lauterbach M, Tsuboi N, Mayadas TN. Neutrophil-selective CD18 silencing using RNA interference in vivo. Blood. 2008;111:3591–3598. doi: 10.1182/blood-2007-12-127837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 62.Robinson SD, Frenette PS, Rayburn H, Cummiskey M, Ullman-Cullere M, Wagner DD, Hynes RO. Multiple, targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment. Proc Natl Acad Sci U S A. 1999;96:11452–11457. doi: 10.1073/pnas.96.20.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P-and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 64.Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 65.Forlow SB, Schurr JR, Kolls JK, Bagby GJ, Schwarzenberger PO, Ley K. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood. 2001;98:3309–3314. doi: 10.1182/blood.v98.12.3309. [DOI] [PubMed] [Google Scholar]
- 66.Horwitz BH, Mizgerd JP, Scott ML, Doerschuk CM. Mechanisms of granulocytosis in the absence of CD18. Blood. 2001;97:1578–1583. doi: 10.1182/blood.v97.6.1578. [DOI] [PubMed] [Google Scholar]
- 67.Weinmann P, Scharffetter-Kochanek K, Forlow SB, Peters T, Walzog B. A role for apoptosis in the control of neutrophil homeostasis in the circulation: insights from CD18-deficient mice. Blood. 2003;101:739–746. doi: 10.1182/blood-2002-01-0239. [DOI] [PubMed] [Google Scholar]
- 68.Bunting M, Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. Leukocyte adhesion deficiency syndromes: adhesion and tethering defects involving beta 2 integrins and selectin ligands. Curr Opin Hematol. 2002;9:30–35. doi: 10.1097/00062752-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Bergmeier W, Goerge T, Wang HW, Crittenden JR, Baldwin AC, Cifuni SM, Housman DE, Graybiel AM, Wagner DD. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117:1699–1707. doi: 10.1172/JCI30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pasvolsky R, Feigelson SW, Kilic SS, Simon AJ, Tal-Lapidot G, Grabovsky V, Crittenden JR, Amariglio N, Safran M, Graybiel AM, Rechavi G, Ben-Dor S, Etzioni A, Alon R. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204:1571–1582. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98:1226–1230. doi: 10.1182/blood.v98.4.1226. [DOI] [PubMed] [Google Scholar]
- 72.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 73.Ma W, Mishra S, Gee K, Mishra JP, Nandan D, Reiner NE, Angel JB, Kumar A. Cyclosporin A and FK506 inhibit IL-12p40 production through the calmodulin/calmodulin-dependent protein kinase-activated phosphoinositide 3-kinase in lipopolysaccharide-stimulated human monocytic cells. J Biol Chem. 2007;282:13351–13362. doi: 10.1074/jbc.M611522200. [DOI] [PubMed] [Google Scholar]
- 74.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 75.Wiekowski MT, Leach MW, Evans EW, Sullivan L, Chen SC, Vassileva G, Bazan JF, Gorman DM, Kastelein RA, Narula S, Lira SA. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol. 2001;166:7563–7570. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- 76.Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J Immunol. 2007;179:8274–8279. doi: 10.4049/jimmunol.179.12.8274. [DOI] [PubMed] [Google Scholar]
- 77.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 78.Smith E, Stark MA, Zarbock A, Burdin TL, Bruce AC, Vaswani D, Foley P, Ley K. IL-17A inhibits the expansion of IL-17A-producing T cells in mice through “short-loop” inhibition via IL-17 receptor. Journal of Immunology. 2008 doi: 10.4049/jimmunol.181.2.1357. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ley K, Smith E, Stark MA. IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunol Res. 2006;34:229–242. doi: 10.1385/IR:34:3:229. [DOI] [PubMed] [Google Scholar]
- 81.Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486–492. doi: 10.7326/0003-4819-146-7-200704030-00004. [DOI] [PubMed] [Google Scholar]
- 82.Ruggiero C, Metter EJ, Cherubini A, Maggio M, Sen R, Najjar SS, Windham GB, Ble A, Senin U, Ferrucci L. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2007;49:1841–1850. doi: 10.1016/j.jacc.2007.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: what is a normal absolute neutrophil count? J Lab Clin Med. 1999;133:15–22. doi: 10.1053/lc.1999.v133.a94931. [DOI] [PubMed] [Google Scholar]
- 84.Mouse Phenome Database. The Jackson Laboratory. Bar Harbor; Maine USA: [May 13 th, 2008]. Accessed at phenome.jax.org on. [Google Scholar]
- 85.Peters LL, Cheever EM, Ellis HR, Magnani PA, Svenson KL, Von Smith R, Bogue MA. Large-scale, high-throughput screening for coagulation and hematologic phenotypes in mice. Physiol Genomics. 2002;11:185–193. doi: 10.1152/physiolgenomics.00077.2002. [DOI] [PubMed] [Google Scholar]
- 86.Peters LL, Zhang W, Lambert AJ, Brugnara C, Churchill GA, Platt OS. Quantitative trait loci for baseline white blood cell count, platelet count, and mean platelet volume. Mamm Genome. 2005;16:749–763. doi: 10.1007/s00335-005-0063-6. [DOI] [PubMed] [Google Scholar]
- 87.Reiner G, Fischer R, Hepp S, Berge T, Kohler F, Willems H. Quantitative trait loci for white blood cell numbers in swine. Anim Genet. 2008;39:163–168. doi: 10.1111/j.1365-2052.2008.01700.x. [DOI] [PubMed] [Google Scholar]
- 88.Wattrang E, Almqvist M, Johansson A, Fossum C, Wallgren P, Pielberg G, Andersson L, Edfors-Lilja I. Confirmation of QTL on porcine chromosomes 1 and 8 influencing leukocyte numbers, haematological parameters and leukocyte function. Anim Genet. 2005;36:337–345. doi: 10.1111/j.1365-2052.2005.01315.x. [DOI] [PubMed] [Google Scholar]
- 89.Stock W, Hoffman R. White blood cells 1: non-malignant disorders. Lancet. 2000;355:1351–1357. doi: 10.1016/S0140-6736(00)02125-5. [DOI] [PubMed] [Google Scholar]
- 90.Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117:1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sunyer J, Munoz A, Peng Y, Margolick J, Chmiel JS, Oishi J, Kingsley L, Samet JM. Longitudinal relation between smoking and white blood cells. Am J Epidemiol. 1996;144:734–741. doi: 10.1093/oxfordjournals.aje.a008997. [DOI] [PubMed] [Google Scholar]
- 92.Brown DW, Ford ES, Giles WH, Croft JB, Balluz LS, Mokdad AH. Associations between white blood cell count and risk for cerebrovascular disease mortality: NHANES II Mortality Study, 1976–1992. Ann Epidemiol. 2004;14:425–430. doi: 10.1016/j.annepidem.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 93.Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, Grimm RH, Jr, Howard BV, Assaf AR, Prentice R. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women’s Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 94.Weiss ST, Segal MR, Sparrow D, Wager C. Relation of FEV1 and peripheral blood leukocyte count to total mortality. The Normative Aging Study. Am J Epidemiol. 1995;142:493–498. doi: 10.1093/oxfordjournals.aje.a117665. discussion 499–503. [DOI] [PubMed] [Google Scholar]
- 95.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. Jama. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 96.Loimaala A, Rontu R, Vuori I, Mercuri M, Lehtimaki T, Nenonen A, Bond MG. Blood leukocyte count is a risk factor for intima-media thickening and subclinical carotid atherosclerosis in middle-aged men. Atherosclerosis. 2006;188:363–369. doi: 10.1016/j.atherosclerosis.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 97.Weijenberg MP, Feskens EJ, Kromhout D. White blood cell count and the risk of coronary heart disease and all-cause mortality in elderly men. Arterioscler Thromb Vasc Biol. 1996;16:499–503. doi: 10.1161/01.atv.16.4.499. [DOI] [PubMed] [Google Scholar]
- 98.Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97:993–996. doi: 10.1016/j.amjcard.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 99.Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, Jeffrey RR, Buchan KG, El-Shafei H, Hillis GS. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154:995–1002. doi: 10.1016/j.ahj.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 100.Erlinger TP, Muntner P, Helzlsouer KJ. WBC count and the risk of cancer mortality in a national sample of U.S. adults: results from the Second National Health and Nutrition Examination Survey mortality study. Cancer Epidemiol Biomarkers Prev. 2004;13:1052–1056. [PubMed] [Google Scholar]
- 101.Bovill EG, Bild DE, Heiss G, Kuller LH, Lee MH, Rock R, Wahl PW. White blood cell counts in persons aged 65 years or more from the Cardiovascular Health Study. Correlations with baseline clinical and demographic characteristics. Am J Epidemiol. 1996;143:1107–1115. doi: 10.1093/oxfordjournals.aje.a008687. [DOI] [PubMed] [Google Scholar]