Abstract
Flaxseed (FS) has high contents of omega-3 fatty acids and lignans with antioxidant properties. Its use in preventing thoracic X-ray radiation therapy (XRT)-induced pneumonopathy has never been evaluated. We evaluated FS supplementation given to mice given before and post-XRT. FS-derived lignans, known for their direct antioxidant properties, were evaluated in abrogating ROS generation in cultured endothelial cells following gamma radiation exposure. Mice were fed 10% FS or isocaloric control diet for three weeks and given 13.5 Gy thoracic XRT. Lungs were evaluated at 24 hours for markers of radiation-induced injury, three weeks for acute lung damage (lipid peroxidation, lung edema and inflammation), and at four months for late lung damage (inflammation and fibrosis). FS-Lignans blunted ROS generation in vitro, resulting from radiation in a dose-dependent manner. FS-fed mice had reduced expression of lung injury biomarkers (Bax, p21, and TGF-beta1) at 24 hours following XRT and reduced oxidative lung damage as measured by malondialdehyde (MDA) levels at 3 weeks following XRT. In addition, FS-fed mice had decreased lung fibrosis as determined by hydroxyproline content and decreased inflammatory cell influx into lungs at 4 months post XRT. Importantly, when Lewis Lung carcinoma cells were injected systemically in mice, FS dietary supplementation did not appear to protect lung tumors from responding to thoracic XRT. Dietary FS is protective against pulmonary fibrosis, inflammation and oxidative lung damage in a murine model. Moreover, in this model, tumor radioprotection was not observed. FS lignans exhibited potent radiation-induced ROS scavenging action. Taken together, these data suggest that dietary flaxseed may be clinically useful as an agent to increase the therapeutic index of thoracic XRT by increasing the radiation tolerance of lung tissues.
Keywords: Flaxseed, radiation pneumonopathy, TGF-β1, lung fibrosis, antioxidant, flaxseed lignans, lung injury, ROS, inflammation, mouse model
INTRODUCTION
The effectiveness of radiotherapy for thoracic malignancies is limited by the low tolerance of normal lung parenchyma for ionizing radiation (1, 2). Clinically significant radiation lung injury occurs in 30% of patients irradiated for lung cancer (3) and 10−15% of other thoracic oncology patients (4). A greater proportion of patients have subclinical adverse effects of radiation on the lung, identifiable by imaging and/or physiologic testing (5). Highly reactive compounds known as reactive oxygen species (ROS) and reactive nitrogen species (RNS) are induced in large quantities by radiation therapy (XRT)and have been implicated in this form of lung injury (6).
There is currently no known effective pharmacologic therapy for the prevention of acute or chronic radiation pneumonopathy. To date, the only means to avoid life-threatening radiation pneumonopathy is to modify the irradiation technique to minimize the volume of normal lung that receives a significant radiation dose. A safe and effective biologic radioprotector would thus be extremely useful. Preclinical data suggest that antioxidant molecules and/or enzymes might offer protection of the lung (7-9). Our group has recently shown that systemic administration of polyethylene glycol conjugated antioxidant enzymes at the time of XRT alters several early biomarkers of lung injury, decreases apoptosis, and ameliorates late pulmonary fibrosis in a murine model (10). Although encouraging, this potential therapeutic is far from human clinical trials, and a safe, more accessible radioprotector is therefore needed.
In this study, we explore the use of dietary flaxseed (FS) as a potential radioprotector against radiation induced lung injury in a murine model of thoracic XRT. FS, a non-toxic whole grain used as a nutritional supplement, has gained popularity because it is a rich source of natural antioxidants. FS is composed of high concentrations of omega-3 fatty acids and lignans. While omega-3 fatty acids reduce inflammation, lignans possess antioxidant properties. Specifically, secoisolariciresinol diglucoside (SDG), isolated from FS was shown in vitro to have direct hydroxyl radical scavenging properties and to inhibit lipid peroxidation (11-13) as verified recently by our group in the context of oxidative lung injury (14, 15). We first examined the effects of the lignan precursor SDG on abrogation of radiation-induced ROS generation in vitro and of dietary FS on early biomarkers of lung injury after thoracic XRT. Given the central role of oxidative stress in radiation pneumonopathy, we also evaluated the effects of dietary FS on acute pneumonitis and late radiation fibrosis in a murine model. Finally, we performed a preliminary evaluation to determine whether FS supplementation decreases lung tumor response to XRT using an orthotopic model.
MATERIALS AND METHODS
Animals
Our studies used female C57/Bl6 mice, a strain well characterized in the field of pulmonary radioprotection (16-18). Mice were obtained from Charles River (Wilmington, MA) and irradiated at 6−8 weeks of age under animal protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania.
Diets and dietary treatments
Semi-purified AIN-93G diet was used as the base diet which was supplemented with 10% (w/w) FS as described in our previous publications (14, 15). Control and experimental diets were isocaloric and identical in physiological fuel value. Whole ground yellow FS (Lot# 1012338) was kindly provided by Dr. Jack Carter (North Dakota State University, Fargo, ND). Mice were maintained on control (0% FS) or treatment (10% FS) diets given ad libitum for three weeks prior to XRT and for the entire duration of the experiment, unless otherwise noted in the text.
Radiation procedure
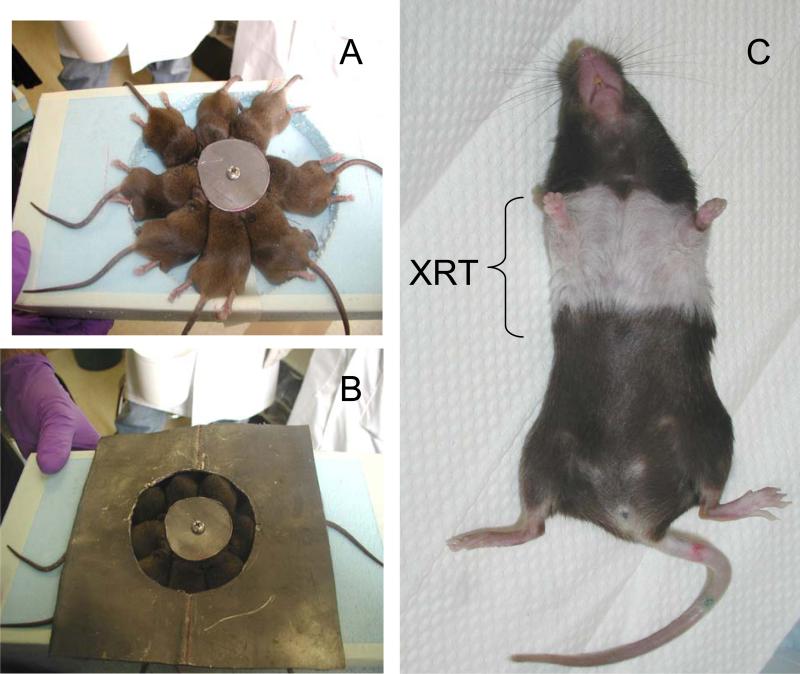
Mice were irradiated as previously described (10). Briefly, using a customized jig that allows thoracic irradiation (Figure 1) of up to 8 mice simultaneously while shielding the head and abdomen/pelvis, 13.5 Gy was delivered to mid-plane using a 250kVp orthovoltage machine with a 2mm copper filter and a tube current of 13mA. Dosimetric analysis confirmed that dose heterogeneity in the animals’ lungs was within 15%.
Figure 1. Single fraction thoracic XRT treatment.
Panels A-C: Mice are anesthetized mildly and irradiated in a custom-made immobilization chamber that allows exposure of the lungs (bilateral) with shielding of the head, abdomen, and extremities (A,B). The dose of irradiation is a single fraction delivered via single AP (anterior-posterior) approach. The dose used is 13.5 Gy (roughly corresponding to LD50). For quality assurance, theromoluminescent dosimeters are placed over selected mice, to verify correct dose administration. Hair loss and discoloration designates and confirms the site or irradiation.
RNA preparation and semi-quantitative RTPCR – Confirmation of selected genes
Mice were sacrificed for semi-quantitative analysis of mRNA expression as described previously (10) for p21, Bax and TGF-β1 at 24 hours post irradiation. The amount of cDNA was normalized using β-actin levels since β-actin gene expression levels have been shown not to change with radiation (19). A minimum of 3 samples from control and irradiated lungs were pooled and analyzed in triplicate. The relative expression level based on cycle number was compared between groups.
Tissue harvesting and evaluation of oxidative lung injury
Radiation experiments were terminated after four months, corresponding to the earliest time point when radiation-induced fibrosis is detectable in our model (10). Separate animals were used for short-term experiments intended for molecular studies or determination of inflammatory parameters (24 hours and 3 weeks post XRT, respectively) (10). Malondialdehyde (MDA), an indicator of oxidative stress (20) was measured in homogenized lung tissues using a commercially available kit (OXIS International, Portland, OR) according to manufacturer's recommendations. The results were expressed as μmol MDA/g lung protein.
ROS imaging in irradiated mouse lung endothelial cells
Pulmonary microvascular cells (PMVEC) were isolated from murine lungs as described previously (21, 22). Briefly, freshly harvested mouse lungs were treated with collagenase followed by isolation of cells by adherence to magnetic beads coated with mAb to platelet endothelial cell adhesion molecule (PECAM). PMVEC were propagated in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), non essential amino acids, and penicillin/streptomycin and maintained under static culture conditions. The endothelial phenotype of the preparation was routinely confirmed by monitoring the expression of endothelial markers such as PECAM (CD31), VE-cadherin, von Willebrandt factor, Acetylated low density lipoprotein (AcLDL) etc. Cells grown to confluence on culture dishes were treated with increasing concentrations of SDG and exposed to 2 Gy of gamma radiation using a JL Shepherd Mark 1 137Cs Irradiator at a dose rate of 1.14 Gy/min. Cells were then labeled with 5 microM H2DCF-DA by addition of the compound to the medium (15). The fluorophore was added to the cells immediately after irradiation for about 10 min and cells were imaged. Imaging studies were carried out as previously described (15) with epifluorescence microscopy. MetaMorph Imaging Software (Universal Imaging, West Chester, PA) was used for image processing on a preset scale of arbitrary units ranging from 0 to 4095. All images were captured at identical time and exposure settings. Data from multiple fields as indicated over several experiments were used to quantitate ROS generation.
Quantitative and semi-quantitative assessment of fibrosis
a) Whole collagen content of mouse lung was evaluated quantitatively by determining hydroxyproline content using acid hydrolysis (10) according to Woessner et al.(23). The data is expressed as μg hydroxyproline/whole lung.
b) Semiquantitative evaluation of fibrosis was done histologically by determining a radiation Fibrotic Index (FI). The FI was generated by a lung pathologist blinded to treatment groups using a scale of 1−4, with 4 signifying maximal fibrosis, was also assessed histologically. Briefly, 0 = Normal Lung; 1 = Fibrosis involving less than 5% of the lung, involving predominantly subpleural areas with minimal areas of inflammation, epithelial hyperplasia and; 2 = fibrosis involving from 5% to 20% of the lung, involving subpleural and peribroncheal areas with areas of inflammation, epithelial hyperplasia and; 3 = fibrosis involving from 21% to 50% of the lung, involving subpleural and peribroncheal areas with areas of inflammation, epithelial hyperplasia and 4 = fibrosis involving more than 50% of the lung, involving subpleural and peribroncheal areas with areas of inflammation, epithelial hyperplasia and. Five to 14 slides per group were assessed and mean values ± SEM of the fibrotic scores were calculated.
Analysis of malignant tumor radioprotection
In order to test for potential tumor protection from radiation by FS feeding, mice were injected with 2 million murine Lewis Lung carcinoma cells (LLC cells) (10). Multiple metastatic lung tumors were established within ten days post injection as judged by parallel animals that were sacrificed and evaluated histologically. Mice were started on 0% or 10% FS diets two weeks prior to tumor cell injection and 10 days prior to receiving a single dose XRT to the thorax (13.5 Gy). Lungs were evaluated for tumor burden 31 days post XRT. Lung weight was also recorded.
Tumor morphometry
Quantitative morphometric analysis was performed on 5-μm serial lung sections stained with H&E using Image Pro-Plus image-analysis software (Spot 3.0 driver software: RTkE Diagnostic, Inc., Image-pro plus 4.0: Media Cybernetics, Silver Spring, MD). Images were obtained with an Olympus BX41 light microscope fitted with a high-resolution RTkE Diagnostic camera. Each image was captured under the same reproducible conditions and digitized. Values, originally expressed in pixels, were converted to micrometers squared and expressed as percentage of the total area.
Statistical analysis
Results are expressed as mean ± SEM. Statistical differences among groups were determined using one-way analysis of variance (ANOVA). When statistically significant differences were found (p<0.05) individual comparisons were made using the Bonferoni/Dunn test (Statview 4.0).
RESULTS
The flaxseed SDG blunts radiation-induced H2O2 generation in lung endothelial cells
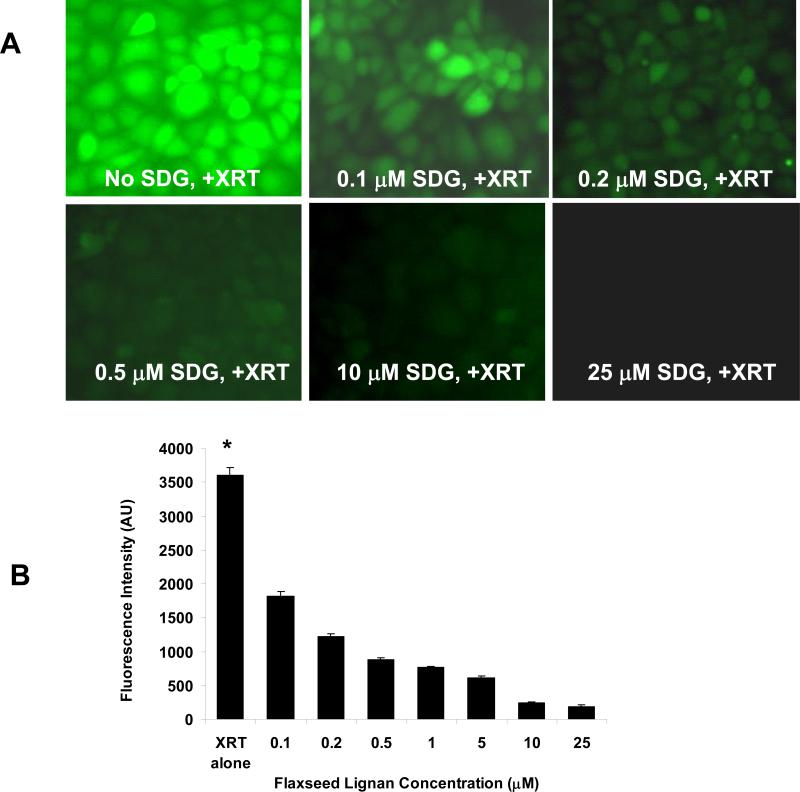
We evaluated SDG in an in vitro model of radiation-induced oxidative stress generation to detect ROS production by the pulmonary endothelium identified by H2DCF-DA fluorescence. Confluent monolayers of PMVEC were challenged with gamma radiation in the presence of physiological (0.1−50 μM) levels of SDG. (Figure 2, Panel A). Quantification of these images show a significant increase in ROS generation shortly after radiation (fluorescent imaging panels of unirradiated cells are completely black-not shown) which was blunted in a dose-dependent manner by SDG (p<0.001). (Figure 2, Panel B).
Figure 2. Antioxidant action of Flaxseed in irradiated lung cells.
Panel A: ROS generation was measured in mouse PMVEC treated with SDG shortly prior to 2 Gy gamma irradiation. Fluorescence intensity was measured in the absence of SDG or treatment with 0.1−25 μM SDG. The ROS-specific dye H2DCF-DA was used to monitor ROS generation. Panel B: 8 random fields were selected for the quantification of fluorescence (B). *P<0.001 for irradiated control as compared to all doses of SDG given to gamma-irradiated cells shortly prior to irradiation.
Dietary FS supplementation ameliorates radiation–induced oxidative lung damage
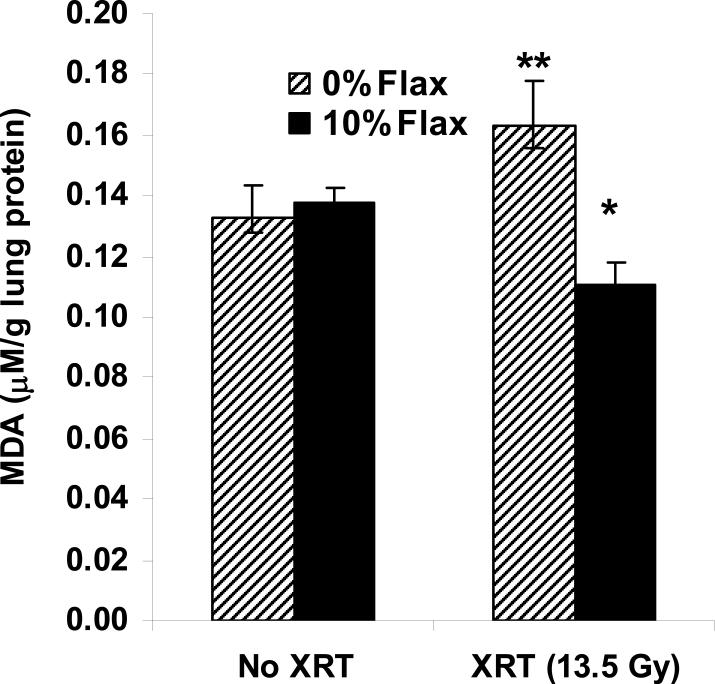
C57/Bl6 mice develop acute pneumonitis within 2−3 weeks after a single fraction of XRT (13.5 Gy) characterized by inflammatory cell influx quantified by BALF evaluation of white blood cell (WBC) counts and by oxidative tissue damage measured by lung MDA (10). Irradiated mouse lungs from animals pre-fed 10% FS, sustained a significantly lower level of lipid peroxidation as compared to irradiated mice fed control diet (n=10−15 mice per group; p=0.001) (Figure 3).
Figure 3. Antioxidant action of Flaxseed in irradiated lung tissues.
Malondialdehyde (MDA) levels were evaluated in whole mouse lung homogenates from non-irradiated, baseline controls and compared to control or 10% Flaxseed fed mice at three weeks after thoracic XRT. Values represent mean ± SEM. n=10−15 mice per group. *p=0.001 for 0% FS vs. 10% Flaxseed + XRT and **p=0.04 for irradiated 0% FS vs. non-irradiated 0% FS.
Dietary FS supplementation reduces the early expression of the Bax, p21, and TGF-β1 after thoracic XRT
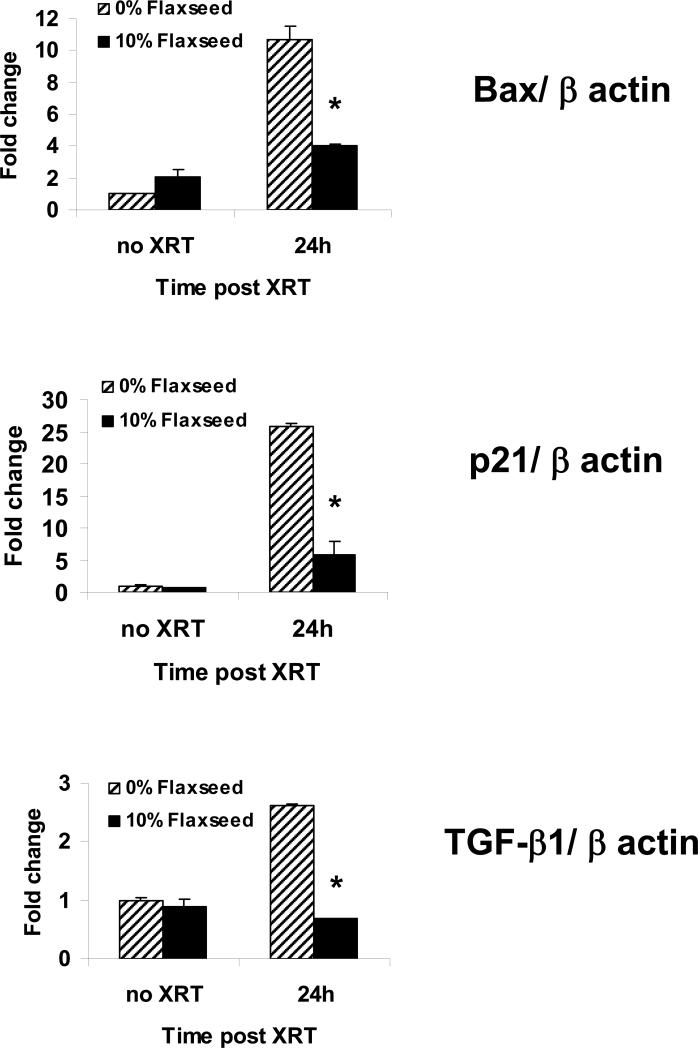
Thoracic irradiation increases the expression of Bax, p21 and TGF-β1, and these biomarkers show strong correlation with the severity of radiation-induced lung injury in both animal studies (10, 24, 25) and human (26, 27). To examine the effect of FS feeding on biomarkers of radiation-induced lung injury, mice were sacrificed and their lungs processed for RT-PCR analysis of Bax, p21 and TGF-β1 expression levels at 24 hours post XRT (Figure 4). Radiation caused an 11- and 26-fold increase of both Bax and p21 gene expression respectively at 24 hours post XRT over non-irradiated control diet fed mice. 10% FS-fed mice had significantly reduced expression of Bax and p21 by 2.8- and 4.3-fold, respectively, compared to control fed irradiated mice, p<0.001. Our group also examined the kinetics of TGF-β1 gene expression levels in irradiated mouse lungs following a single-fraction radiation treatment (10) and observed that a 2.6 fold increase in TGF-β1 levels is seen at 24 hours post thoracic XRT, confirming previously published work (28). In the current experiment, we showed a similar early increase of TGF-β1 expression at 24 hours post-XRT in control diet fed mice and a 3.9-fold decrease in expression in the 10% FS fed mice compared to control fed irradiated mice (p<0.001) (Figure 4).
Figure 4. Flaxseed modulates post XRT gene expression involved with apoptosis, cell cycle regulation, and inflammation.
RTPCR of Bax, p21, and TGFβ-1 at 24 hours post XRT in mice fed control or 10% Flaxseed diets. Values represent means of triplicate samples of pooled cDNA from n=3 mice per group. Values were normalized to beta actin gene levels and compared to unirradiated controls. Error bars represent mean ± SEM. *p<0.001 vs. 0% Flaxseed at 24h post XRT for Bax, p21, and TGF-β1.
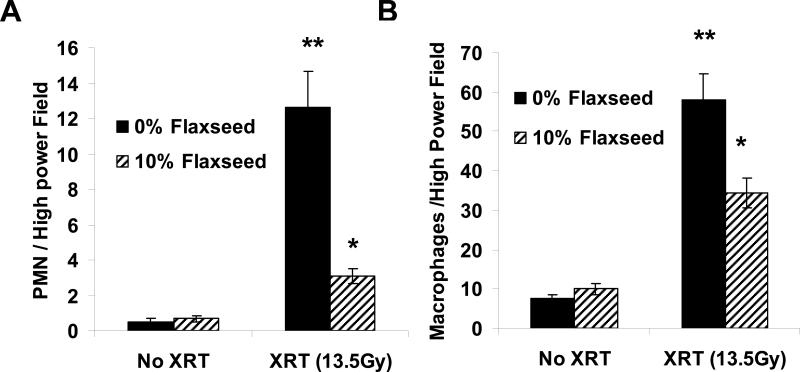
Dietary FS supplementation abrogates long term inflammatory cell infiltration and fibrosis in lungs after single fraction thoracic XRT
Compared to control diet fed mice, mice pre-fed 10% FS prior to radiation did not have significant differences in BALF measures of early inflammation and injury. Specifically, mouse lung lavages 3 weeks post XRT, the earliest time of detectable lung inflammation and injury in this model (10), showed no significant differences in BAL WBC, % infiltrating PMN or BAL proteins between irradiated mice on control 0% FS or 10% FS diet (n=10−15 mice per group). To evaluate the effect of FS on radiation pneumonitis, histopathologic changes of irradiated animals 4 months post XRT in each diet group (0% and 10% FS) were evaluated by quantification of alveolar neutrophils (PMN) and macrophages (MF) from H&E lung sections from all animals that survived the 4-month long-term experiments (Figure 5, Panels A and B respectively). A pathologist blinded to the experimental groups evaluated 5 high-power fields (HPF) per slide and recorded the average for each lung. While XRT induced a significant influx of both PMN and MF in 0% FS animals, the effect was ameliorated by FS supplementation.
Figure 5. Evaluation of FS in abrogating XRT-induced inflammation.
Mice we re fed a 0% or 10% FS-supplemented diet for 3 weeks and then exposed to a single fraction thoracic XRT (13.5 Gy). Mice were kept on the respective diets and sacrificed after 4 months (Late phase) for evaluation of lung inflammation and injury. Panels A,B: Comparison of alveolar PMN and Macrophage influx in lungs 4 months post-XRT. Cells were quantified from histological H&E-stained lung sections in 5 high-power fields per lung in all animals from 3 separate long-term studies. *p<0.0001 for 0% FS vs. 10% FS XRT and **p<0.0001 vs. 0% FS no XRT in the late phase.
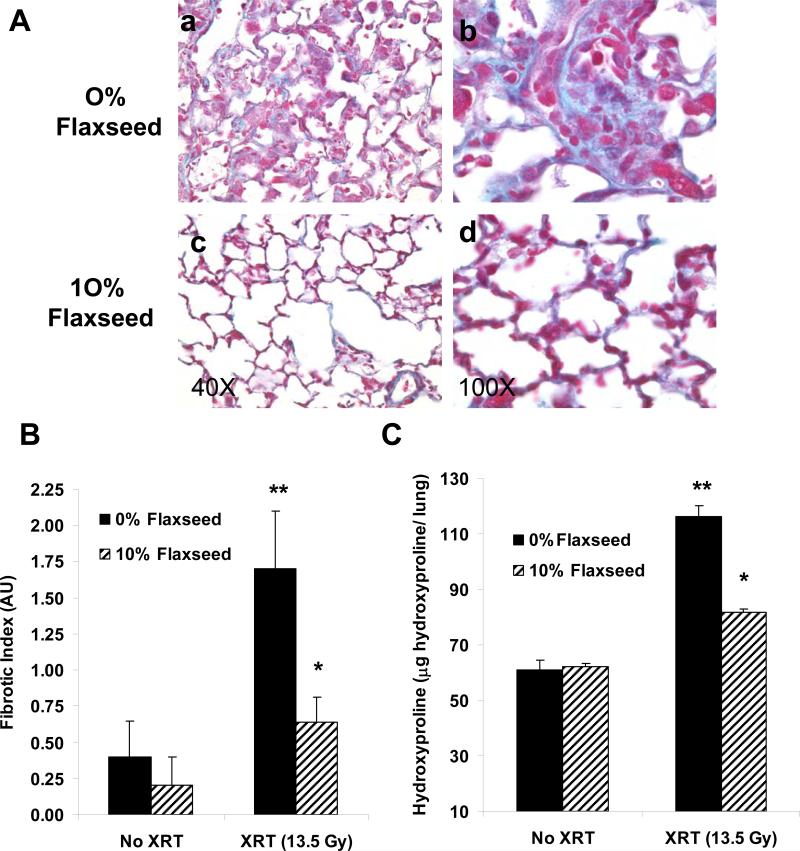
Moreover, when the degree of radiation fibrosis was evaluated using Trichrome staining, the 10% FS treated group of irradiated lungs had less severe fibrosis and less structural damage compared to control diet-fed irradiated mice as shown in representative animals (Figure 6, Panel A, subpanels c and d vs. a and b ). Semi-quantitative analysis of the degree of late-phase pneumonopathy characterized by both inflammation and fibrotic changes using the radiation Fibrotic Index (FI) score indicated significant decrease in lung fibrosis in irradiated lungs from 10% FS vs. 0% FS fed mice (p=0.005) (Figure 6, Panel B). Similarly, mice prefed 10% FS supplemented diets before and after thoracic XRT had significantly decreased lung hydroxyproline content four months after XRT as compared to irradiated mice fed control diet (n=10 mice per group; **p=0.01 vs. 0% Flaxseed + XRT) while no diet-related difference in animal weight was observed. This represents a 10.5% vs. 39% increase in hydroxyproline content as compared to non-irradiated, age-matched control mice placed on the respective diets (10% FS and 0% FS diets respectively). (Figure 6, Panel C).
Figure 6. Lung fibrosis four months after thoracic irradiation.
Panel A: Evaluation of collagen deposition using Mason's trichrome blue stain (MTB) in lungs from mice fed control or 10% FS diets at four months ± thoracic XRT. Characteristic morphology of each group is shown at 100x (Panel a,c) and at higher magnification at 400x (Panels b, d). Panel B: Semi-quantitative assessment of lung fibrosis using a radiation Fibrotic Index (FI) generated by a lung pathologist, with 4 signifying maximal fibrosis. Values represent mean ± SEM of n=5−14 slides per group, **p=0.008 vs. 0% FS, No XRT and *p=0.005 vs.10% + XRT. Panel C: Evaluation of hydroxyproline content in mice treated as in A and B. Values indicate mean ± SEM of n=10 mice per group. (**p=0.0004 vs. 0% FS, No XRT and *p=0.002).
Dietary FS supplementation at the time of thoracic XRT does not affect tumor response to radiation
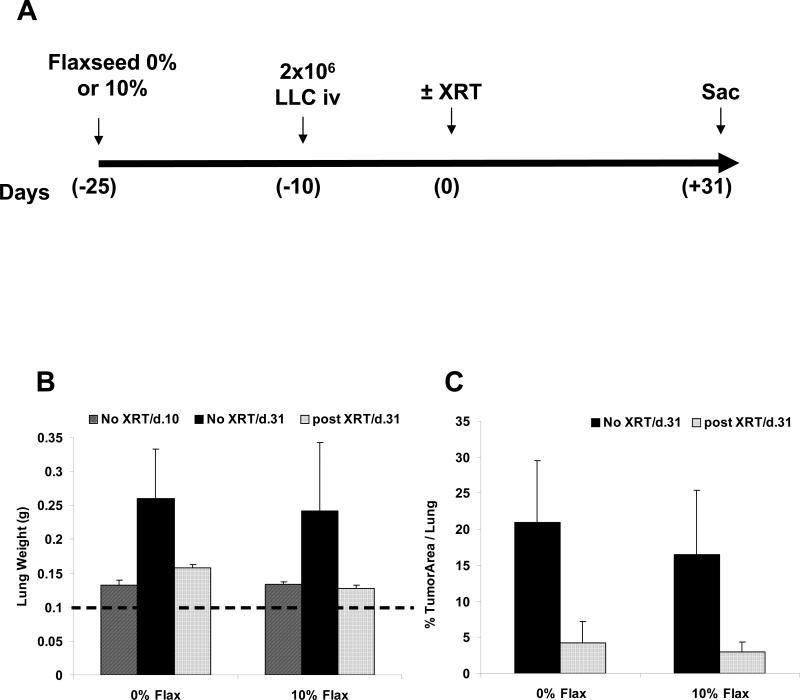
To determine whether FS feeding alters lung tumor response to thoracic XRT, an orthotopic murine lung cancer model was used as previously described (10). C57/Bl6 mice were injected with LLC and pulmonary metastases were established within 10 days. Control or 10% FS diet was started >3 weeks prior to thoracic XRT (13.5 Gy) and continued for the duration of the experiment (Figure 7, Panel A). This ensured that flaxseed-mediated upregulation of antioxidant defenses in the lung would be adequately induced as observed by Lee et al. (15). Four weeks after XRT, mice were sacrificed and lungs were weighed and assessed histologically to determine tumor burden. There was no statistical difference in lung weight observed with 0% vs. 10% FS feeding in irradiated, tumor-bearing lungs (Figure 7, Panel B). Morphometric analysis from 0% and 10% FS fed mice who received tumor injections and irradiation revealed no significant difference in % tumor area per slide (Figure 7, Panel C). These data indicate that dietary supplementation of flaxseed did not prevent radiation-induced tumor killing. In fact, dietary FS alone showed a trend toward decreased tumor burden, a phenomenon that we are currently investigating.
Figure 7. Dietary Flaxseed treatment and lung tumor protection.
Panel A: Mice were injected with 2x106 LLC cells or saline and lung tumors were established within 10 days post injection. Twenty five days prior to a single dose of XRT (13.5 Gy) to the thorax at T=0, the mice were placed on a 0% or 10% Flaxseed diet. Mice were allowed to live 4 additional weeks and lungs were excised and weighed. (n=4 mice/group). Panel B: Lung weight evaluations of 0% Flaxseed as compared to 10% flaxseed supplemented. The data represent Mean ± SEM. *p=0.04 vs. Lung + tumor, no XRT vs. plus XRT. Dashed line represents non-irradiated, non-treated, control mouse lungs at initiation of the experiments. Panel C: Morphometric analysis of % tumor in total area per treatment group, Mean ± SEM *p=0.005 for 0% FS tumor/no XRT vs. 0% FS Tumor+ XRT.
DISCUSSION
In this study, we demonstrate that FS, a non-toxic nutritional supplement with antioxidant properties, can prevent oxidative reactions in lung cells and tissues induced by radiation. Long term post-XRT fibrosis and inflammation were also improved with FS feeding prior to and after XRT. This normal tissue radioprotection does not come at the expense of tumor protection, suggesting that dietary FS may be clinically useful in increasing the therapeutic index of thoracic radiation therapy. Antioxidant cell and tissue protection from radiation using FS and FS-derived SDG was also demonstrated. Thus, the use of FS in the realm of radiation pneumonopathy is novel and has potential implications for more effective radiotherapy of thoracic malignancies.
Our group has had an interest in exploring the use of natural dietary supplements as a means of ameliorating acute and chronic lung diseases that implicate oxidative stress such as radiation pneumonopathy. Flaxseed is one such dietary supplement, a whole grain that is non-toxic and has both anti-inflammatory and antioxidant properties due to its high concentrations of omega-3 fatty acids and lignans (29). FS and its bioactive metabolic components have been extensively studied in other organ systems and have proven to be beneficial, mostly in regards to cancer (30-33).Our group was the first to show that dietary FS supplementation could also reduce inflammation and lipid peroxidation in murine models of acid aspiration and hyperoxia (14) and more recently, in ischemia/reperfusion injury related to lung transplantation damage (15) .
During the acute phase of radiation-induced lung injury, FS inhibited oxidative lung damage but not inflammatory cell influx. While this may seem contradictory, recent studies by Lee at al. (15) showed that inflammatory cells isolated from lung lavage of FS-fed animals are inhibited in respiratory burst and ROS release when stimulated. This may help explain the antioxidant, protective effects of flaxseed in radiation injury. In addition, this study indicated that lignans inhibit radiation-induced ROS generation in endothelial cells (EC). In vitro ROS release by EC in response to ionizing radiation has been previously reported (34). Since SDG was added shortly prior to irradiation, we can speculate that it acted via direct ROS scavenging rather than enhancement of intracellular antioxidant defense systems as shown by Lee et al. (15). SDG and FS lignans have been reported to have such direct ROS scavenging properties (13).
Our data show that a number of markers of acute radiation effects are altered by FS. TGF-β1 mRNA levels have been demonstrated to surge soon after irradiation and that this elevation is reversible with a single dose of radioprotective antioxidant enzymes (AOEs) administered intravenously (35, 36). The results from the current study are consistent with this previous data in that creating an antioxidant-rich environment at the time of thoracic XRT from FS feeding abrogates the early rise in the proliferative cytokine TGF-β1. Previous studies have shown that both Bax and p21 are upregulated early after irradiation, suggesting that these and similar genes might be implicated in the early molecular response to radiation-induced lung injury (24, 25). Similar to the observed effects of FS feeding on TGF-β1, in the current study, increases in apoptotic (Bax) and cell cycle progression (p21) signals were blunted at 24 hours post XRT, genes known to be responsive to oxidative stimuli.
These effects on biomarkers of radiation pneumonopathy were borne out of the ability of 10% FS dietary supplementation to decrease both pneumonitis and fibrosis in mice. While these results were obtained using a single radiation fraction, the induction of radiation pneumonopathy in rodent models by various single fraction and hypofractionated regimens have been studied in detail by many investigators as models for the radiation pneumonopathy of multifraction clinical irradiation of humans (8, 9, 28).
Lastly, we evaluated whether in addition to protecting normal lung parenchyma, dietary FS supplementation might protect lung tumor, negating its radioprotective benefits. Given published reports of the anti-carcinogenic effects of FS (37, 38), there was a possibility of reducing tumor size due to FS feeding for several days before XRT. We did observe a non-significant trend towards decreased tumor size with FS feeding (20%±8% vs. 15%±8% tumor area for control diet vs. FS supplemented). However, we concluded that FS feeding prior to XRT did not inhibit tumor growth to significantly confound our experimental design. Our data indicated that there that FS successfully and preferentially provides protection of normal lung parenchyma while not radioprotecting established lung tumors.
In summary, this study evaluates a non-toxic, widely available nutritional supplement that offers long term radioprotective effects in the setting of thoracic radiotherapy. Potential mechanisms of the effects of prolonged FS feeding include altering immediate XRT-induced markers of lung damage, creating a baseline radioprotective state prior to XRT, and providing high levels of circulating antioxidants from the continued metabolism is of bioactive lignans. Our long term goal is to be able to provide adequate radioprotection not only to prevent the side effects of radiation on normal lung parenchyma, but also to allow for greater doses of radiation to affect further clinical response and cure against thoracic malignancies.
ACKNOWLEDGEMENTS
The authors wish to thank Drs. Steven M. Albelda and Costas Koumenis for reviewing the manuscript and their valuable advice in evaluating the data as well as Matthew Serotta for his technical assistance in evaluating tumor morphometry.
Funded in part by: NIH-R01 CA133470-01A1, NIH-R21 CA-118111-01, American Institute for Cancer Research, AICR-03B024, the University of Pennsylvania Research Foundation (URF) and by pilot project support from 1P30 ES013508-02 awarded to MCS. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
ABBREVIATIONS
- AU
Arbitrary units
- BAL F
Bronchoalveolar lavage fluid
- DMEM
Dulbeccos's modified Eagle medium
- FI
Fibrotic Index
- FS
Flaxseed
- H2DCF
2',7'-dichlorodihydrofluorescein
- H&E
Hematoxylin and eosin
- LLC
Lewis Lung Carcinoma
- MDA
Malondialdehyde
- PMN
Polymorphonuclear leukocyte
- PMVEC
Pulmonary microvascular endothelial cells
- ROS
Reactive oxygen species
- SDG
Secoisolariciresinol diglucoside
- WBC
White blood cells
- XRT
X-ray Treatment
Reference List
- 1.Machtay M. Pulmonary complications of anticancer treatment. 3rd. ed. Churchill Livingston; 2003. [Google Scholar]
- 2.Wang JY, Chen KY, Wang JT, et al. Outcome and prognostic factors for patients with non-small-cell lung cancer and severe radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2002;54:735–741. doi: 10.1016/s0360-3016(02)02994-2. [DOI] [PubMed] [Google Scholar]
- 3.Robnett TJ, Machtay M, Vines EF, et al. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48:89–94. doi: 10.1016/s0360-3016(00)00648-9. [DOI] [PubMed] [Google Scholar]
- 4.Hughes-Davies L, Tarbell NJ, Coleman CN, et al. Stage IA-IIB Hodgkin's disease: management and outcome of extensive thoracic involvement. Int J Radiat Oncol Biol Phys. 1997;39:361–369. doi: 10.1016/s0360-3016(97)00085-0. [DOI] [PubMed] [Google Scholar]
- 5.Marks LB, Fan M, Clough R, et al. Radiation-induced pulmonary injury: symptomatic versus subclinical endpoints. Int J Radiat Biol. 2000;76:469–475. doi: 10.1080/095530000138466. [DOI] [PubMed] [Google Scholar]
- 6.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 7.Molteni A, Ward WF, Ts'ao CH, et al. Monocrotaline-induced pulmonary fibrosis in rats: amelioration by captopril and penicillamine. Proc Soc Exp Biol Med. 1985;180:112–120. doi: 10.3181/00379727-180-42151. [DOI] [PubMed] [Google Scholar]
- 8.Epperly M, Bray J, Kraeger S, et al. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Ther. 1998;5:196–208. doi: 10.1038/sj.gt.3300580. [DOI] [PubMed] [Google Scholar]
- 9.Vujaskovic Z, Feng QF, Rabbani ZN, et al. Assessment of the protective effect of amifostine on radiation-induced pulmonary toxicity. Exp Lung Res. 2002;28:577–590. doi: 10.1080/01902140290096791. [DOI] [PubMed] [Google Scholar]
- 10.Machtay M, Scherpereel A, Santiago J, et al. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol. 2006;81:196–205. doi: 10.1016/j.radonc.2006.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flax-seed. Mol Cell Biochem. 1997;168:117–123. doi: 10.1023/a:1006847310741. [DOI] [PubMed] [Google Scholar]
- 12.Prasad K. Antioxidant Activity of Secoisolariciresinol Diglucoside-derived Metabolites, Secoisolariciresinol, Enterodiol, and Enterolactone. Int J Angiol. 2000;9:220–225. doi: 10.1007/BF01623898. [DOI] [PubMed] [Google Scholar]
- 13.Kitts DD, Yuan YV, Wijewickreme AN, et al. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol Cell Biochem. 1999;202:91–100. doi: 10.1023/a:1007022329660. [DOI] [PubMed] [Google Scholar]
- 14.Kinniry P, Amrani Y, Vachani A, et al. Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J Nutr. 2006;136:1545–1551. doi: 10.1093/jn/136.6.1545. [DOI] [PubMed] [Google Scholar]
- 15.Lee JC, Bhora F, Sun J, et al. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2008;294:L255–265. doi: 10.1152/ajplung.00138.2007. [DOI] [PubMed] [Google Scholar]
- 16.Dileto CL, Travis EL. Fibroblast radiosensitivity in vitro and lung fibrosis in vivo: comparison between a fibrosis-prone and fibrosis-resistant mouse strain. Radiat Res. 1996;146:61–67. [PubMed] [Google Scholar]
- 17.Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the intermediate and late phases. Radiat Res. 1989;119:15–31. [PubMed] [Google Scholar]
- 18.Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the early phase. Radiat Res. 1989;119:1–14. [PubMed] [Google Scholar]
- 19.Banda M, Bommineni A, Thomas RA, et al. Evaluation and validation of housekeeping genes in response to ionizing radiation and chemical exposure for normalizing RNA expression in real-time PCR. Mutat Res. 2008;649:126–134. doi: 10.1016/j.mrgentox.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee S, Levitan I, Wei Z, et al. KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation. 2006;13:633–644. doi: 10.1080/10739680600930255. [DOI] [PubMed] [Google Scholar]
- 22.Dong QG, Bernasconi S, Lostaglio S, et al. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler Thromb Vasc Biol. 1997;17:1599–1604. doi: 10.1161/01.atv.17.8.1599. [DOI] [PubMed] [Google Scholar]
- 23.Woessner JF., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 24.Bouvard V, Zaitchouk T, Vacher M, et al. Tissue and cell-specific expression of the p53-target genes: bax, fas, mdm2 and waf1/p21, before and following ionising irradiation in mice. Oncogene. 2000;19:649–660. doi: 10.1038/sj.onc.1203366. [DOI] [PubMed] [Google Scholar]
- 25.Kitada S, Krajewski S, Miyashita T, et al. Gamma-radiation induces upregulation of Bax protein and apoptosis in radiosensitive cells in vivo. Oncogene. 1996;12:187–192. [PubMed] [Google Scholar]
- 26.Anscher MS, Murase T, Prescott DM, et al. Changes in plasma TGF beta levels during pulmonary radiotherapy as a predictor of the risk of developing radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1994;30:671–676. doi: 10.1016/0360-3016(92)90954-g. [DOI] [PubMed] [Google Scholar]
- 27.Anscher MS, Peters WP, Reisenbichler H, et al. Transforming growth factor beta as a predictor of liver and lung fibrosis after autologous bone marrow transplantation for advanced breast cancer. N Engl J Med. 1993;328:1592–1598. doi: 10.1056/NEJM199306033282203. [DOI] [PubMed] [Google Scholar]
- 28.Rube CE, Uthe D, K.W. S, et al. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47:1033–1042. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- 29.Thompson L. Flaxseed in Human Nutrition. 2nd ed AOCS Press; Champaign: 2003. [Google Scholar]
- 30.Bergman Jungestrom M, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13:1061–1067. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 31.Bommareddy A, Arasada BL, Mathees DP, et al. Chemopreventive effects of dietary flaxseed on colon tumor development. Nutr Cancer. 2006;54:216–222. doi: 10.1207/s15327914nc5402_8. [DOI] [PubMed] [Google Scholar]
- 32.Dwivedi C, Natarajan K, Matthees DP. Chemopreventive effects of dietary flaxseed oil on colon tumor development. Nutr Cancer. 2005;51:52–58. doi: 10.1207/s15327914nc5101_8. [DOI] [PubMed] [Google Scholar]
- 33.Oikarinen S, Heinonen SM, Nurmi T, et al. No effect on adenoma formation in Min mice after moderate amount of flaxseed. Eur J Nutr. 2005;44:273–280. doi: 10.1007/s00394-004-0521-z. [DOI] [PubMed] [Google Scholar]
- 34.Szotowski B, Antoniak S, Goldin-Lang P, et al. Antioxidative treatment inhibits the release of thrombogenic tissue factor from irradiation- and cytokine-induced endothelial cells. Cardiovasc Res. 2007;73:806–812. doi: 10.1016/j.cardiores.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Anscher MS, Vujaskovic Z. Mechanisms and potential targets for prevention and treatment of normal tissue injury after radiation therapy. Semin Oncol. 2005;32:S86–91. doi: 10.1053/j.seminoncol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Rabbani ZN, Batinic-Haberle I, Anscher MS, et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67:573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demark-Wahnefried W, Price DT, Polascik TJ, et al. Pilot study of dietary fat restriction and flaxseed supplementation in men with prostate cancer before surgery: exploring the effects on hormonal levels, prostate-specific antigen, and histopathologic features. Urology. 2001;58:47–52. doi: 10.1016/s0090-4295(01)01014-7. [DOI] [PubMed] [Google Scholar]
- 38.Thompson LU, Rickard SE, Orcheson LJ, et al. Flaxseed and its lignan and oil components reduce mammary tumor growth at a late stage of carcinogenesis. Carcinogenesis. 1996;17:1373–1376. doi: 10.1093/carcin/17.6.1373. [DOI] [PubMed] [Google Scholar]