Abstract
The mechanism and stimulatory requirements of regulatory T cell (Treg)-mediated suppression are still unclear. To assess the requirement for Treg stimulation by cognate peptide:MHC, we used T cells from OTII and AND TCR transgenic mice that are specific for and restricted by distinct, non-crossreactive peptide:MHC combinations. This allowed us to independently activate Tregs and their conventional T cell (Tconv) targets. Surprisingly, we found that suppression can occur in the absence of peptide:MHC-mediated stimulation of Tregs. This suppression was Treg-dependent and not due to cold-target inhibition. Using Rag1-/- TCR transgenic T cells, we show that regulation of Tconv proliferation by heterogeneous Tregs is not due to alloreactivity or crossreactivity. Finally, using anti-TCR-Vβ8-coated microbeads and Vβ8- Tregs we show that TCR stimulation-independent suppression can occur in the absence of APCs. These data suggest that Tregs may possess constitutive regulatory activity that can be mediated in the absence of cognate peptide:MHC-TCR stimulation.
Keywords: T cells, Suppression, T cell receptor, Activation
Introduction
It has become increasingly clear that regulatory T cells (Treg)3 represent an important subset in the control of the immune response. Not only do they play a significant role in peripheral tolerance and protection against autoimmune disease, they also modulate immune responses to infection and tumors (1). Several studies have assessed the requirements for Treg-mediated suppression in vitro. Tregs have been shown to be non-responsive to TCR stimulation in the absence of exogenous interleukin-2 (IL-2) (2). It is believed that suppression in vitro occurs via a cell-cell contact dependent, cytokine-independent mechanism (3,4); however, a role for short-acting, and/or labile factors has not been ruled out (5). Furthermore, Treg-mediated suppression does not have to be mediated by Tregs that have the same antigen specificity and MHC-restriction at the suppressed T cell population (2,6). Lastly, it has been suggested that Tregs require activation through their TCR in order to exert their suppressive activity (2,4,6). This was observed for both polyclonal and transgenic populations of T cells.
A number of mechanisms of Treg suppression have been proposed using in vitro and in vivo models (7). These include suppression via contact with target cells or antigen presenting cells (2,4,8), soluble factors (9,10) or through cell killing (11,12). While it is still unclear how Tregs exert their activity, growing evidence suggests that multiple mechanisms are used depending upon context, location and disease state (7). Studies investigating the kinetics of Treg activity in vitro suggest Tregs exert their suppressive activity in a narrow kinetic window, within six to ten hours of culture (13,14). These observations suggest that perhaps resting Tregs may exist in a somewhat `pre-activated' state and thus raise the possibility that they possess some constitutive regulatory activity.
This prompted us to re-evaluate the requirements for Treg-mediated suppression. We used purified conventional, naïve T cells (Tconv) and Tregs from two TCR transgenic mouse models, AND and OTII, that have distinct and non-crossreactive specificities and restriction elements. In accordance with what others have seen, we show that suppression by Tregs is neither antigen- nor MHC-restriction dependent. However, surprisingly, we found that suppression by Tregs can occur in the absence of stimulation through the TCR.
Materials and Methods
Mice
Congenic C57BL/6J and B10.BR mice were purchased from The Jackson Laboratory. Foxp3gfp mice (15), OTII TCR transgenic mice (16) and AND TCR transgenic mice (17) (on wild type, Rag1-/- and/or Foxp3gfp crosses) were bred and maintained at St. Jude Children's Research Hospital. All animal experiments were performed in American Association for the Accreditation of Laboratory Animal Care-accredited, specific pathogen-free, helicobacter-free facilities following national, state and institutional guidelines. Animal protocols were approved by the St. Jude Animal Care and Use Committee.
Cell purification and flow cytometry
T cells (Tconv and Treg) from spleen and/or lymph nodes were stained and purified by FACS using anti-CD4, anti-CD25 and, in some experiments, anti-CD45RB. Dendritic cells (DCs) were purified from spleen by FACS using anti-CD11c. Vβ8 Tconv and Treg were purified by FACS using anti-Vβ8 antibody (F23.1). Antibodies against Vβ3, CD3 and Foxp3 were additionally used for flow cytometric analysis. The anti-Foxp3 antibody was purchased from eBioscience. All other antibodies used were from BD Pharmingen.
Proliferation assays
Tconv (CD4+CD25-; 2.5 × 104) were cultured in 96-well, round-bottom plates with DCs (104) and various concentrations of peptide (Ovalbumin326-339 [Ova326-339] and/or pigeon cytochrome c88-104 [PCC88-104]) for 68 h (200 μl final volume). Cells were pulsed with [3H]thymidine for the last 8 h of culture and proliferation measured.
In vitro suppression assays
Tconv (2.5 × 104) were cultured with DCs or irradiated splenocytes and peptide (Ova326-339 and/or PCC88-104) for 68 h with titrating amounts of Tregs. Cells were pulsed with [3H]thymidine for the last 8 h of culture and proliferation measured. For CFSE dilution, Tconv were purified by FACS, washed in PBS, resuspended in PBS/0.1% BSA with 5μM CFSE and incubated for 10 min at 37°C. Cells were washed, resuspended in media and incubated with titrating numbers of Tregs and DCs with peptide for 60 h. CFSE dilution was measured by flow cytometry. For assays using Vβ8+ and Vβ8- purified cells, Tconv and Tregs were incubated with anti-Vβ8 coated sulfate latex microbeads (Molecular Probes) for 68 h, pulsed with [3H]thymidine for the last 8 h of culture and proliferation measured. Percent suppression is calculated by subtracting the c.p.m. of Tconv + Treg from the c.p.m of Tconv alone, divided by c.p.m. of Tconv alone.
Statistics
Statistical analysis was performed using the unpaired Student's t tests.
Results and Discussion
Tregs do not require activation through their TCR to function
To evaluate the stimulatory requirements for Treg-mediated suppression, we co-cultured T cells from OTII TCR transgenic mice (Ovalbumin [Ova]326-339-specific; H-2Ab-restricted) (16) and AND TCR transgenic mice (pigeon cytochrome c peptide [PCC]88-104-specific, H-2Ek-restricted) (17) with dendritic cells (DC) from B10.BR (expressing H-2Ek) and C57BL/6 (expressing H-2Ab) mice. To verify that there was no crossreactivity, we assessed the ability of purified OTII and AND T cells to proliferate in the presence of C57BL/6 or B10BR DCs with either peptide. As is clearly shown, no crossreactivity to the irrelevant peptide or MHC was observed (Fig. 1A). In other words, OTII T cells (Tconv or Tregs) do not respond to PCC88-104 in the presence of either C57BL/6 or B10.BR DCs nor to Ova326-339 when incubated with B10.BR DCs. Likewise, AND T cells do not respond to Ova326-339 in the presence of either C57BL/6 or B10.BR DCs nor to PCC88-104 when incubated with C57BL/6 DCs. Neither T cell proliferated when incubated with MHC Class II-deficient DCs (data not shown).
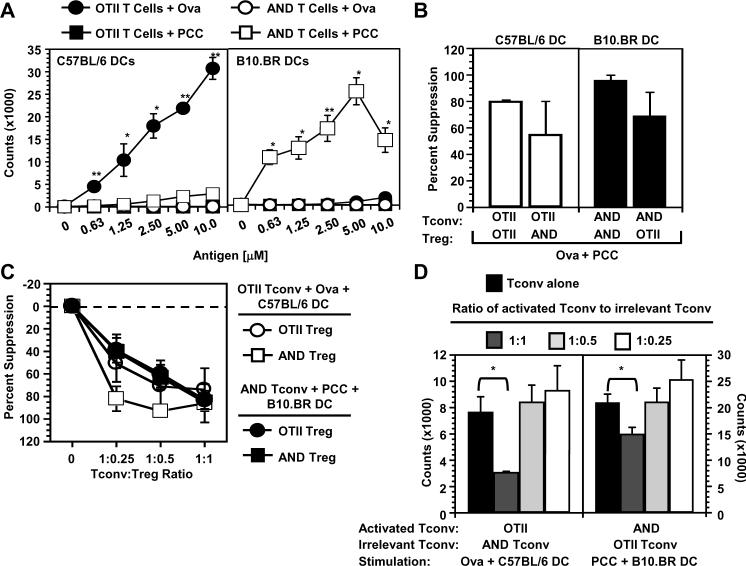
FIGURE 1.
Treg do not require activation by their cognate peptide:DC combination to suppress. A, T cells from OTII and AND TCR transgenic mice were incubated with DCs from C57BL/6 or B10.BR mice in the presence of the peptide indicated at varying concentrations and Tconv proliferation measured. Data is representative of 2 experiments with average ± s.d. of samples in triplicate. B, Tconv were incubated with DCs (6.25 × 103) and Tregs (1.25 × 104) in the presence of peptide (Ova326-339 and PCC88-104; 0.3μM) and Tconv proliferation measured. Percent suppression is shown as the average ± s.e. of 3 experiments. C, Tconv were incubated with DCs as in B with Tregs of varying concentrations in the presence of peptide (Ova326-339 or PCC88-104; 0.3μM) and Tconv proliferation measured. Percent suppression is shown as the average ± s.e. of 2-5 experiments. D, Tconv were incubated with DCs as in B with varying concentrations of Tconv of opposing phenotype (irrelevant Tconv) in the presence of peptide (Ova326-339 and PCC88-104; 0.3μM) and Tconv proliferation measured. The `activated' Tconv to `irrelevant' Tconv ratio is indicated. Data are the average ± s.e. of 2-4 experiments. All experiments used T cells isolated from spleens. *, p>0.05, **, p<0.005.
Previous studies have suggested that Tregs require activation through their TCR to function as polyclonal populations of Tregs are unable to suppress TCR transgenic T cells proliferating in response to their cognate antigen. However, this inability can be overcome with the addition of anti-CD3 (2,4). Furthermore, it was suggested, but not shown, that antigen-specific Treg were able to suppress Tconv of different antigen specificity but only in the presence of the antigen for which the Tregs were specific (4,6). To re-examine this requirement, we performed co-culture experiments to assess the ability of Tregs to suppress Tconv of different antigen specificities in the absence of their cognate MHC:peptide interaction. As expected, OTII Tregs were able to suppress proliferation of OTII Tconv when cultured with B6 DCs and both stimulating peptides (Fig. 1B and Supplemental Fig. 1A). Likewise, AND Tregs suppressed AND Tconv in the presence of peptide-pulsed B10.BR DCs. Surprisingly, AND Treg were also capable of suppressing OTII Tconv when cultured with B6 DCs and OTII Tregs were capable of suppressing AND Tconv in the presence of B10.BR DCs. This suggested that Tregs do not need to be stimulated through their TCR to function as in each system the Treg-stimulating DC and restriction element is absent. To confirm these results, and alleviate any concerns about potential activation of Tregs by their cognate peptide, these assays were repeated with cultures containing only the Tconv-stimulating peptide (Fig. 1C and Supplemental Fig. 1B). Again, Tregs were able to suppress Tconv of different antigen specificity in the absence of any Treg-specific TCR-activation signal. On the whole, the `opposing' Tregs were slightly less efficient than Tregs with the same transgenic TCR but were nonetheless quite effective regulators in vitro. These observations were very surprising and clearly contrasted with previous observations (2,4).
A potential concern with these experiments is that suppression of Tconv proliferation may be occurring due to cold-target inhibition, in which T cell contact with stimulatory APCs is sterically limited by the third party cell population. To rule out this possibility, we cultured Tconv with their cognate peptide:DC combination and replaced the Tregs in these assays with irrelevant, non-stimulated Tconv cells (ie. stimulation of OTII Tconv with Ova peptide plus C57BL/6 DCs in the presence or absence of increasing numbers of AND Tconv). Suppression was observed at a 1:1 ratio of stimulated Tconv to non-stimulated Tconv, demonstrating that this ratio should not be used to assess Treg activity (Fig. 1D). However, no suppression was observed at lower ratios suggesting that cold-target inhibition cannot account for the ability of Tregs to suppress in the absence of cognate MHC:peptide stimulation.
TCR stimulation-independent suppression by transgenic T cells is not due to crossreactivity or alloreactivity
Several studies have suggested that the TCR repertoire utilized by Tconv and Tregs is distinct and that Tregs in TCR transgenic mice may utilize non-clonotypic TCR during selection (18,19), although this has been contested by others (20). Furthermore, it has recently been shown that high affinity, autoreactive TCR are rare on both Tconv and Tregs (21). Nevertheless, the possibility remains that Tregs in these assays may be stimulated by the non-cognate or allo-MHC while their Tconv brethren are not. To perform these assays in the absence of any potential alloreactivity or crossreactivity due to the expression of non-clonotypic TCR chains, we crossed the OTII and AND TCR transgenic mice onto a Rag1-/- background. While Rag1-/- OTII TCR transgenic mice appeared to have a negligible Foxp3+ Treg population, Rag1-/- AND TCR transgenic mice do have a clearly definable, if reduced, Treg population as determined by intracellular staining for Foxp3 (Fig. 2A and Supplemental Fig. 2A). This was further verified by crossing the Rag1-/- AND TCR transgenic mice with Foxp3gfp reporter mice (15). Flow cytometric analysis of both the spleen and thymus demonstrates that the CD4+CD25+CD45RBlo population of AND Tregs used in suppression assays are Foxp3+ (Fig. 2B and Supplemental Fig. 2B) as well as essentially 100% Vβ3 clonotype positive (Supplemental Fig. 3). Although the Rag1-/- OTII TCR transgenic mice had a minimal number of Foxp3+ T cells, there was a small but detectable CD4+CD25+CD45RBlo population that appeared to have potent regulatory activity as measured by their ability to inhibit CD4+CD25-CD45RBhi Rag1-/- OTII Tconv proliferation in a CFSE dilution assay (Fig. 3 and Supplemental Fig. 4). Strikingly, Rag1-/- AND Tregs were also capable of suppressing the proliferation of Rag1-/- OTII Tconv in the absence of the appropriate Treg stimulatory peptide:DC combination.
FIGURE 2.
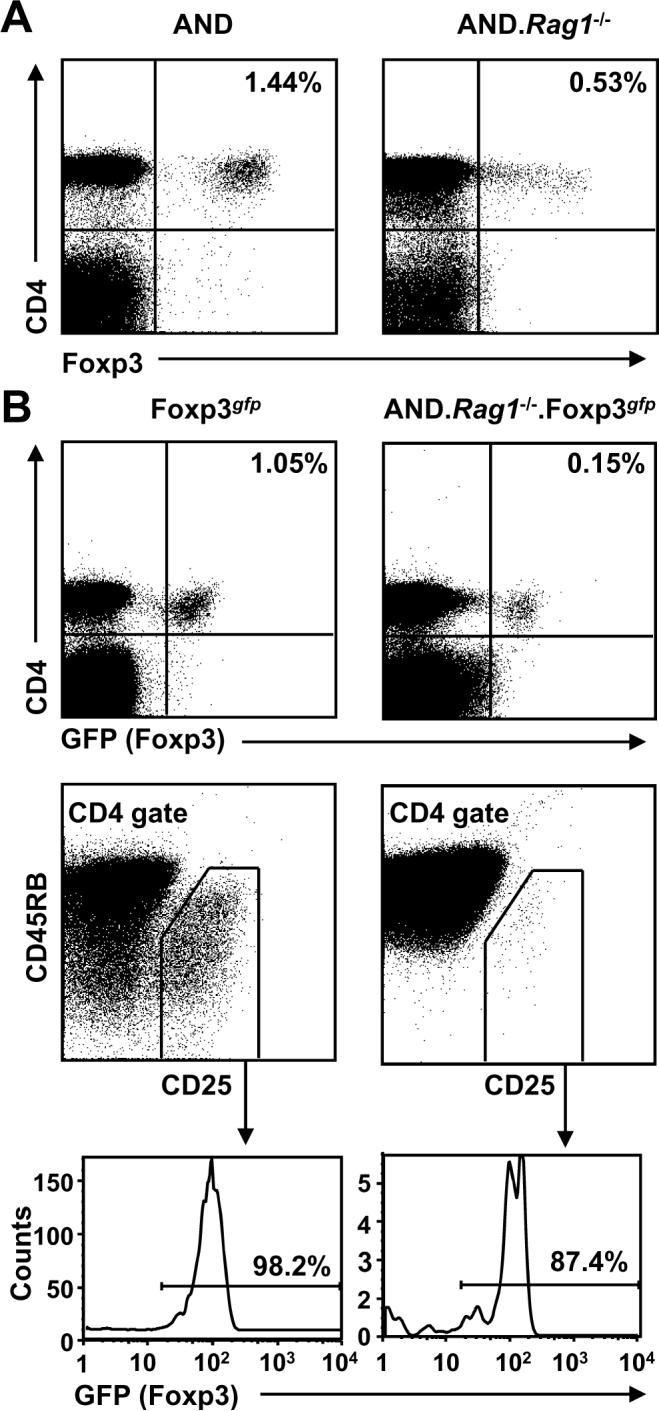
TCR transgenic mice on a Rag1-/- background develop Foxp3+ Tregs. A, AND TCR transgenic mice were crossed onto a Rag1-/- background (A) with Foxp3gfp mice (B). Spleens and lymph nodes were pooled and stained with antibodies against CD4 and Foxp3 (A) and CD4, CD25 and CD45RB (B).
FIGURE 3.
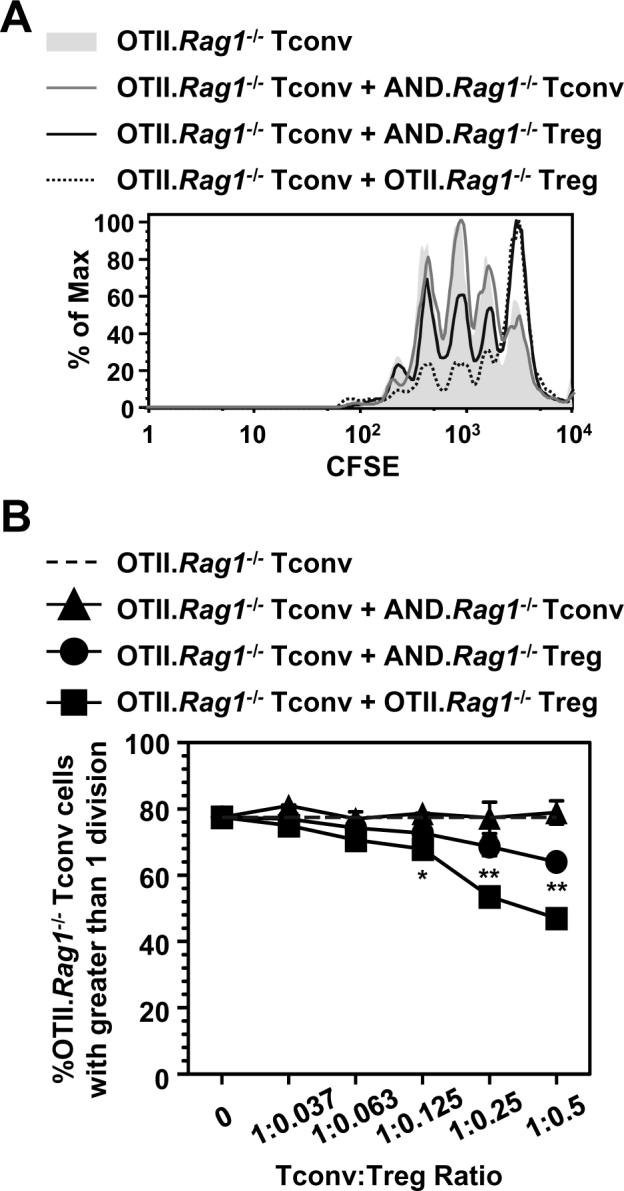
TCR-ligation-independent Treg suppression is not due to alloreactivity/crossreactivity. CFSE-labeled OTII.Rag1-/- Tconv were incubated with C57BL/6 DCs (6.25 × 103) and varying concentrations of Tregs or control Tconv in the presence of peptide (Ova326-339; 0.3μM) for 68 h and analyzed by flow cytometry. CFSE dilution of OTII.Rag1-/- Tconv incubated with various Treg populations or AND.Rag1-/- Tconv (0.5 to 1 `Treg':Tconv ratio) (A) and the percentage of dividing cells during the titration (B) are shown. Data is average ± s.d of samples in triplicate. All experiments used T cells isolated from spleen and lymph nodes. *, p>0.05, **, p<0.005 (statically significant differences between groups with OTII.Rag1-/- Teff + Tregs compared to OTII.Rag1-/- Teff + Tconv.)
Finally, we used two additional experimental systems to further verify the ability of Tregs to function in the absence of TCR stimulation. First, purified OTII Tconv were incubated with Tregs from either OTII or C57BL/6 mice in the presence of Ova peptide. The polyclonal population of C57BL/6 Tregs was able to suppress OTII Tconv proliferation almost as well as the OTII Tregs under conditions that would only activate Ova-reactive T cells (Fig. 4A and Supplemental Fig. 5A). Second, in order to eliminate MHC+ APCs from the experimental system, we used Vβ8+ Tconv stimulated with anti-Vβ8 coated microbeads. Purified Vβ8- Tregs were equally capable of suppressing Vβ8+ Tconv proliferation as the Vβ8+ Tregs without TCR stimulation (Fig. 4B and Supplemental Fig. 5B). Taken together, these data suggest that Tregs can mediate some degree of regulatory activity in the absence of TCR or MHC stimulation suggesting that they possess constitutive regulatory activity.
FIGURE 4.
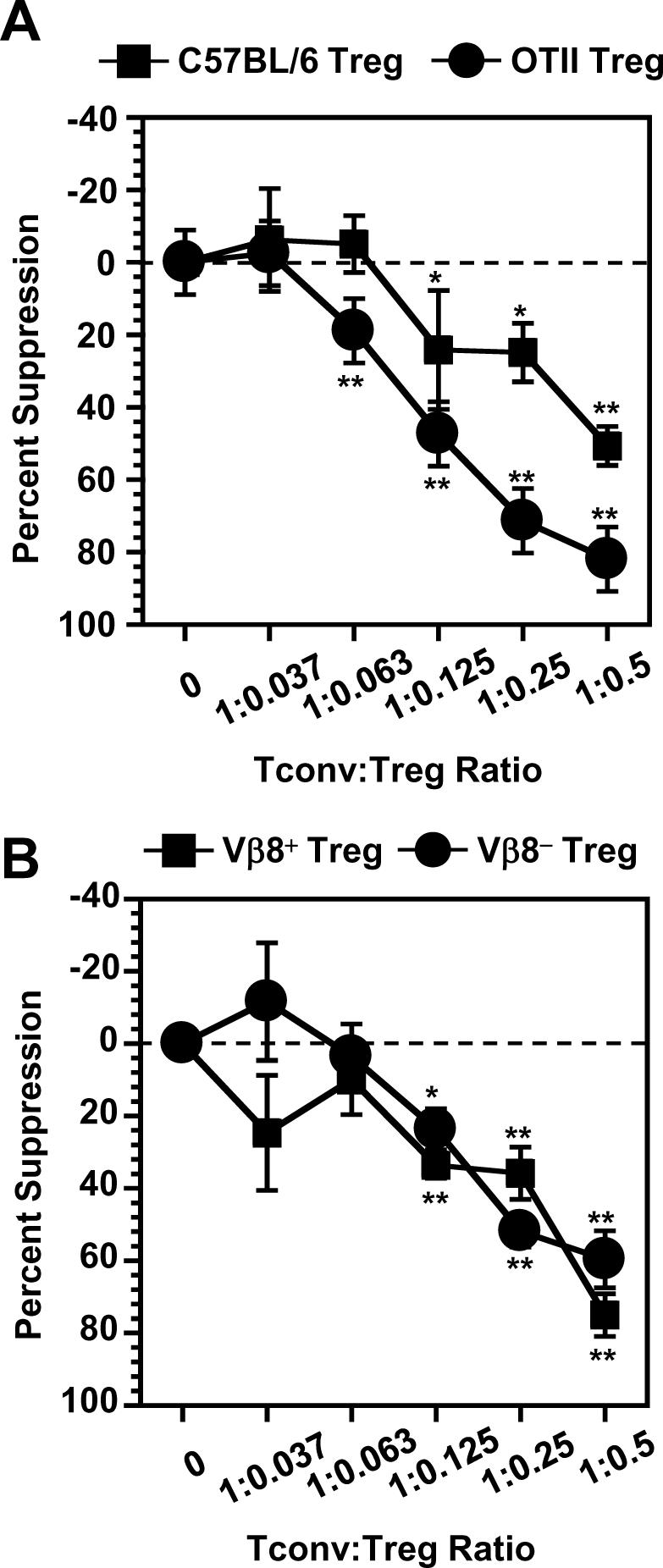
Tregs do not require stimulation through their TCR to suppress Tconv proliferation. A, OTII Tconv were incubated with irradiated splenocytes (C57BL/6; 5 × 104) and Tregs (1.25 × 104) in the presence of peptide (Ova326-339; 2.0μM) and Tconv proliferation measured. B, Vβ8+ Tconv were incubated with anti-Vβ8 coated microbeads and Tregs of varying concentrations and Tconv proliferation measured. Data are the average ± s.e. of 2-3 experiments with each sample in triplicate. All experiments used T cells isolated from spleen and lymph nodes. *, p>0.05, **, p<0.005.
Tregs possess constitutive regulatory activity
Consistent with previous findings, our data confirm that Tregs need not be specific for, or restricted by, the same peptide:MHC combination as the target Tconv (2,6). However, to our surprise, activation of the Tregs via their TCR does not appear to be an absolute requirement, contrary to previous suggestions (2,4,6). While the reason behind these discrepancies is unclear, it could be due to differences in the antigen presenting populations and/or the transgenic/background combinations used. For instance, in most assays we used purified DCs as opposed to T cell-depleted splenocytes, which may be better suited to mediate Treg activity as DCs have been suggested to play a role in Treg-mediated suppression in vivo (8). Therefore, their `contribution' may be amplified in our assays. It is important to note that in all assays, suppression in the absence of the Treg-activating peptide:MHC is seen further validating our results.
The ability of Tregs to mediate suppression without prerequisite TCR-mediated activation may not be without precedent. Indeed, unmanipulated Tregs, directly ex vivo, appear to have a somewhat activated phenotype, suggesting that they might be `primed for action' (22). For instance, a number of molecules shown to be upregulated on Tregs, such as CD25, CTLA-4, OX40 and LAG-3, are also found on activated Tconv (23-25). Furthermore, several studies have suggested that the kinetics of Treg activity in vitro are very rapid (six to ten hours), further supporting the notion that Tregs can mediate some regulatory activity in the absence of initiating a complete activation program (13,14). Although it seems unlikely, we cannot completely rule out the possibility that the sorting and manipulation of the Tregs led to some pre-activation which might have affected their performance in the in vitro Treg assays. It has been suggested that Tregs possess a distinct TCR repertoire and have a higher reactivity to self peptide:MHC (18,19), although this notion has been contested (20). However, it could be argued that the Rag1-/- AND Treg used our assays mediate suppression due to stimulation by the self peptide:MHC expressed by the APCs. While we cannot completely rule out this possibility, it is noteworthy that we reached similar conclusions regarding the ability of Tregs to suppress without TCR stimulation using anti-Vβ8-stimulated Vβ8+ Tconv with Vβ8- Tregs. In this system there are no DCs thus no MHC by which Tregs might be stimulated. Thus, our data provide evidence that Tregs can suppress in the absence of cognate peptide:MHC-mediated stimulation suggesting that further reevaluation of the requirements of Treg-mediated suppression may be required. Whether Tregs need to be stimulated via their TCR in vivo to mediate their regulatory activity remains to be determined. It is conceivable that some measure of constitutive regulatory activity afforded by Tregs in vivo may be critical in mediating the timely control of immune responses.
Supplementary Material
Acknowledgments
We would like to thank Steve Schoenberger, Bill Heath, Terry Geiger and Alexander Rudensky for TCR transgenic and Foxp3gfp reporter mice, and Richard Cross, Jennifer Rogers and Greig Lennon for assistance with flow cytometry. We are also very grateful to Karen Forbes and the staff in the St. Jude Animal Resource Center for mouse colony management.
This work was supported by the NIH (AI39480, AI058156), the St Jude Cancer Center Support CORE grant (CA-21765) and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Abbreviations used in the paper: DC, dendritic cell; Ova, ovalbumin; PCC, pigeon cytochrome c; Tconv, conventional T cell; Treg, regulatory T cell.
References
- 1.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 2.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McHugh RS, Shevach EM, Thornton AM. Control of organ-specific autoimmunity by immunoregulatory CD4(+)CD25(+) T cells. Microbes. Infect. 2001;3:919–927. doi: 10.1016/s1286-4579(01)01453-8. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 5.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 7.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 11.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J. Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 12.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sojka DK, Hughson A, Sukiennicki TL, Fowell DJ. Early kinetic window of target T cell susceptibility to CD25+ regulatory T cell activity. J. Immunol. 2005;175:7274–7280. doi: 10.4049/jimmunol.175.11.7274. [DOI] [PubMed] [Google Scholar]
- 14.Sukiennicki TL, Fowell DJ. Distinct molecular program imposed on CD4+ T cell targets by CD4+CD25+ regulatory T cells. J. Immunol. 2006;177:6952–6961. doi: 10.4049/jimmunol.177.10.6952. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 19.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR Repertoires to Self-Peptides in Regulatory and Nonregulatory CD4+ T Cells. J Immunol. 2007;178:7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 21.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi H, Zhen Y, Jiang L, Zheng J, Zhao Y. The phenotypic characterization of naturally occurring regulatory CD4+CD25+ T cells. Cell Mol. Immunol. 2006;3:189–195. [PubMed] [Google Scholar]
- 23.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 25.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.