Abstract
Despite their origin from self-tissue, tumor cells can be immunogenic and trigger immune responses that can profoundly influence tumor growth and development. Clinically, it may be possible to amplify or induce anti-tumor immune responses to achieve tumor rejection in patients. Increasing data over the last 8 years suggest that the human telomerase reverse transcriptase hTERT is immunogenic both in vitro and in vivo and may be a suitable target for novel cancer immunotherapy. Peptides derived from hTERT are naturally processed by tumors and presented on MHC molecules and trigger effector functions of specific T lymphocytes. Vaccination of cancer patients against hTERT epitopes induces anti-tumor T cells without clinical toxicity. If second-generation vaccines and other strategies are able to generate optimal cellular immunity against hTERT without toxicity in humans, the possibility of broad-spectrum immunotherapy or even immunoprevention therapy of cancer may be possible.
Keywords: Telomerase, cytotoxic T lymphocyte, tumor immunology, immunotherapy
1. Introduction
Although cancer is fundamentally a genetic disease, it has become increasingly clear that additional pressures within the tumor microenvironment, particularly cellular immune responses, profoundly influence tumor growth and development [1]. In the wake of genomic instability and aberrant gene expression, tumor cells express antigens that make them immunologically distinct and potential targets for the host immune system [2]. Indeed, cellular immunity has long been postulated as a mechanism of tumor suppression [3], with compelling evidence for this hypothesis coming most recently from studies in mice deficient for key regulators of cellular immune responses [4-7].
For humans with cancer, careful clinicopathological studies demonstrate that the presence and type of T lymphocytes that infiltrate tumor lesions independently predict clinical outcome across a broad range of histologies [8-12]. In particular, cytotoxic T lymphocytes (CTL) are considered chief mediators of tumor immuno-surveillance [1,2] via the recognition of tumor-associated antigens (TAA) as cognate peptides bound to major histocompatibility molecules expressed on the surface of tumor cells. Like viral or other microbial antigens, TAA are degraded by the proteasome into short peptides, transported into the endoplasmic reticulum, packaged in the groove of newly synthesized MHC molecules, and delivered as peptide-MHC (pMHC) complexes to the cell membrane. Engagement of a specific TCR by these pMHC complexes activates CTL to proliferate, produce cytokines, and seek out and lyse target cells presenting the same antigen.
A major achievement in the field of tumor immunology over the last 20 years has been the clear demonstration that TAA mediate specific anti-cancer T lymphocyte responses. In pioneering studies dating from the early 1990s, the molecular targets of anti-cancer T cell responses have been characterized by comprehensive analyses of patient derived T cells [13,14]. Although this work initially focused on melanoma, it was quickly extended to most other malignancies, raising the hypothesis that most tumors express antigens that T lymphocytes can potentially attack [14-17]. The notion that tumor antigen-specific immune responses can lead to tumor regression has now been borne out extensively in animal models and is being actively tested in human clinical trials [18].
Dozens of TAA have by now been described [19]. Unfortunately, the expression of most TAA is restricted to a few tumor types and to a fraction of patients with these types of tumors, and the appearance of antigen-loss mutations in tumor cells in the face of immune pressure is well-described [20-22]. To circumvent this issue, a class of TAA termed “universal tumor antigens” has been proposed that are hypothesized to not only trigger T cell reactivity against a broad range of tumor types but also play critical functional roles in tumor growth and development [23]. Such universal TAA would:
be expressed in the vast majority of human cancers with minimal expression in normal tissue,
include peptide sequences that bind to MHC molecules,
be processed by tumor cells such that antigen-derived peptides are available for binding to MHC molecules,
be recognized by the T cell repertoire in an MHC-restricted fashion, and
permit the expansion of CTL precursors expressing specific T cell receptors.
2. Telomerase as a universal tumor antigen
A prototype antigen for this hypothesis is the telomerase reverse transcriptase (hTERT) [24]. Human telomeres are comprised of non-coding, repetitive DNA at the ends of chromosomes featuring 3-20 kilobase regions of the nucleotide repeat TTAGGG. The cell biology of telomerase and its associated proteins, including hTERT, has been reviewed elsewhere [25-27]. Telomerase maintains chromosomal integrity by protecting telomeric DNA that would otherwise be lost during successive rounds of cell division in rapidly dividing cells such as cancer cells [25,28]. Most human cells do not express telomerase [29] and lose telomeric DNA with each cell division [30,31]. In contrast, most human tumors exhibit strong telomerase activity [29], express hTERT [32,33], and maintain telomere length [34]. hTERT is the rate-limiting component of the complex, and its expression tightly correlates with telomerase activity [32,33,35,36].
Unlike most other TAA, the expression of hTERT in tumor cells has been linked to tumor growth and development, and the expression of hTERT contributes critically to oncogenic transformation by permitting unlimited replicative potential. Ectopic expression of hTERT in combination with the simian virus 40 large-T oncoprotein and an oncogenic allele of H-ras results in tumorigenic conversion of normal human epithelial and fibroblast cells, demonstrating that disruption of the intracellular pathways regulated by large-T, oncogenic ras and telomerase is sufficient to create a human tumor cell [37]. Moreover, inhibition of telomerase activity in hTERT-positive tumor cells leads to telomere shortening and cell death by apoptosis [38,39]. This is an important feature of hTERT as a TAA because it is already well-established that therapeutic strategies targeting antigens not involved in tumor growth can result in the selection of antigen-loss tumor mutants that are clinically progressive [20-22].
3. Immunological characterization of hTERT-derived T cell epitopes
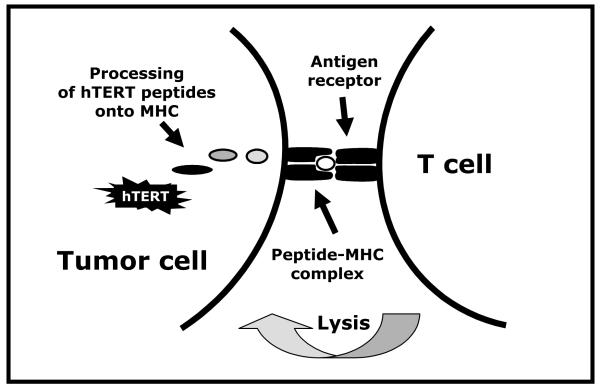
Studies over the last 8 years have demonstrated that peptides derived from hTERT are naturally processed by tumors and presented on MHC molecules and can trigger effector functions of specific CTL (Fig. 1). The first immunogenic peptide described from hTERT — I540 (ILAKFLHWL) — is restricted to the MHC class I allele HLA-A2, found among nearly 50% of Caucasian, Asians, and Hispanics and 33% of African-Americans. The I540 peptide was deduced from the sequence of hTERT based on computer-assisted analysis of MHC-binding motifs and was subsequently shown to bind strongly to HLA-A2 [40]. As demonstrated independently by several groups [40-43], CTL specific for I540 peptide can be generated in vitro that kill a range of hTERT+ tumor cell lines and primary tumors in a peptide-specific, MHC-restricted fashion. On the other hand, Parkhurst et al [44] evaluated several I540-specific T cell clones and found that none were able to recognize HLA-A2+ hTERT+ tumor cells. Similar data was also reported by another group [45] and the conclusion reached by these investigators was that the hTERT I540 peptide is not presented on the surfaces of tumor cells in the context of HLA-A2. Most recently, Sadelain and colleagues [42] make note of these discrepancies and address it using a novel antigen-presenting cell (APC) system. These investigators found that the I540 epitope is naturally processed on the surface of both tumor cells and APCs, and I540-specific CD8+ T cells efficiently lyse tumors expressing endogenous hTERT in a HLA-restricted manner. Indeed, the direct isolation of the I540 peptide from the groove of HLA-A2 on primary tumor cells [46] as well as K562 cells expressing both HLA-A2 and hTERT [47] has been demonstrated by mass spectroscopy.
Fig. 1.
Graphical representation for the proposed mechanism of T cell recognition of hTERT+ tumor cells. hTERT is processed by the proteasome into short peptides, presented in the groove of newly synthesized MHC molecules, and delivered as peptide-MHC complexes to the cell surface. T cells bearing a T-cell receptor of the appropriate specificity bind to these peptide-MHC complexes, leading to T cell activation.
In addition to the I540 epitope, multiple other HLA binding epitopes derived from hTERT have been identified (Table 1). These include epitopes restricted to HLA-A2 as well as HLA-A1, -A3, -A24, and -B7 [41,48-56]. More than 90% of humans express at least one of these five MHC class I alleles. In each case, candidate peptide epitopes were shown to bind to MHC, and used to generate T cells in vitro that lyse targets and tumors in an antigen-specific, MHC class I-restricted fashion. In another experimental approach, hTERT-specific CTL were generated ex vivo from cancer patients using autologous dendritic cells transduced with hTERT mRNA as the stimulating APC [57]. These CTL were shown to lyse primary human tumors in an antigen-specific fashion. Finally, Vieweg and colleagues have generated polyclonal anti-tumor CTL ex vivo from patients with prostate or renal cell carcinoma following stimulation with autologous dendritic cells transduced with whole tumor mRNA [58,59]. These CTL, as designed, had multiple antigen specificities, a significant portion of which was against hTERT [58,59].
Table 1.
hTERT-derived T cell epitopes
| Epitope | Sequence | Restricting MHC allele | Reference |
|---|---|---|---|
| I540 | ILAKFLHWL | HLA-A2 | [40] |
| R865 | RLVDDFLLV | HLA-A2 | [41] |
| 572Y* | YLFFYRKSV | HLA-A2 | [50] |
| 988Y* | YLQVNSLQTV | HLA-A2 | [50] |
| R38* | RLGPQGWRV | HLA-A2 | [56] |
| K973 | KLFGVLRLK | HLA-A3 | [48] |
| V324 | VYAETKHFL | HLA-A24 | [49] |
| V461 | VYGFVRACL | HLA-A24 | [49] |
| Y325 | YAETKHFLY | HLA-A1 | [53] |
| M1 | MPRAPRCRA | HLA-B7 | [52] |
| A4 | APRCRAVRSL | HLA-B7 | [52] |
| A68 | APSFRQVSCL | HLA-B7 | [52] |
| R277 | RPAEEATSL | HLA-B7 | [52] |
| R342 | RPSFLLSSL | HLA-B7 | [52] |
| R351 | RPSLTGARRL | HLA-B7 | [52] |
| L1123 | LPSDFKTIL | HLA-B7 | [55] |
| T1088 | TYVPLLGSL | HLA-A24 | [54] |
| C845 | CYGDMENKL | HLA-A24 | [54] |
| A167 | AYQVCGPPL | HLA-A24 | [54] |
| L766 | LTDLQPYMRQFVAHL | DR4, DR11, DR15 | [63] |
| R672 | RPGLLGASVLGLDDI | DR1, DR7, DR15 | [62] |
| E611 | EARPALLTSRLRFIPK | Promiscuous DR, DP, DQ | [64,65] |
Underlined amino acids are mutated
Because T cell epitopes such as hTERT I540 may be influenced by immune tolerance due to its high affinity and stability with HLA-A2, there have also been efforts to characterize low-affinity epitopes derived from hTERT that may escape tolerance induction. Classically considered poorly immunogenic, low-affinity epitopes can induce robust CTL responses after modification from wild type sequence, for example, by changing position 1 of HLA-A2-restricted peptide epitopes to tyrosine [60]. Two such low-affinity HLA-A2-restricted epitopes derived from hTERT are 572Y (YLFFYRKSV) and 988Y (YLQVNSLQTV) [50,51] and each exhibits strong affinity for HLA-A2 and stimulates specific CTL in vitro. These CTL specifically lyse hTERT-expressing tumor cells of various histologies but not HLA-A2-negative or hTERT-negative tumors [50,51]. In HLA-A2 transgenic mice, vaccination with 572Y or 988Y generates specific T cell responses that protect animals from a lethal challenge with TERT-expressing tumor cells without toxicity [61].
Beyond MHC class I epitopes for CD8+ T cells, hTERT also includes multiple epitopes restricted to MHC class II that drive CD4+ T cell responses (Table 1). Particular CD4+ hTERT epitopes include R672 and L766 peptides, each shown to be naturally processed and presented and recognized by the T cell repertoire in patients and healthy individuals [62,63]. Both of these epitopes as well as a third epitope (E611) [64,65], induce T cell activation promiscuously such that they can induce a CD4+ response in the context of multiple MHC Class II alleles. Transfection of APCs with full-length hTERT constructs also triggers hTERT-specific CD4+ T cells in vitro [66,67].
4. Naturally occurring immune responses to hTERT
In healthy individuals, the precursor frequency of hTERT-specific CTL in peripheral blood is thought to be very low, or at least undetectable with state-of-the art immunoassessment assays. This situation may be different for cancer patients. For example, naturally occurring CD8+ T cells specific for the hTERT I540 peptide have been observed in high numbers in blood from certain populations of cancer patients in remission following standard therapies [43,46]. For example, >80% of HLA-A2+ patients with chronic myelogenous leukemia in durable remission following treatment with imatinib, interferon-alpha, or stem cell transplantation harbor large numbers of circulating, tetramer-positive I540-specific CTL in peripheral blood, as high as 13.2% of freshly isolated CD8+ T cells [46]. Similarly, >90% of HLA-A2+ patients with prostate cancer in remission following prostatectomy demonstrate I540-specific CD8+ T cells (up to 1.4%) that recognize HLA-matched or autologous tumor and specifically secrete IFN-γ in vitro [43]. HLA-A2+ patients with multiple myeloma also exhibit, albeit rarely, naturally occurring CTL specific for I540 hTERT peptide in blood [68]. Beyond the I540 epitope, Mizukoshi et al reported in a study of 72 hepatocellular carcinoma patients, naturally occurring T cell responses to five different HLA-A24-restricted epitopes derived from hTERT, with up to 12% of patients responding to any one particular peptide [54]. These CTL were isolated in vitro and shown to specifically secrete IFN-γ and mediate cytotoxicity against hTERT+ HLA-A24+ tumor cells. Finally, hTERT-specific auto-antibodies have also been detected in cancer patients. For example, an unexpectedly high fraction of patients with hepatocellular carcinoma and other cancers, but not healthy individuals, exhibit hTERT reactive antibody in the serum [69].
Taken together, these studies suggest that hTERT can be an immunogenic protein and that immune tolerance to hTERT, despite being a self antigen, is not complete. In acute viral infections, expansion of antigen-specific CD8+ T cells in peripheral blood is typically associated with immune control of disease. In cancer, however, even extensive expansion of tumor antigen-specific CD8+ T cells in vivo is not thought to guarantee a therapeutic effect [70]. In such cases, antigen-specific cytolytic function has been shown to be impaired [71]. It remains an important question whether the naturally occurring precursor frequency of hTERT-specific T cells is significantly higher in patients in remission than in patients with bulky burden of disease. If it is, one implication would be that naturally occurring hTERT-specific T cell responses actively contribute to tumor immunosurveillance; however, it would remain equally possible that the existence of naturally occurring hTERT-specific T cell responses only represents a late consequence of T priming by APC that have cross-presented antigen from tumor cells killed by other processes.
5. Clinical trials of hTERT immunotherapy
Multiple phase 1 clinical trials of hTERT immunotherapy have already been conducted in patients with advanced cancer, each of which test the hypothesis that hTERT-specific vaccination can overcome immunological tolerance and trigger anti-tumor T cell responses in vivo (Table 2). Chief findings include the induction of hTERT-specific T cells in the absence of toxicity. Objective clinical responses have been rarely reported, but no study yet has been statistically powered to measure this endpoint adequately.
Table 2.
Clinical trials of hTERT-based vaccines in advanced or metastatic cancer patients
| Vaccine Formulation |
Cancer type | Number of patients |
Immune response rate* |
Clinical Outcomes |
Reference |
|---|---|---|---|---|---|
| Dendritic cells with I540 peptide |
Breast and prostate |
7 | 57% | One minor clinical response | [72] |
| I540 peptide in Montanide with GM-CSF |
Breast | 19 | 68% | No objective responses; correlation of overall survival with immune response |
[73] |
| I540 peptide in Montanide |
Kidney, colon, or melanoma |
14 | 50% | No objective responses | [44] |
| I540 and E611 peptides and GM- CSF |
NSCLC | 26 | 46% | One complete response | [64] |
| E611 peptide and GM-CSF |
Pancreas | 48 | 63% | Correlation of overall survival with immune response |
[74] |
| 572Y and 572 peptides in Montanide |
Multiple | 19 | 95% | No objective responses | [75] |
| 572Y and 572 peptides in Montanide |
NSCLC | 22 | 76% | No objective responses; correlation of overall survival with immune response |
[76] |
| B cells loaded with I540 and 572Y peptides |
Prostate | 15 | Variable depending on dose |
No objective responses | [77] |
| Dendritic cells transfected with full- length hTERT mRNA |
Prostate | 20 | 95% | Reduction of prostate-specific antigen velocity and molecular clearance of circulating micrometastases |
[78] |
As defined by investigators based on various laboratory immune assessment assays
Abbreviation: NSCLC, non-small cell lung cancer
In the first hTERT vaccination trial, conducted at the Dana Farber Cancer Institute (Boston, MA, USA), 7 HLA-A2 patients with advanced breast or prostate cancer were treated with autologous monocyte-derived DC pulsed ex vivo with hTERT I540 peptide and keyhole limpet hemocyanin [72]. Immune responses and clinical evidence of anti-tumor activity were observed without toxicity. Tetramer-guided high-speed sorting and polyclonal expansion was used ex vivo to obtain highly enriched populations of hTERT-specific cells and these T cells specifically killed hTERT+ tumor cells in an MHC-restricted fashion.
In another trial at the Abramson Cancer Center of University of Pennsylvania (Philadelphia, PA, USA), 19 HLA-A2+ women with metastatic breast cancer were vaccinated subcutaneously with hTERT I540 peptide emulsified in Montanide adjuvant and administered with granulocyte macrophage colony-stimulating factor (GM-CSF) up to eight times [73]. Based on in vitro analyses performed on peripheral blood obtained before and after treatment, 68% of patients were found to have responded immunologically. Tumor-infiltrating lymphocytes (TIL) were evident after, but not before vaccination, with 4%-13% of post-vaccine CD8+ TIL specific for hTERT I540. Induction of TIL manifested clinically with tumor-site pain and pruritis and pathologically with marked tumor necrosis. Peripheral blood hTERT-specific CD8+ T cells were also induced and shown in vitro to proliferate, produce IFN-gamma, and lyse tumors. An exploratory landmark analysis revealed an association between an hTERT-specific CD8+ T cell immune response and overall survival in these patients. Interestingly, these clinical and immunological findings were not observed in a study at the National Cancer Institute (Bethesda, USA) of 14 patients with metastatic cancers injected with hTERT I540 in Montanide without GM-CSF. Although induction of I540-specific CTL was observed in 50% of patients without toxicity, CTL isolated ex vivo failed to lyse tumors endogenously expressed telomerase and no clinical benefit was observed [44].
With the goal of inducing both CD4 and CD8 T cells responses in patients, a trial at Norwegian Radium Hospital (Oslo, Norway) enrolled 26 patients with non-small cell lung cancer who were repeatedly injected with I540 and E611 peptide and GM-CSF [64]. Only a fraction of all patients treated in this trial, however, were HLA-A2+. Treatment was well-tolerated and immune responses against E611 were detected in 46% of evaluable patients. A complete tumor response was observed in one patient who developed an immune response following treatment. In a second trial at the same institution, patients with non-resectable pancreatic cancer were immunized with E611 peptide alone, with GM-CSF, with specific T cell responses detected in 63% of patients [74]. Median survival for immune responders was significantly higher than that for non-responders.
To test the immunogenicity of low-affinity hTERT peptides, two trials at the University General Hospital of Heraklion (Heraklion, Crete) enrolled HLA-A2+ patients. Patients were given subcutaneous injections of 572Y modified peptide in Montanide adjuvant. Nineteen patients with chemotherapy refractory and progressing malignant tumors advanced were treated in a phase I trial [75], and 22 patients with advanced non-small cell lung cancer were treated in a follow-up expanded safety trial [76]. Peptide specific T cell responses were observed in the majority of patients without toxicity. In the second trial, estimated overall survival was 30 months for immunological responders vs. 4 months for non-responders [76]. A trial testing both I540 and 572Y peptides loaded onto autologous B cells as a vaccine was tested in 15 patients with advanced prostate cancer at the University of California San Diego (San Diego, USA). Again, immune responses were observed without toxicity [77].
Finally, in the first clinical trial evaluating immunotherapy with full-length hTERT [78], 20 patients with metastatic prostate cancer at Duke University Medical Center (Durham, NC, USA) were administered autologous dendritic cells transfected with mRNA encoding hTERT with or without the inclusion of a chimeric construct of lysosome-associated membrane protein-1 (LAMP). Induction of hTERT-specific CD8+ T cells was observed in all but one patient, with up to 1.8% of CD8+ T cells exhibiting hTERT specificity. Antigen-specific immune measurements were higher in patients immunized with chimeric LAMP hTERT. Treatment was associated with an increase in the prostate-specific antigen doubling time and molecular clearance of circulating micrometastases.
6. Prospects for building a cancer vaccine targeting telomerase
For the most part, published reports of hTERT vaccination offer preliminary evidence to support the notion that hTERT can function as a TAA target for novel vaccines. If, in further studies with second-generation vaccines, optimal immunity can be successfully elicited in cancer patients without the induction of severe autoimmunity, hTERT clearly becomes a prime candidate for a widely applicable cancer vaccine. The major advantages of using hTERT as tumor antigen include its nearly universal expression across all types of tumor histologies as well as the critical role hTERT plays in oncogenesis. The latter feature may help to circumvent the difficulties of immune escape because tumor downregulation of hTERT might itself be incompatible with sustained tumor growth. In two trials targeting the hTERT I540 peptide in patients with breast and prostate cancer, loss of hTERT mRNA in tumors was not observed after vaccination despite the induction of hTERT-specific T cell responses [72,73].
Finally, because hTERT can be predicted to be associated with >85% of human cancers, the opportunity for vaccinating individuals as an immunoprevention strategy can be envisioned for hTERT-based therapies. This not only includes the testing of hTERT vaccination in the adjuvant (minimal residual disease) clinical setting, for example, but also ultimately in healthy individuals considered at high risk for cancer based on genetic factors and medical history. Although new candidate antigens need to be tested for safety in a therapeutic clinical setting (as has now been extensively done for hTERT-based formulations), it is important to note that post-exposure vaccination is rarely clinically effective in any medical setting. In cancer patients, tumor burden negatively impacts attempts at therapeutic vaccination, and accordingly, there is considerable effort to rapidly apply strategies that pass phase I safety testing to patients with less tumor burden. Of course, any preventative cancer vaccine would require a very narrow toxicity profile, and whether or not this is achievable for hTERT or any other universal tumor anatigen is an important question for future studies. Nevertheless, the potential for significant clinical impact would be great.
7. Challenges for building a cancer vaccine targeting telomerase
The major challenge with regard to hTERT and other TAA derived from the human genome is to develop therapies that generate an immune response as robust and as safe as those that can be generated against viruses. It has often been cited that the amplitude of T cell responses achieved in patients so far against any of a number of TAA, including hTERT, are 10-fold or more lower than T cell responses the offer protection and clearance after viral infection in humans. A number of mechanisms of immuno-resistance have been described over the last 10 years, as extensively reviewed elsewhere [79]. These mechanisms include not only tumor-derived factors that antagonize cellular immunity, but also host factors that dampen any cellular immune response. The cellular and molecular basis of many of these mechanisms are being increasing understood and are the subject of intense efforts to target these mechanisms clinically in combination with novel cancer vaccines. For hTERT in particular, a number of novel immunological approaches have been reported in the last year alone [80-84].
Assuming that robust and optimal T cell responses against hTERT can one day be generated in patients, a major remaining issue is the potential lysis of rare normal cell types in which telomerase has been detected. Telomerase activity is absent in most major organs but activity or hTERT mRNA expression has been reported in hematopoietic stem cells, activated lymphocytes, basal keratinocytes, gonadal cells, and certain epithelial cells [25,26]. hTERT-specific CTL do not lyse telomerase-positive CD34+ hematopoietic progenitor cells or activated T lymphocytes in vitro [40,41]. Likewise, no CTL-mediated toxicity has been observed against normal bone marrow cells using immunodeficient mice reconstituted with patient hematopoeitic progenitors after exposure to hTERT-specific CTL [85]. In mouse models, TERT-specific vaccination generates robust protective immunity without the development of autoimmunity against TERT-expressing cells [57]. These findings may reflect relatively low levels of TERT protein in normal cells, or alternatively, inefficient processing TERT peptide in normal cells. Without long-term safety data after hTERT immunotherapy, however, it remains conceivable that sustained hTERT-specific immune responses may cause toxicity in normal hTERT-expressing cells. Indeed, the clinical consequences of long-term telomerase insufficiency in patients with inherited mutations in hTERT or associated genes are well-described and serious [86-89]. These concerns argue for prudent design and execution of clinical trials targeting hTERT for immunotherapy.
8. Summary
Immunological analysis of the telomerase reverse transcriptase hTERT suggests that the enzyme is a potentially important and widely applicable target for anti-cancer T cell immunotherapy. Initial clinical trials of multiple vaccine formulations demonstrate that hTERT-specific immune responses can be safely induced in patients. If second-generation vaccines and other strategies are able to generate optimal cellular immunity against hTERT without toxicity in humans, the possibility of broad-spectrum cancer immunotherapy or even immunoprevention therapy based on this and similar antigens could be considered.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 CA111377) and the Beckman Foundation. Dr. Vonderheide reports being an inventor on a patent application that relates to hTERT as a tumor-associated antigen for cancer immunotherapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- [2].Van Der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, Chapiro J, Van Den Eynde BJ, Brasseur F, Boon T. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol. Rev. 2002;188:51–64. doi: 10.1034/j.1600-065x.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- [3].Burnet F. Cancer—a biological approach. Brit. Med. J. 1957;1:841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- [5].Van Den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- [8].Clark WH, Jr., Elder DE, Guerry DT, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC. Model predicting survival in stage I melanoma based on tumor progression. J. Natl. Cancer. Inst. 1989;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- [9].Marrogi AJ, Munshi A, Merogi AJ, Ohadike Y, El-Habashi A, Marrogi OL, Freeman SM. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer. 1997;74:492–501. doi: 10.1002/(sici)1097-0215(19971021)74:5<492::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [10].Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. New Engl J Med. 2003;348:201–211. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- [11].Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- [12].Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- [13].Van Pel A, Van Der Bruggen P, Coulie P, Brichard V, Lethe B, Van Den Eynde B, Uyttenhove C, Renauld J-C, Boon T. Genes coding for tumor antigens recognized by cytolytic T lymphocytes. Immunol. Rev. 1995;145:229–250. doi: 10.1111/j.1600-065x.1995.tb00084.x. [DOI] [PubMed] [Google Scholar]
- [14].Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997;18:175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- [15].Van Pel A, Van Der Bruggen P, Coulie PG, Brichard VG, Lethe B, Van Den Eynde B, Uyttenhove C, Renauld JC, Boon T. Genes coding for tumor antigens recognized by cytolytic T lymphocytes. Immunol. Rev. 1995;145:229–250. doi: 10.1111/j.1600-065x.1995.tb00084.x. [DOI] [PubMed] [Google Scholar]
- [16].Van Den Eynde BJ, Van Der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684–693. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- [17].Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- [18].Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–411. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- [19].Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jager E, Ringhoffer M, Karbach J, Arand M, Oesch F, Knuth A. Inverse relationship of melanocyte differentiation antigen expression in melanoma tissues and CD8+ cytotoxic-T-cell responses: evidence for immunoselection of antigen-loss variants in vivo. Int J Cancer. 1996;66:470–476. doi: 10.1002/(SICI)1097-0215(19960516)66:4<470::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [21].Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, Kenyon K, Davis MM, Riddell SR, Greenberg PD. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell-mediated vitiligo. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schmollinger JC, Vonderheide RH, Hoar KM, Maecker B, Schultze JL, Hodi FS, Soiffer RJ, Jung K, Kuroda MJ, Letvin NL, Greenfield EA, Mihm M, Kutok JL, Dranoff G. Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc Natl Acad Sci USA. 2003;100:3398–3403. doi: 10.1073/pnas.0530311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schultze JL, Vonderheide RH. From cancer genomics to cancer immunotherapy: toward second-generation tumor antigens. Trends Immunol. 2001;22:516–523. doi: 10.1016/s1471-4906(01)02015-4. [DOI] [PubMed] [Google Scholar]
- [24].Vonderheide RH. Telomerase as a universal tumor-associated antigen for cancer immunotherapy. Oncogene. 2002;21:674–679. doi: 10.1038/sj.onc.1205074. [DOI] [PubMed] [Google Scholar]
- [25].Hahn WC, Meyerson M. Telomerase activation, cellular immortalization and cancer. Ann Med. 2001;33:123–129. doi: 10.3109/07853890109002067. [DOI] [PubMed] [Google Scholar]
- [26].Shay JW, Wright WE. Telomerase: a target for cancer therapeutics. Cancer Cell. 2002;2:257–265. doi: 10.1016/s1535-6108(02)00159-9. [DOI] [PubMed] [Google Scholar]
- [27].Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- [28].Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407–425. doi: 10.1128/MMBR.66.3.407-425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- [30].Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- [31].Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- [32].Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up- regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- [33].Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- [34].Counter CM, Botelho FM, Wang P, Harley CB, Bacchetti S. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein-Barr virus-transformed human B lymphocytes. J Virol. 1994;68:3410–3414. doi: 10.1128/jvi.68.5.3410-3414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- [36].Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- [37].Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- [38].Hahn WC, Stewart SA, Brooks MW, York SG, Eaton E, Kurachi A, Beijersbergen RL, Knoll JH, Meyerson M, Weinberg RA. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- [39].Herbert B, Pitts AE, Baker SI, Hamilton SE, Wright WE, Shay JW, Corey DR. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- [41].Minev B, Hipp J, Firat H, Schmidt JD, Langlade-Demoyen P, Zanetti M. Cytotoxic T cell immunity against telomerase reverse transcriptase in humans. Proc Natl Acad Sci USA. 2000;97:4796–4801. doi: 10.1073/pnas.070560797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dupont J, Latouche JB, Ma C, Sadelain M. Artificial antigen-presenting cells transduced with telomerase efficiently expand epitope-specific, human leukocyte antigen-restricted cytotoxic T cells. Cancer Res. 2005;65:5417–5427. doi: 10.1158/0008-5472.CAN-04-2991. [DOI] [PubMed] [Google Scholar]
- [43].Filaci G, Fravega M, Setti M, Traverso P, Millo E, Fenoglio D, Negrini S, Ferrera F, Romagnoli A, Basso M, Contini P, Rizzi M, Ghio M, Benatti U, Damonte G, Ravetti JL, Carmignani G, Zanetti M, Indiveri F. Frequency of telomerase-specific CD8+ T lymphocytes in patients with cancer. Blood. 2006;107:1505–1512. doi: 10.1182/blood-2005-01-0258. [DOI] [PubMed] [Google Scholar]
- [44].Parkhurst MR, Riley JP, Igarashi T, Li Y, Robbins PF, Rosenberg SA. Immunization of patients with the hTERT:540-548 peptide induces peptide-reactive T lymphocytes that do not recognize tumors endogenously expressing telomerase. Clin Cancer Res. 2004;10:4688–4698. doi: 10.1158/1078-0432.CCR-04-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ayyoub M, Migliaccio M, Guillaume P, Lienard D, Cerottini JC, Romero P, Levy F, Speiser DE, Valmori D. Lack of tumor recognition by hTERT peptide 540-548-specific CD8(+) T cells from melanoma patients reveals inefficient antigen processing. Eur J Immunol. 2001;31:2642–2651. doi: 10.1002/1521-4141(200109)31:9<2642::aid-immu2642>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [46].Gannage M, Abel M, Michallet AS, Delluc S, Lambert M, Giraudier S, Kratzer R, Niedermann G, Saveanu L, Guilhot F, Camoin L, Varet B, Buzyn A, Caillat-Zucman S. Ex vivo characterization of multiepitopic tumor-specific CD8 T cells in patients with chronic myeloid leukemia: implications for vaccine development and adoptive cellular immunotherapy. J. Immunol. 2005;174:8210–8218. doi: 10.4049/jimmunol.174.12.8210. [DOI] [PubMed] [Google Scholar]
- [47].Hirano N, Butler MO, Xia Z, Berezovskaya A, Murray AP, Ansen S, Kojima S, Nadler LM. Identification of an immunogenic CD8+ T-cell epitope derived from gamma-globin, a putative tumor-associated antigen for juvenile myelomonocytic leukemia. Blood. 2006;108:2662–2668. doi: 10.1182/blood-2006-04-017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vonderheide RH, Anderson KS, Hahn WC, Butler MO, Schultze JL, Nadler LM. Characterization of HLA-A3-restricted cytotoxic T lymphocytes reactive against the widely expressed tumor antigen telomerase. Clin Cancer Res. 2001;7:3343–3348. [PubMed] [Google Scholar]
- [49].Arai J, Yasukawa M, Ohminami H, Kakimoto M, Hasegawa A, Fujita S. Identification of human telomerase reverse transcriptase-derived peptides that induce HLA-A24-restricted antileukemia cytotoxic T lymphocytes. Blood. 2001;97:2903–2907. doi: 10.1182/blood.v97.9.2903. [DOI] [PubMed] [Google Scholar]
- [50].Scardino A, Gross DA, Alves P, Schultze JL, Graff-Dubois S, Faure O, Tourdot S, Chouaib S, Nadler LM, Lemonnier FA, Vonderheide RH, Cardoso AA, Kosmatopoulos K. HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J. Immunol. 2002;168:5900–5906. doi: 10.4049/jimmunol.168.11.5900. [DOI] [PubMed] [Google Scholar]
- [51].Hernandez J, Garcia-Pons F, Lone YC, Firat H, Schmidt JD, Langlade-Demoyen P, Zanetti M. Identification of a human telomerase reverse transcriptase peptide of low affinity for HLA A2.1 that induces cytotoxic T lymphocytes and mediates lysis of tumor cells. Proc Natl Acad Sci USA. 2002;99:12275–12280. doi: 10.1073/pnas.182418399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Adotevi O, Mollier K, Neuveut C, Cardinaud S, Boulanger E, Mignen B, Fridman WH, Zanetti M, Charneau P, Tartour E, Lemonnier F, Langlade-Demoyen P. Immunogenic HLA-B*0702-restricted epitopes derived from human telomerase reverse transcriptase that elicit antitumor cytotoxic T-cell responses. Clin Cancer Res. 2006;12:3158–3167. doi: 10.1158/1078-0432.CCR-05-2647. [DOI] [PubMed] [Google Scholar]
- [53].Schreurs MW, Kueter EW, Scholten KB, Kramer D, Meijer CJ, Hooijberg E. Identification of a potential human telomerase reverse transcriptase-derived, HLA-A1-restricted cytotoxic T-lymphocyte epitope. Cancer Immunol Immunother. 2005;54:703–712. doi: 10.1007/s00262-004-0611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mizukoshi E, Nakamoto Y, Marukawa Y, Arai K, Yamashita T, Tsuji H, Kuzushima K, Takiguchi M, Kaneko S. Cytotoxic T cell responses to human telomerase reverse transcriptase in patients with hepatocellular carcinoma. Hepatology. 2006;43:1284–1294. doi: 10.1002/hep.21203. [DOI] [PubMed] [Google Scholar]
- [55].Cortez-Gonzalez X, Sidney J, Adotevi O, Sette A, Millard F, Lemonnier F, Langlade-Demoyen P, Zanetti M. Immunogenic HLA-B7-restricted peptides of hTRT. Int Immunol. 2006;18:1707–1718. doi: 10.1093/intimm/dxl105. [DOI] [PubMed] [Google Scholar]
- [56].Thorn M, Wang M, Kloverpris H, Schmidt EG, Fomsgaard A, Wenandy L, Berntsen A, Brunak S, Buus S, Claesson MH. Identification of a new hTERT-derived HLA-A*0201 restricted, naturally processed CTL epitope. Cancer Immunol Immunother. 2007 doi: 10.1007/s00262-007-0319-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, Vieweg J, Gilboa E. Induction of cytotoxic T lymphocyte responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med. 2000;6:1011–1017. doi: 10.1038/79519. [DOI] [PubMed] [Google Scholar]
- [58].Heiser A, Maurice MA, Yancey DR, Coleman DM, Dahm P, Vieweg J. Human dendritic cells transfected with renal tumor RNA stimulate polyclonal T-cell responses against antigens expressed by primary and metastatic tumors. Cancer Res. 2001;61:3388–3393. [PubMed] [Google Scholar]
- [59].Heiser A, Maurice MA, Yancey DR, Wu NZ, Dahm P, Pruitt SK, Boczkowski D, Nair SK, Ballo MS, Gilboa E, Vieweg J. Induction of polyclonal prostate cancer-specific CTL using dendritic cells transfected with amplified tumor RNA. J. Immunol. 2001;166:2953–2960. doi: 10.4049/jimmunol.166.5.2953. [DOI] [PubMed] [Google Scholar]
- [60].Tourdot S, Scardino A, Saloustrou E, Gross DA, Pascolo S, Cordopatis P, Lemonnier FA, Kosmatopoulos K. A general strategy to enhance immunogenicity of low-affinity HLA-A2. 1-associated peptides: implication in the identification of cryptic tumor epitopes. Eur J Immunol. 2000;30:3411–3421. doi: 10.1002/1521-4141(2000012)30:12<3411::AID-IMMU3411>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [61].Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Bennaceur-Griscelli A, Faure O, Guillaume P, Firat H, Chouaib S, Lemonnier FA, Davoust J, Miconnet I, Vonderheide RH, Kosmatopoulos K. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest. 2004;113:425–433. doi: 10.1172/JCI19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Schroers R, Huang XF, Hammer J, Zhang J, Chen SY. Identification of HLA DR7-restricted epitopes from human telomerase reverse transcriptase recognized by CD4+ T-helper cells. Cancer Res. 2002;62:2600–2605. [PubMed] [Google Scholar]
- [63].Schroers R, Shen L, Rollins L, Rooney CM, Slawin K, Sonderstrup G, Huang XF, Chen SY. Human telomerase reverse transcriptase-specific T-helper responses induced by promiscuous major histocompatibility complex class II-restricted epitopes. Clin Cancer Res. 2003;9:4743–4755. [PubMed] [Google Scholar]
- [64].Brunsvig PF, Aamdal S, Gjertsen MK, Kvalheim G, Markowski-Grimsrud CJ, Sve I, Dyrhaug M, Trachsel S, Moller M, Eriksen JA, Gaudernack G. Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2006;55:1553–1564. doi: 10.1007/s00262-006-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kokhaei P, Palma M, Hansson L, Osterborg A, Mellstedt H, Choudhury A. Telomerase (hTERT 611-626) serves as a tumor antigen in B-cell chronic lymphocytic leukemia and generates spontaneously antileukemic, cytotoxic T cells. Exp Hematol. 2007;35:297–304. doi: 10.1016/j.exphem.2006.10.006. [DOI] [PubMed] [Google Scholar]
- [66].Su Z, Vieweg J, Weizer AZ, Dahm P, Yancey D, Turaga V, Higgins J, Boczkowski D, Gilboa E, Dannull J. Enhanced induction of telomerase-specific CD4(+) T cells using dendritic cells transfected with RNA encoding a chimeric gene product. Cancer Res. 2002;62:5041–5048. [PubMed] [Google Scholar]
- [67].Frolkis M, Fischer MB, Wang Z, Lebkowski JS, Chiu CP, Majumdar AS. Dendritic cells reconstituted with human telomerase gene induce potent cytotoxic T-cell response against different types of tumors. Cancer Gene Ther. 2003;10:239–249. doi: 10.1038/sj.cgt.7700563. [DOI] [PubMed] [Google Scholar]
- [68].Maecker B, Von Bergwelt-Baildon MS, Anderson KS, Vonderheide RH, Anderson KC, Nadler LM, Schultze JL. Rare naturally occurring immune responses to three epitopes from the widely expressed tumour antigens hTERT and CYP1B1 in multiple myeloma patients. Clin Exp Immunol. 2005;141:558–562. doi: 10.1111/j.1365-2249.2005.02879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Masutomi K, Kaneko S, Yasukawa M, Arai K, Murakami S, Kobayashi K. Identification of serum anti-human telomerase reverse transcriptase (hTERT) auto-antibodies during progression to hepatocellular carcinoma. Oncogene. 2002;21:5946–5950. doi: 10.1038/sj.onc.1205788. [DOI] [PubMed] [Google Scholar]
- [70].Marincola FM, Wang E, Herlyn M, Seliger B, Ferrone S. Tumors as elusive targets of T-cell-based active immunotherapy. Trends Immunol. 2003;24:335–342. doi: 10.1016/s1471-4906(03)00116-9. [DOI] [PubMed] [Google Scholar]
- [71].Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- [72].Vonderheide RH, Domchek SM, Schultze JL, George DJ, Hoar KM, Chen DY, Stephans KF, Masutomi K, Loda M, Xia Z, Anderson KS, Hahn WC, Nadler LM. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004;10:828–839. doi: 10.1158/1078-0432.ccr-0620-3. [DOI] [PubMed] [Google Scholar]
- [73].Domchek SM, Fox K, Recio A, Schuchter LM, Davidson R, Demichele A, Mick R, Feldman M, Vonderheide RH. Immunological and clinical outcomes following telomerase peptide vaccination in patients with metastatic breast cancer. Proc AACR. 2006:4003a. [Google Scholar]
- [74].Bernhardt SL, Gjertsen MK, Trachsel S, Moller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer. 2006;95:1474–1482. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mavroudis D, Bolonakis I, Cornet S, Myllaki G, Kanellou P, Kotsakis A, Galanis A, Nikoloudi I, Spyropoulou M, Menez J, Miconnet I, Niniraki M, Cordopatis P, Kosmatopoulos K, Georgoulias V. A phase I study of the optimized cryptic peptide TERT(572y) in patients with advanced malignancies. Oncology. 2006;70:306–314. doi: 10.1159/000096252. [DOI] [PubMed] [Google Scholar]
- [76].Bolonaki I, Kotsakis A, Papadimitraki E, Aggouraki D, Konsolakis G, Vagia A, Christophylakis C, Nikoloudi I, Magganas E, Galanis A, Cordopatis P, Kosmatopoulos K, Georgoulias V, Mavroudis D. Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J Clin Oncol. 2007;25:2727–2734. doi: 10.1200/JCO.2006.10.3465. [DOI] [PubMed] [Google Scholar]
- [77].Cortez-Gonzalez X, Zanetti M. Telomerase immunity from bench to bedside: round one. J Transl Med. 2007;5:12. doi: 10.1186/1479-5876-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, Sichi S, Niedzwiecki D, Boczkowski D, Gilboa E, Vieweg J. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J. Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- [79].Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- [80].Lin X, Zhou C, Wang S, Wang D, Ma W, Liang X, Lin C, Wang Z, Li J, Guo S, Zhang Y, Zhang S. Enhanced antitumor effect against human telomerase reverse transcriptase (hTERT) by vaccination with chemotactic-hTERT gene-modified tumor cell and the combination with anti-4-1BB monoclonal antibodies. Int J Cancer. 2006;119:1886–1896. doi: 10.1002/ijc.22048. [DOI] [PubMed] [Google Scholar]
- [81].Chen L, Liang GP, Tang XD, Chen T, Cai YG, Fang DC, Yu ST, Luo YH, Yang SM. In vitro anti-tumor immune response induced by dendritic cells transfected with hTERT recombinant adenovirus. Biochem Biophys Res Commun. 2006;351:927–934. doi: 10.1016/j.bbrc.2006.10.165. [DOI] [PubMed] [Google Scholar]
- [82].Yamano T, Kaneda Y, Hiramatsu SH, Huang S, Tran AN, Giuliano AE, Hoon DS. Immunity against breast cancer by TERT DNA vaccine primed with chemokine CCL21. Cancer Gene Ther. 2007;14:451–459. doi: 10.1038/sj.cgt.7701035. [DOI] [PubMed] [Google Scholar]
- [83].Kim CH, Yoon JS, Sohn HJ, Kim CK, Paik SY, Hong YK, Kim TG. Direct vaccination with pseudotype baculovirus expressing murine telomerase induces anti-tumor immunity comparable with RNA-electroporated dendritic cells in a murine glioma model. Cancer Lett. 2007;250:276–283. doi: 10.1016/j.canlet.2006.10.022. [DOI] [PubMed] [Google Scholar]
- [84].Guo H, Hao J, Wu C, Shi Y, Zhao XY, Fang DC. A novel peptide-nucleotide dual vaccine of human telomerase reverse transcriptase induces a potent cytotoxic T-cell response in vivo. Biochem Biophys Res Commun. 2007;357:1090–1095. doi: 10.1016/j.bbrc.2007.04.056. [DOI] [PubMed] [Google Scholar]
- [85].Danet-Desnoyers G, Luongo J, Bonnet D, Domchek S, Vonderheide R. Telomerase vaccination has no detectable effect on SCID-repopulating and colony-forming activities in the bone marrow of cancer patients. Exp. Hematology. 2005;33:1275–1280. doi: 10.1016/j.exphem.2005.07.011. [DOI] [PubMed] [Google Scholar]
- [86].Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- [87].Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- [88].Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- [89].Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]