Abstract
Regulated upon activation, normal T-cell expressed, and secreted (RANTES, CCL-5) is an important immunoregulatory mediator that is suppressed in children with malarial anemia (MA). Although proinflammatory (e.g. TNF-α, IL-1β and IFN-γ) and anti-inflammatory (e.g., IL-4, IL-10 and IL-13) cytokines regulate RANTES production, their effect on RANTES in children with MA has not been determined. Since intraleukocytic malarial pigment, hemozoin (Hz), causes dysregulation in chemokine and cytokine production, the impact of naturally-acquired Hz (pfHz) on RANTES and RANTES-regulatory cytokines (TNF-α, IFN-γ, IL-1β, IL-4, IL-10, and IL-13) was examined. Circulating RANTES levels progressively declined with increasing levels of pigment-containing monocytes (PCM) (P=0.035). Additional experiments in cultured peripheral blood mononuclear cells (PBMC) showed that monocytic-acquisition of pfHz (in vivo) was associated with suppression of RANTES under baseline (P=0.001) and stimulated conditions (P=0.072). Although high PCM levels were associated with decreased circulating IFN-γ (P=0.003) and IL-10 (P=0.010), multivariate modeling revealed that only PCM (P=0.048, β=-0.171) and IL-10 (P<0.0001, β=-0.476) were independently associated with RANTES production. Subsequent in vitro experiments revealed that blockade of endogenous IL-10 significantly increased RANTES production (P=0.028) in PBMC from children with naturally-acquired Hz. Results here demonstrate that monocytic-acquisition of Hz suppresses RANTES production in children with MA through an IL-10-dependent mechanism.
Keywords: Malaria, Hemozoin, Monocytes, Rantes, IL-10
1. Introduction
The host innate immune response to P. falciparum products is mediated through the production of cytokines and chemokines from phagocytes (i.e. monocytes/macrophages and neutrophils) that condition the development and outcomes of malarial anemia (MA) [1]. Following infection by P. falciparum, pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interferon (IFN)-γ are produced during the early stages of the host immune response [2]. Although pro-inflammatory mediators elicit anti-parasitic activities, their over-production also promotes enhanced pathogenesis [3]. Elevated levels of TNF-α, interleukin (IL)-1β and IFN-γ have been associated with SMA and hyperparasitemia in children with malaria [4, 5]. Conversely, antiinflammatory cytokines such as IL-10 are produced during the later stages of a malarial infection and down-regulate the potentially pathogenic inflammatory responses that are important for controlling parasitemia [6]. Although previous studies showed decreased IL-10 levels in children with SMA, a low IL-10/TNF-α ratio is associated with enhanced severity of MA [4, 5, 7], suggesting that the timing and magnitude of pro-inflammatory cytokine production, relative to the anti-inflammatory cytokine response, mediates the clinical outcomes of malaria.
Accumulating evidence illustrates that hemozoin (Hz)-acquisition by phagocytes suppresses cellular immunity and enhances malaria severity [8, 9]. Hz is synthesized by trophozoites and early schizonts and is acquired by phagocytic and endothelial cells [9]. Our previous studies demonstrated that P. falciparum-derived hemozoin (pfHz) and synthetic hemozoin (sHz) cause dysregulation in inflammatory mediator release from human monocytes/macrophages [10-12]. Additional investigations in our laboratory and those of others showed that RANTES suppression was associated with enhanced severity of MA, suppression of erythropoiesis, and increased mortality in children with CM [12, 13]. Moreover, we also demonstrated that both pfHz and sHz suppress RANTES transcripts and protein in peripheral blood mononuclear cells (PBMC) from malaria-naïve donors [12]. Thus, optimal RANTES production appears important for regulating the immune and erythropoietic responses during a malarial infection.
RANTES biosynthesis is differentially regulated by pro- and anti-inflammatory cytokines. Previous studies showed that TNF-α, IL-1β, and IFN-γ induced RANTES transcript and protein expression in human endothelial and glial cells [14, 15]. Conversely, IL-10 down-regulates TNF-α- and IFN-γ-induced RANTES release by human monocytes and microglial cells [16, 17]. Consistent with these observations, IL-4, IL-10, and IL-13 inhibit RANTES expression in human endothelial cells [15], suggesting that immunoregulation of RANTES production depends on the microenvironment and/or the cellular sources in the inflammatory milieu.
To more fully characterize the role of RANTES in malaria pathogenesis, we investigated the impact of naturally-acquired Hz (pfHz) on RANTES production in children with MA (Hb<11.0 g/dL) residing in a holoendemic P. falciparum transmission area of western Kenya. The role of intramonocytic pfHz in mediating the production of the primary pro- (i.e., TNF-α, IL-1β, and IFN-γ) and anti-inflammatory (i.e., IL-4, IL-10, and IL-13) cytokines that regulate RANTES expression was also investigated. Additional studies were conducted in cultured PBMC from children with naturally acquired intraleukocytic pfHz to determine the cytokine(s) responsible for suppression of mononuclear cell-derived RANTES suppression.
2. Materials and Methods
2.1. Study site and population
The study was conducted at the Siaya District Hospital, a rural health facility located in a malaria holoendemic region of western Kenya. The study population consisted of children (n=194; age 3-31 mos) with a primary diagnosis of MA defined by Hb<11.0 g/dL and presence of asexual P. falciparum parasitemia. In addition, all study participants were HIV-negative and abacteremic. Individuals with HbSS were also excluded from the study since this genetic variant is associated with severe anemia in African children with malaria [18]. Details of MA in this geographic region are presented in our recent report [19].
2.2. Laboratory measures
Venous blood (<3 mL) was collected into EDTA vacutainer® tubes (Becton-Dickinson, New Jersey, USA) and used for hematological (AcT diff2™ Coulter, Beckman-Coulter Inc., Fullerton, USA) and laboratory investigations. Asexual stages of malaria parasites were counted/300 leukocytes in thick Giemsa-stained blood films and parasitemia was expressed per μL of blood using the patient's absolute leukocyte count. Intracellular pigment in monocytes and neutrophils was determined as previously described [4, 9]. The erythropoietic response was determined by calculating the reticulocyte production index (RPI) using our previous methods [13] and the absolute reticulocyte number (ARN) was calculated using reticulocyte and erythrocyte counts.
2.3. Measurement of RANTES and cytokines
Plasma samples were obtained from venous blood and stored at -70°C until chemokine and cytokine measurements were performed. RANTES and cytokine (i.e., IL-1β, IL-4, IL-10, IL-13, TNF-α, and IFN-γ) concentrations were evaluated in plasma using a 25-plex cytokine assay (Biosource International, Camarillo, USA) according to the manufacturer's instructions. Detection limits (pg/mL) for the cytokines were as follows: IL-1β (2.66); IL-4 (0.49); IL-10 (5.70); IL-13 (0.76); TNF-α (0.20); and IFN-γ (0.30). The detection limit for RANTES was 0.002 ng/mL. Production of RANTES from PBMC was determined using ELISA according to the manufacturer's protocol (Biosource International, Camarillo, USA), with a sensitivity of >0.015 ng/mL.
2.4. Isolation and culture of peripheral blood mononuclear cells
PBMC were cultured using our previously described methods [10, 20]. Briefly, PBMC were cultured at 1×106 cells/mL in media alone or stimulated with lipopolysaccharide (LPS) (Escherichia coli LPS 0127B8, 100 ng/mL) (Alexis Corp., California, USA) and IFN-γ (200 U/mL) (Pharmingen, New Jersey, USA) in the presence/absence of either recombinant human IL-10 protein (rhIL-10, 2.0 ng/mL; Endogen, Rockford, USA) or IL-10 neutralizing antibodies (1.0 μg/mL; R&D Systems, Minneapolis, USA). Cultures were incubated at 37°C in 5% CO2 atmosphere for 48 hrs, after which culture supernatants were harvested, centrifuged, and stored at -70°C until experimental measures were obtained.
2.5. Statistical analyses
Statistical analyses were conducted with SPSS, version 15.0 (SPSS Inc., Chicago, USA). Kruskal-Wallis test was used to determine across group differences, and where significant, post-hoc Mann-Whitney U-tests with the Bonferroni correction were performed. Chi-square analyses were used for comparing proportions between groups. Stepwise linear regression analyses were used to determine the impact of PCM and cytokine concentrations on circulating RANTES. Variables associated with RANTES in the bivariate analyses at (P<0.10) were entered into the model as independent variables. Before conducting inferential analyses, the following variables that deviated from univariate normality were transformed towards normality using logarithmic transformation: RANTES; TNF-α; IL-1β; IFN-γ; IL-10; parasitemia; age; gender; Hb levels; total PCM/μL; platelets; lymphocytes; monocytes; and granulocytes. P<0.05 was used to determine statistical significance.
3. Results
3.1. Presence of pfHz deposition in monocytes and neutrophils
Phagocytosis of pfHz enhances malaria severity and causes dysregulation in inflammatory mediator production [4, 8, 9, 11]. In addition, we have previously shown higher baseline RANTES production in healthy, malaria-exposed aparasitemic children compared to children with malarial anemia in the same cohort [13]. Thus, Hz deposition in monocytes and neutrophils was examined in P. falciparum-infected children with MA (i.e., Hb<11.0 g/dL). PCM and PCN levels in the cohort were 0-86.7% [median (Q1-Q3)=1.7% (0-14.2%)] and 0-12.0% [median (Q1-Q3)=0% (0%)], respectively. Due to a substantially higher percentage of PCM vs. PCN in the cohort, we investigated the effect of naturally-acquired intramonocytic Hz on dysregulation of both RANTES and RANTES-regulatory cytokines (i.e., TNF-α, IL-1β, IFN-γ, IL-4, IL-10, and IL-13). As shown in Table 1, children were stratified according to intramonocytic pfHz deposition: 0% [PCM(-), n=97]; ≤10.0% (low PCM, n=41); >10.0<26.7% (moderate PCM, n=28); and ≥26.7% PCM (high PCM, n=28). Division of the categories was based on percentile distributions of PCM in the population, and the fact that the denominator for calculating PCM levels for each individual is 30. Analysis of the three groups that contained intramonocytic Hz revealed that total PCM levels (/μL) were elevated in the high PCM group relative to both the low (P<0.0001) and moderate PCM (P<0.0001) groups.
Table 1.
Clinical and demographic characteristics of the study participants
| Characteristic | PCM (%) | P | |||
|---|---|---|---|---|---|
| PCM(-) (0%) | Low PCM (≤10%) | Moderate PCM (>10<26.7%) | High PCM (≥26.7%) | ||
| No. of subjects | 97 | 41 | 28 | 28 | |
| Total PCM/μL | 0 | 113 (44-264) | 1258 (749-1950)a | 6082 (3450-11747)a,b,c | <0.0001 |
| Gender, male/female (%) | 63/37 | 44/56 | 61/39 | 29/71 | 0.006 |
| Age, months | 10.0 (7.0-16.0) | 10.0 (7.0-16.0) | 8.0 (5.0-13.8) | 8.0 (5.3-10.8) | 0.059 |
| Axillary temperature, °C | 38.3 (36.8-38.8) | 37.4 (36.8-38.4) | 37.8 (36.7-38.2) | 37.0 (36.3-38.0) | 0.058 |
| Glucose, mM | 4.9 (4.4-5.5) | 4.8 (4.6-5.5) | 5.0 (4.4-5.9) | 4.9 (4.2-5.7) | 0.957 |
| Parasites/μL | 17,893 (7,216-46,967) | 25,935 (9,660-56,839) | 47,222 (14,850-101,745) | 46,481 (10,774-122,583) | 0.011 |
| Geometric mean/μL | 15,991 | 23,680 | 31,193 | 35,206 | |
| HDP, n (%) | 64 (66.0) | 31 (75.6) | 23 (82.1) | 22 (78.6) | 0.256 |
| Lymphocytes (×103/μL) | 6.8 (4.0-7.4) | 4.5 (3.6-7.5) | 6.7 (5.1-8.1) | 7.4 (5.5-9.1)a,c | 0.008 |
| Monocytes (×103/μL) | 1.0 (0.7-1.6) | 0.9 (0.5-1.5) | 1.2 (0.9-2.0) | 1.2 (0.9-2.1) | 0.090 |
| Granulocytes (×103/μL) | 4.3 (3.2-5.4) | 4.2 (3.1-5.6) | 6.0 (2.8-9.2) | 4.7 (3.3-7.8) | 0.130 |
| Hemoglobin, g/dL | 7.50 (5.50-8.80) | 5.50 (4.80-7.30)c | 5.70 (5.03-8.0) | 4.75 (4.03-5.60)a,b,c | <0.0001 |
| SMA (Hb<6.0 g/dL), n(%) | 27 (27.8) | 29 (70.7) | 17 (60.7) | 26 (92.6) | <0.0001 |
| RPI | 1.02 (0.49-1.76) | 1.30 (0.48-2.11) | 0.84 (0.33-1.54) | 0.73 (0.39-1.93) | 0.380 |
| ARN (×109/L) | 59.7 (31.0-102.8) | 59.1 (25.7-102.8) | 39.4 (16.3-84.2) | 28.5 (17.7-87.1)a | 0.003 |
| Platelets (×103/μL) | 159 (108-231) | 137 (92-203) | 154 (104-194) | 128 (109-216) | 0.292 |
| Thrombocytopenia, n (%) | 41 (42.3) | 24 (58.5) | 13 (46.4) | 18 (64.3) | 0.115 |
Data are presented as median (Q1-Q3) unless otherwise indicated. Data analysis was performed by χ2-test and, Kruskal-Wallis tests for across group differences, and post-hoc Mann-Whitney U-tests with Bonferroni correction for paired comparisons. PCM, pigment-containing monocytes; HDP, high-density parasitemia (≥10,000 parasites/μL); SMA, severe malarial anemia (Hb<6.0 g/dL); RPI, Reticulocyte production index; ARN, reticulocyte production number; Thrombocytopenia, platelets <150×103/μL;
P<0.00833 vs. low PCM;
P<0.00833 vs. moderate PCM;
P<0.00833 vs. PCM(-).
3.2. Demographic and clinical characteristics of the study participants
The clinical and demographic features of the study participants are summarized in Table 1. Gender distribution differed across the groups (P=0.006). However, age, axillary temperature, and plasma glucose were not significantly different (P=0.059, P=0.058, and P=0.957, respectively). Although parasitemia differed across the groups (P=0.011), proportions of high-density parasitemia (HDP, ≥10,000 parasites/μL) were not significant (P=0.256). Lymphocyte counts differed across the groups (P=0.008), but monocyte and granulocyte counts were not significantly different (P=0.090 and P=0.130, respectively). Consistent with decreasing Hb levels in the presence of increasing pfHz deposition, prevalence of SMA (Hb<6.0 g/dL) was most pronounced in the high PCM group (P<0.05 vs. all groups). The reticulocyte production index (RPI) non-significantly decreased with increasing monocytic-acquisition of pfHz (P=0.380), such that the RPI was 28.4% and 43.8% less in the high PCM group, relative to the PCM(-) and low PCM groups, respectively. Consistent with the decreasing RPI, the absolute reticulocyte number (ARN) was reduced with increasing pfHz-deposition (P=0.003). Platelet counts (P=0.292), and prevalence of thrombocytopenia (<150×103/μL, P=0.115) were not significantly different across the groups.
3.3. Suppression of RANTES is associated with monocytic acquisition of pfHz
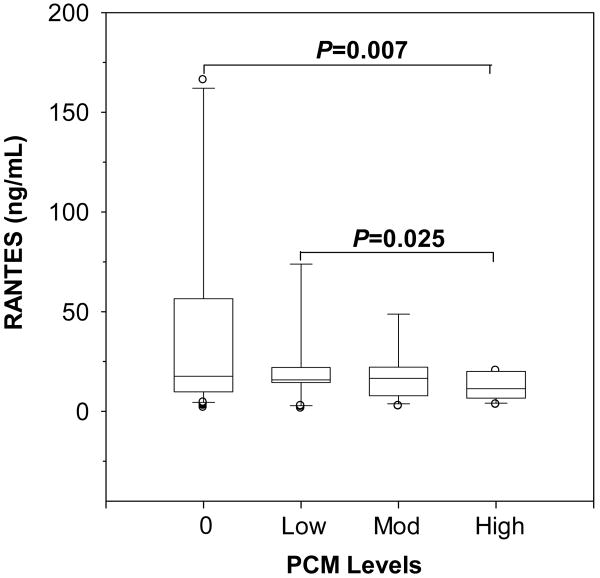
Our previous studies showing that pfHz and sHz suppress RANTES production in PBMC from malaria-naïve individuals [12] led us to determine the impact of naturally-acquired monocytic pfHz (i.e., PCM) on circulating RANTES. Circulating RANTES progressively declined with increasing PCM levels (P=0.035, across group difference), and were lowest in the high PCM group relative to the PCM(-) group (P=0.007, Fig. 1). RANTES was also inversely associated with the total PCM/μL (r=-0.258, P<0.01), suggesting that increasing monocytic deposition of pfHz is associated with significant reductions in circulating RANTES levels in children with malarial anemia.
Figure 1. Circulating RANTES stratified according to intramonocytic pfHz.
Children with malarial anemia (Hb<11.0 g/dL) were stratified based on PCM levels: PCM(-) (0%, n=97); low PCM (≤10%, n=41); moderate (Mod) PCM (>10<26.7%, n=28); and high PCM (≥26.7%, n=28). Data are presented as box plots, where the box represents the interquartile range, the line through the box represents the median, whiskers indicate the 10th and 90th percentiles, and the open circles represent outliers. RANTES levels (ng/mL) decreased with PCM level (P=0.035, Kruskal-Wallis test), and were significantly lower in children with high PCM relative to the PCM(-) group (P=0.007, post-hoc Mann-Whitney U-test with the Bonferroni correction).
3.4. Reduced RANTES biosynthesis in cultured PBMC is associated with in vivo phagocytosis of pfHz
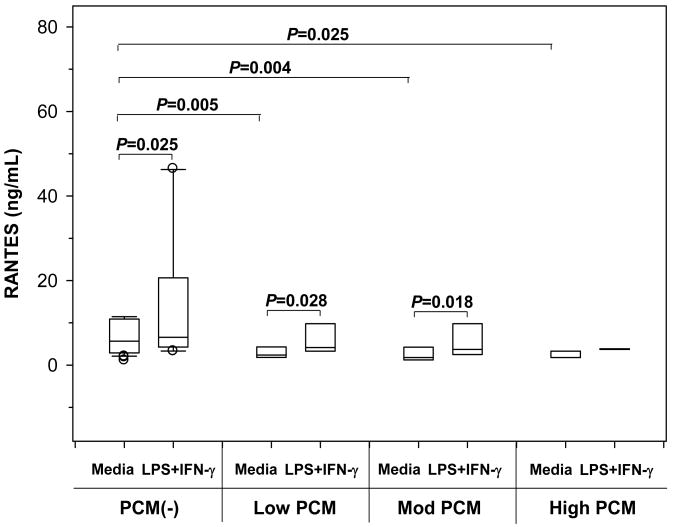
To further examine the effect of pfHz on RANTES production, PBMC from children with MA were cultured in either media alone or concomitant stimulation with LPS+IFN-γ. Children were stratified into: PCM(-) (0%, n=36); low PCM (≤10%, n=7); moderate (Mod) PCM (>10<26.7%, n=6); and high PCM (≥26.7%, n=3). RANTES production under baseline (P=0.001) and stimulated (P=0.072) conditions decreased with increasing PCM level (Fig. 2). Baseline RANTES production was reduced in the low (P=0.005), moderate (P=0.004), and high PCM (P=0.021) groups relative to the PCM(-) group (Fig. 2). These in vitro results support the in vivo findings showing that reduced RANTES in circulation is associated with monocytic acquisition of pfHz.
Figure 2. Effect of naturally-acquired pfHz on PBMC RANTES production.
RANTES synthesis (ng/mL) by PBMC from children with malarial anemia (Hb<11.0 g/dL) was stratified according to pfHz-containing monocytes: [PCM(-), (0%, n=36)]; low PCM (≤10%, n=7); moderate (Mod) PCM (>10<26.7%, n=6); and high PCM (≥26.7%, n=3)]. PBMC were cultured at 1×106 cells/mL in media alone or stimulated with a combination of lipopolysaccharide (LPS, 100 ng/mL) and interferon (IFN)-γ (200 U/mL). Data are presented as box plots, where the box represents the interquartile range, the line through the box represents the median, whiskers indicate the 10th and 90th percentiles, and the open circles represent outliers. RANTES production under baseline (P=0.001, Kruskal-Wallis test) and stimulated (P=0.072, Kruskal-Wallis test) conditions decreased with increasing PCM level. Post-hoc Mann-Whitney U-test showed that baseline RANTES production was lower in the low (P=0.005), moderate (P=0.004), and high PCM (P=0.025) groups relative to the PCM(-) group. RANTES biosynthesis was higher in stimulated conditions in the PCM(-) (P=0.025, Wilcoxon rank sum test), low (P=0.028, Wilcoxon rank sum test), and moderate PCM (P=0.018, Wilcoxon rank sum test) groups.
3.5. Monocytic acquisition of pfHz alters pro-inflammatory cytokine profiles
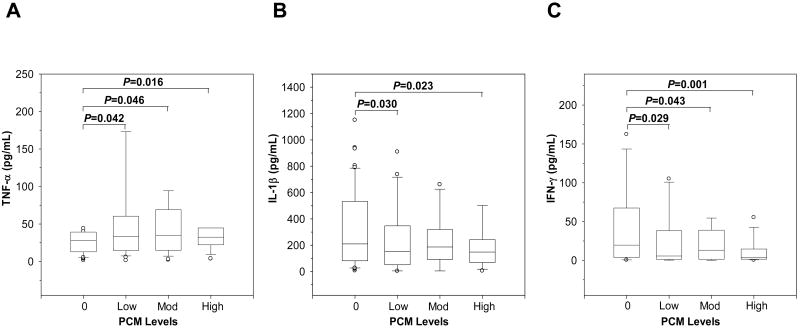
Since we have previously shown that phagocytosis of pfHz by monocytes is associated with dysregulation in soluble inflammatory mediators in children with MA [10, 11], the impact of PCM on circulating pro-inflammatory cytokines (i.e., TNF-α, IL-1β, and IFN-γ) known to up-regulate RANTES expression [14, 15] was investigated. TNF-α increased with elevated PCM levels (P=0.029, Fig. 3A), while IL-1β (P=0.05, Fig. 3B) and IFN-γ (P=0.003, Fig. 3C) decreased with increasing percentages of PCM. In addition, IFN-γ was lower in the high PCM group relative to the PCM(-) group (P=0.001, Fig. 3C). Thus, acquisition of pfHz by monocytes is associated with significant changes in circulating levels of TNF-α, IL-1β, and IFN-γ.
Figure 3. Circulating pro-inflammatory cytokine profiles in children with naturally-acquired pfHz.
Children with malarial anemia (Hb<11.0 g/dL) were stratified based on PCM level as follows: PCM(-) (0%, n=97); low PCM (≤10%, n=41); moderate (Mod) PCM (>10<26.7%, n=28); and high PCM (≥26.7%, n=28). Data are presented as box plots, where the box represents the interquartile range, the line through the box represents the median, whiskers indicate the 10th and 90th percentiles, and the open circles represent outliers. (A) Circulating TNF-α levels (pg/mL) in children with varying PCM levels differed across groups (P=0.029, Kruskal-Wallis test). (B) Circulating IL-1β concentrations (pg/mL) differed in children with increasing PCM levels (P=0.05, Kruskal-Wallis test). (C) Plasma IFN-γ levels (pg/mL) in children with different PCM levels. IFN-γ levels decreased gradually with increasing levels of PCM (P=0.003, Kruskal-Wallis test) and were significantly lower in the high PCM group compared to the PCM(-) group (P=0.001, post-hoc Mann-Whitney U-test with the Bonferroni correction).
3.6. Monocytic acquisition of pfHz alters anti-inflammatory cytokine profiles
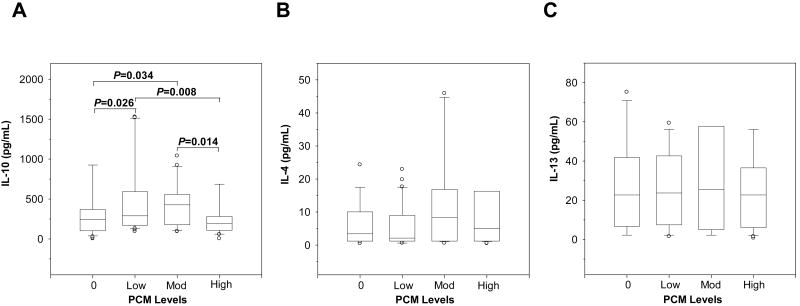
To further define the inflammatory milieu associated with altered RANTES production, circulating antiinflammatory cytokines (i.e., IL-4, IL-10, and IL-13) known to down-regulate RANTES expression [16, 17] were examined. IL-10 differed across the groups (P=0.010) and was significantly reduced in children with high PCM levels relative to the low PCM group (P=0.008, Fig. 4A). Neither IL-4 (P=0.568, Fig. 4B) nor IL-13 (P=0.741, Fig. 4C) differed significantly across the groups. These results illustrate that monocytic acquisition of pfHz may alter circulating IL-10 production, a cytokine with potent down-regulatory effects on RANTES [16, 17].
Figure 4. Circulating anti-inflammatory cytokine profiles in children with naturally-acquired pfHz.
Children with malarial anemia (Hb<11.0 g/dL) were stratified based on PCM level as follows: PCM(-) (0%, n=97); low PCM (≤10%, n=41); moderate PCM (>10<26.7%, n=28); and high PCM (≥26.7%, n=28). Data are presented as box plots, where the box represents the interquartile range, the line through the box represents the median, whiskers indicate the 10th and 90th percentiles, and the open circles represent outliers. (A) Plasma IL-10 levels (pg/mL) differed in children with varying PCM levels (P=0.010, Kruskal-Wallis test). IL-10 levels decreased in the high PCM group relative to the low (P=0.008, post-hoc Mann-Whitney U-test with the Bonferroni correction). (B) Plasma IL-4 (pg/mL) concentrations were not significantly different across the groups (P=0.568, Kruskal-Wallis test). (C) IL-13 levels (pg/mL) were also not significantly different across the groups (P=0.741, Kruskal-Wallis test).
3.7. Modeling of pro- and anti-inflammatory cytokines reveal that IL-10 is associated with circulating RANTES
Since phagocytosis of pfHz by monocytes caused dysregulation in a number of cytokines known to regulate RANTES production, the association between these cytokines and RANTES was further explored. To establish the predictor(s) of circulating RANTES in children with MA, stepwise linear regression analyses were used to examine the impact of pro- (i.e., TNF-α, IL-1β, and IFN-γ) and anti-inflammatory (i.e., IL-10) cytokines, acquisition of monocytic pfHz (i.e., PCM), and clinical variables associated with circulating RANTES. Only variables with differences at the P<0.10 level in the bivariate analyses were included in the model. Circulating RANTES levels were entered in the model as the dependent variable, followed by the block of predictor variables: age; gender; parasitemia; Hb levels; TNF-α; IL-1β; IFN-γ; IL-10; total PCM/μL; platelets; monocytes; lymphocytes; and granulocytes. The overall model was significant (R=0.507, R2=0.257, P=0.048) with PCM (standardized regression coefficient, β=-0.171, P=0.048) and IL-10 (β=-0.476, P<0.0001) independently associated with circulating RANTES. These analyses suggest that suppression of circulating RANTES occurs, at least in part, through acquisition of PCM and altered IL-10 production.
3.8. Suppression of RANTES biosynthesis is due to endogenous production of IL-10
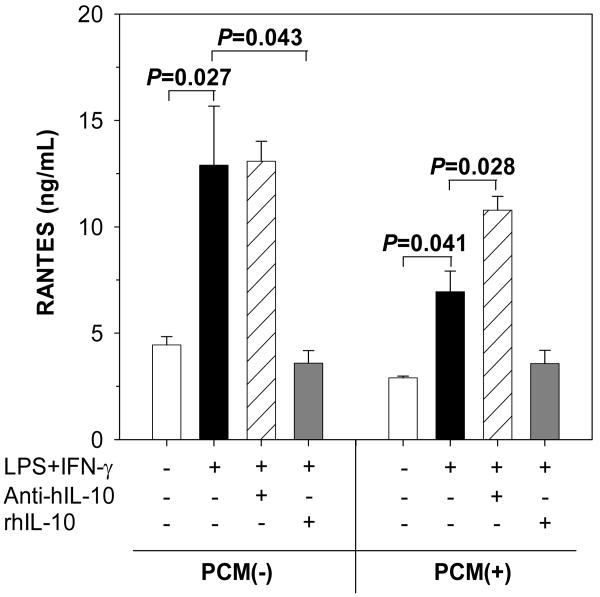
Based on the modeling results suggesting that IL-10 is closely associated with circulating RANTES, in vitro experiments examined the effect of IL-10 on RANTES production in PBMC isolated from children without [PCM(-), n=6] and with [PCM(+), n=6] monocytic pfHz accumulation. PBMC were cultured in media alone or concomitant stimulation with LPS (100 ng/mL) and IFN-γ (200 U/mL) with either rhIL-10 (2.0 ng/mL) or IL-10 neutralizing antibodies (1.0 μg/mL), for 48 hrs. Culture conditions were based on our previous studies demonstrating that monocytic phagocytosis of pfHz results in maximal inflammatory mediator dysregulation at 48 hrs [10, 20]. As shown in Fig. 5, concomitant stimulation with LPS+IFN-γ increased RANTES production in both the PCM(-) and PCM(+) groups (P=0.027 and P=0.041, respectively). RANTES production in stimulated PBMC from the PCM(-) group was not significantly altered by blockade of endogenous IL-10 production (P=0.752, Fig. 5). However, RANTES production in stimulated cells from the PCM(+) group were significantly elevated (P=0.043) in the presence of IL-10 neutralizing antibodies (Fig. 5). Addition of exogenous rhIL-10 did not significantly decrease RANTES production in stimulated cells from the PCM(+) group (P=0.141). In contrast, rhIL-10 caused significant reductions (P=0.028) in RANTES biosynthesis in stimulated cells from the PCM(-) group (Fig. 5). Taken together, these results suggest that suppression of RANTES production in children with intramonocytic acquisition of pfHz is due, at least in part, to endogenous IL-10 production.
Figure 5. Effect of IL-10 on PBMC RANTES production.
Effect of IL-10 on RANTES production in PBMC with PCM [PCM(+), range 6.7–23.3% (n=6)] and without PCM [PCM(-), 0%, n=6]. PBMC were cultured at 1×106 cells/mL in media alone or lipopolysaccharide (LPS, 100 ng/mL) and interferon (IFN)-γ (200 U/mL) in the presence of either recombinant human IL-10 protein (rhIL-10, 2.0 ng/mL) or IL-10 neutralizing antibodies (Anti-hIL-10, 1.0 μg/mL) for 48hrs. Data are shown as mean (SEM). RANTES biosynthesis in LPS- and IFN-γ-stimulated cells was higher in the PCM(-) and PCM(+) groups (P=0.027 and P=0.041, respectively, Wilcoxon rank sum test) relative to baseline conditions. Compared to cells stimulated with LPS and IFN-γ, exogenous IL-10 decreased RANTES production only in the PCM(-) group (P=0.043, Wilcoxon rank sum test), while IL-10 neutralizing antibody increased RANTES production in the PCM(+) group (P=0.028, Wilcoxon rank sum test).
4. Discussion
Our recent reports were the first to demonstrate that RANTES is suppressed in children with falciparum malaria [12, 13]. Subsequent investigations revealed that reduced circulating RANTES in children with cerebral malaria is associated with enhanced mortality [21]. Results presented here extend previous studies by illustrating that naturally-acquired pfHz by monocytes is, at least in part, an important mechanism through which RANTES levels are reduced in children with MA.
Stratification of children according to pfHz accumulation in their monocytes revealed that circulating RANTES levels progressively declined with increasing PCM. In addition, presence of intramonocytic pfHz was associated with significantly elevated rates of SMA. These results are consistent with our previous investigation illustrating that pfHz deposition in monocytes is a significant predictor of SMA [11]. Findings presented here also support our previous study showing that circulating RANTES levels decrease with increasing severity of MA [13], and that circulating RANTES is suppressed in children with acute falciparum malaria relative to healthy, malaria-exposed controls [12]. Additional results presented here demonstrate that naturally-acquired pfHz by monocytes is associated with reduced RANTES expression in cultured PBMC from children with MA under both baseline conditions and stimulated conditions. This finding parallels our previous in vitro study in malaria-naïve individuals showing that phagocytosis of hemozoin (pfHz and synthetic Hz) by PBMC suppresses RANTES transcription and protein expression [12]. Thus, results presented here, and in our previous investigation, suggest that hemozoin deposition in monocytes may be an important mechanism for both reduced RANTES biosynthesis and enhanced severity of malarial anemia, at least in part, through suppression and/or reduced erythropoiesis.
The immunopathology of malaria infection involves interactions between a number of cell types important for both innate and adaptive immunity including monocytes, dendritic, γδ T and NK cells, and conventional and regulatory T cell activities [22-27]. Although a number of these cell populations, and their respective interactions, were not directly examined here due to limited blood volumes available from small, anemic children, our previous investigation showed that cytokine dysregulation in peripheral blood following acquisition of pfHz by leukocytes occurs primarily through monocytes (i.e., CD14+ cell populations) [28]. In addition, we have previously demonstrated that phagocytosis of pfHz by cultured PBMC and CD14+ cells causes dysregulation in cytokine (IL-10, IL-12, and TNF-α) and chemokine (MIF, MIP-1α, MIP-1β, and RANTES) production [10, 12, 28], suggesting that altered cytokine and chemokine profiles during a malaria infection are largely due to acquisition of hemozoin by monocytes.
To explore the potential mechanism(s) through which pfHz suppresses RANTES, we examined a panel of pro- (i.e., TNF-α, IL-1β, and IFN-γ) and anti-inflammatory (i.e., IL-4, IL-10, and IL-13) cytokines known to regulate RANTES [14-17]. These studies demonstrated that children with elevated concentrations of PCM had reduced circulating IL-10 levels. The pattern of IL-10 production in children with MA in the current study is consistent with our previous investigation showing that PCM(+) children had decreased IL-10 levels [28]. These results also parallel our previous study in women with placental malaria showing that high levels of pfHz in intervillous blood mononuclear cells (IVBMC) was associated with decreased IL-10 production [29]. Thus, high levels of intramonocytic pfHz accumulation may render mononuclear cells anergic and/or refractory to IL-10 production. It is important to note that the relative expression of IL-10 in the environment will impact on RANTES production. Thus, even in the presence of low IL-10 levels, these reduced levels may be sufficient to suppress RANTES production. Data presented here in children with malarial anemia also parallel previous investigations in placental malaria illustrating a dose-dependent suppression of IFN-γ production in the presence of increasing intraleukocytic pfHz-deposition [30]. Taken together, these studies and results presented here illustrate that there are both common and differing effects of naturally-acquired pfHz on inflammatory cytokine pathways in various mononuclear cells.
After determining that TNF-α, IL-1β, IFN-γ, and IL-10 differed in children with varying amounts of intramonocytic pfHz, a model was developed to determine the relationship between cytokine dysregulation and circulating RANTES. Stepwise linear regression analyses revealed that IL-10 and PCM were the only significant predictors of circulating RANTES levels. The negative β-weight for IL-10 in the analyses suggests that IL-10 may be responsible, at least in part, for decreased production of RANTES in children with MA. These results are consistent with the fact that IL-10 suppresses RANTES production from human monocytes [17].
Based on results obtained in the cytokine modeling, the effect of IL-10 on RANTES production was directly examined by culturing PBMC from children with MA stratified into two categories: PCM(-) and PCM(+). Children in the PCM(+) were not further stratified due to limited sample size. The in vitro experiments demonstrated that blockade of endogenous IL-10 in stimulated cells from PCM(+) children caused a significant increase in RANTES production, providing further evidence that IL-10 is an important mechanism for decreasing RANTES biosynthesis in pigmented mononuclear cells. In contrast, stimulated cells from children in the PCM(-) group produced very high levels of RANTES that were unaffected by IL-10 neutralizing antibodies, suggesting that LPS and IFN-γ stimulation induce maximal levels of RANTES in the absence of intramonocytic pigment that cannot be further augmented through blockade of IL-10. Additional experiments revealed that addition of exogenous IL-10 decreased stimulated RANTES production in both the PCM(-) and PCM(+) groups, although to a greater degree in the PCM(-) group. This finding appears to be due to the fact that intramonocytic pfHz has a strong suppressive effect on RANTES production that cannot be further downregulated by exogenous IL-10 since RANTES levels are already substantially reduced. Taken together, the in vitro results suggest that suppression of RANTES following natural-acquisition of pfHz by monocytes is mediated, at least in part, by IL-10.
The precise underlying molecular mechanism(s) by which IL-10 suppresses RANTES remains to be defined. IL-10 could potentially suppress RANTES production through inhibition of transcriptional factors and/or indirectly through suppression of RANTES-regulatory cytokines [16, 17]. This hypothesis is supported by our previous results and those of others showing that Hz-induced up-regulation of IL-10 suppresses cytokine production and cellular responses of monocytes and PBMC [28]. Although not examined in the current studies, we have previously shown that TGF-β, an anti-inflammatory cytokine that decreases TNF-α-induced RANTES production in a manner similar to IL-10 [16], is suppressed in children with severe malaria [31], suggesting that TGF-β may not be a primary source of reduced RANTES in the cohort.
Previous studies demonstrated that altered production of erythropoietic mediators and acquisition of hemozoin by phagocytic cells are associated with enhanced pathogenesis of MA [8], suggesting that perturbations in hematopoietic cytokine production plays an important role in MA pathogenesis. We recently showed that reduced RANTES production in children with MA was associated with suppression of erythropoiesis [13]. These investigations further revealed that decreased RANTES was due, at least in part, to reduced numbers of platelets, an important source of circulating RANTES production [32]. Results presented here extend the previous findings by demonstrating that naturally-acquired intramonocytic pfHz also suppresses RANTES biosynthesis through an IL-10-dependent mechanism, illustrating that there are, at least, several different mechanisms for decreased RANTES in children with MA: thrombocytopenia and monocytic phagocytosis of pfHz. It is important to note that additional parasite-derived products, such as glycosylphosphatidylinositol and malarial antigens were not examined in the current study. Since these parasitic products can cause dysregulation in the inflammatory cascade [33], it is possible that these molecules may also contribute to altered RANTES production in children with MA. Subsequent studies investigating the impact of additional parasite-derived products and the molecular mechanism(s) through which phagocytosis of hemozoin generates altered cytokine and RANTES production may provide additional insights into the pathogenesis of MA.
Acknowledgments
This study was supported by grants from the NIH (R01-AI051305, DJP) and Fogarty International Center (2D43-TW005884-05, DJP). The author(s) declare that they have no conflict of interest.
We thank all of the parents, guardians, and children for participating in this study. We thank the University of New Mexico-KEMRI staff and the Siaya District Hospital staff for their support during this study. We are grateful to the Director of Kenya Medical Research Institute (KEMRI) for approving this manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdalla SH, Pasvol G. Malaria: a hematological perspective. Imperial College Press; Distributed by World Scientific Pub.; London, River Edge, NJ: 2004. [Google Scholar]
- 2.Scragg IG, Hensmann M, Bate CA, Kwiatkowski D. Early cytokine induction by Plasmodium falciparum is not a classical endotoxin-like process. Eur J Immunol. 1999;29:2636–2644. doi: 10.1002/(SICI)1521-4141(199908)29:08<2636::AID-IMMU2636>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 3.Kremsner PG, Winkler S, Brandts C, Wildling E, Jenne L, Graninger W, Prada J, Bienzle U, Juillard P, Grau GE. Prediction of accelerated cure in Plasmodium falciparum malaria by the elevated capacity of tumor necrosis factor production. Am J Trop Med Hyg. 1995;53:532–538. doi: 10.4269/ajtmh.1995.53.532. [DOI] [PubMed] [Google Scholar]
- 4.Luty AJ, Perkins DJ, Lell B, Schmidt-Ott R, Lehman LG, Luckner D, Greve B, Matousek P, Herbich K, Schmid D, Weinberg JB, Kremsner PG. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect Immun. 2000;68:3909–3915. doi: 10.1128/iai.68.7.3909-3915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perkins DJ, Weinberg JB, Kremsner PG. Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J Infect Dis. 2000;182:988–992. doi: 10.1086/315762. [DOI] [PubMed] [Google Scholar]
- 6.Ho M, Schollaardt T, Snape S, Looareesuwan S, Suntharasamai P, White NJ. Endogenous interleukin-10 modulates proinflammatory response in Plasmodium falciparum malaria. J Infect Dis. 1998;178:520–525. doi: 10.1086/515640. [DOI] [PubMed] [Google Scholar]
- 7.Othoro C, Lal AA, Nahlen B, Koech D, Orago AS, Udhayakumar V. A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis. 1999;179:279–282. doi: 10.1086/314548. [DOI] [PubMed] [Google Scholar]
- 8.Casals-Pascual C, Kai O, Cheung JO, Williams S, Lowe B, Nyanoti M, Williams TN, Maitland K, Molyneux M, Newton CR, Peshu N, Watt SM, Roberts DJ. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood. 2006;108:2569–2577. doi: 10.1182/blood-2006-05-018697. [DOI] [PubMed] [Google Scholar]
- 9.Lyke KE, Diallo DA, Dicko A, Kone A, Coulibaly D, Guindo A, Cissoko Y, Sangare L, Coulibaly S, Dakouo B, Taylor TE, Doumbo OK, Plowe CV. Association of intraleukocytic Plasmodium falciparum malaria pigment with disease severity, clinical manifestations, and prognosis in severe malaria. Am J Trop Med Hyg. 2003;69:253–259. [PubMed] [Google Scholar]
- 10.Awandare GA, Hittner JB, Kremsner PG, Ochiel DO, Keller CC, Weinberg JB, Clark IA, Perkins DJ. Decreased circulating macrophage migration inhibitory factor (MIF) protein and blood mononuclear cell MIF transcripts in children with Plasmodium falciparum malaria. Clin Immunol. 2006;119:219–225. doi: 10.1016/j.clim.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Awandare GA, Ouma Y, Ouma C, Were T, Otieno R, Keller CC, Davenport GC, Hittner JB, Vulule J, Ferrell R, Ong'echa JM, Perkins DJ. Role of monocyte-acquired hemozoin in suppression of macrophage migration inhibitory factor in children with severe malarial anemia. Infect Immun. 2007;75:201–210. doi: 10.1128/IAI.01327-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochiel DO, Awandare GA, Keller CC, Hittner JB, Kremsner PG, Weinberg JB, Perkins DJ. Differential regulation of beta-chemokines in children with Plasmodium falciparum malaria. Infect Immun. 2005;73:4190–4197. doi: 10.1128/IAI.73.7.4190-4197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Were T, Hittner JB, Ouma C, Otieno RO, Orago AS, Ong'echa JM, Vulule JM, Keller CC, Perkins DJ. Suppression of RANTES in children with Plasmodium falciparum malaria. Haematologica. 2006;91:1396–1399. [PubMed] [Google Scholar]
- 14.Li QQ, Bever CT. Th1 cytokines stimulate RANTES chemokine secretion by human astroglial cells depending on de novo transcription. Neurochem Res. 2001;26:125–133. doi: 10.1023/a:1011042711631. [DOI] [PubMed] [Google Scholar]
- 15.Marfaing-Koka A, Devergne O, Gorgone G, Portier A, Schall TJ, Galanaud P, Emilie D. Regulation of the production of the RANTES chemokine by endothelial cells. Synergistic induction by IFN-gamma plus TNF-alpha and inhibition by IL-4 and IL-13. J Immunol. 1995;154:1870–1878. [PubMed] [Google Scholar]
- 16.Hu S, Chao CC, Ehrlich LC, Sheng WS, Sutton RL, Rockswold GL, Peterson PK. Inhibition of microglial cell RANTES production by IL-10 and TGF-beta. J Leukoc Biol. 1999;65:815–821. doi: 10.1002/jlb.65.6.815. [DOI] [PubMed] [Google Scholar]
- 17.Marfaing-Koka A, Maravic M, Humbert M, Galanaud P, Emilie D. Contrasting effects of IL-4, IL-10 and corticosteroids on RANTES production by human monocytes. Int Immunol. 1996;8:1587–1594. doi: 10.1093/intimm/8.10.1587. [DOI] [PubMed] [Google Scholar]
- 18.Ambe JP, Fatunde JO, Sodeinde OO. Associated morbidities in children with sickle-cell anaemia presenting with severe anaemia in a malarious area. Trop Doct. 2001;31:26–27. doi: 10.1177/004947550103100109. [DOI] [PubMed] [Google Scholar]
- 19.Ong'echa JM, Keller CC, Were T, Ouma C, Otieno RO, Landis-Lewis Z, Ochiel D, Slingluff JL, Mogere S, Ogonji GA, Orago AS, Vulule JM, Kaplan SS, Day RD, Perkins DJ. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am J Trop Med Hyg. 2006;74:376–385. [PubMed] [Google Scholar]
- 20.Keller CC, Hittner JB, Nti BK, Weinberg JB, Kremsner PG, Perkins DJ. Reduced peripheral PGE2 biosynthesis in Plasmodium falciparum malaria occurs through hemozoin-induced suppression of blood mononuclear cell cyclooxygenase-2 gene expression via an interleukin-10-independent mechanism. Mol Med. 2004;10:45–54. doi: 10.2119/2004-00035.perkins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John CC, Opika-Opoka R, Byarugaba J, Idro R, Boivin MJ. Low levels of RANTES are associated with mortality in children with cerebral malaria. J Infect Dis. 2006;194:837–845. doi: 10.1086/506623. [DOI] [PubMed] [Google Scholar]
- 22.Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, Flavell RA, de Souza JB, Riley EM. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 2008;4:e1000004. doi: 10.1371/journal.ppat.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Ombrain MC, Hansen DS, Simpson KM, Schofield L. gammadelta-T cells expressing NK receptors predominate over NK cells and conventional T cells in the innate IFN-gamma response to Plasmodium falciparum malaria. Eur J Immunol. 2007;37:1863–1873. doi: 10.1002/eji.200636889. [DOI] [PubMed] [Google Scholar]
- 24.Good MF, Xu H, Wykes M, Engwerda CR. Development and regulation of cell-mediated immune responses to the blood stages of malaria: implications for vaccine research. Annu Rev Immunol. 2005;23:69–99. doi: 10.1146/annurev.immunol.23.021704.115638. [DOI] [PubMed] [Google Scholar]
- 25.Newman KC, Korbel DS, Hafalla JC, Riley EM. Cross-talk with myeloid accessory cells regulates human natural killer cell interferon-gamma responses to malaria. PLoS Pathog. 2006;2:e118. doi: 10.1371/journal.ppat.0020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nie CQ, Bernard NJ, Schofield L, Hansen DS. CD4+ CD25+ regulatory T cells suppress CD4+ T-cell function and inhibit the development of Plasmodium berghei-specific TH1 responses involved in cerebral malaria pathogenesis. Infect Immun. 2007;75:2275–2282. doi: 10.1128/IAI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 28.Keller CC, Yamo O, Ouma C, Ong'echa JM, Ounah D, Hittner JB, Vulule JM, Perkins DJ. Acquisition of hemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: in vivo and in vitro findings in severe malarial anemia. Infect Immun. 2006;74:5249–5260. doi: 10.1128/IAI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins DJ, Moore JM, Otieno J, Shi YP, Nahlen BL, Udhayakumar V, Lal AA. In vivo acquisition of hemozoin by placental blood mononuclear cells suppresses PGE2, TNF-alpha, and IL-10. Biochem Biophys Res Commun. 2003;311:839–846. doi: 10.1016/j.bbrc.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 30.Moore JM, Chaisavaneeyakorn S, Perkins DJ, Othoro C, Otieno J, Nahlen BL, Shi YP, Udhayakumar V. Hemozoin differentially regulates proinflammatory cytokine production in human immunodeficiency virus-seropositive and -seronegative women with placental malaria. Infect Immun. 2004;72:7022–7029. doi: 10.1128/IAI.72.12.7022-7029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaiyaroj SC, Rutta AS, Muenthaisong K, Watkins P, Na Ubol M, Looareesuwan S. Reduced levels of transforming growth factor-beta1, interleukin-12 and increased migration inhibitory factor are associated with severe malaria. Acta Trop. 2004;89:319–327. doi: 10.1016/j.actatropica.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70:6524–6533. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]