Abstract
Objective
Macrophages are key players in the pathogenesis of rheumatoid synovitis as well as in atherosclerosis. To determine whether atherogenic oxidized phospholipids potentially contribute to synovial inflammation and subsequent monocyte/macrophage recruitment, we examined the effects of oxidized 1-palmitoyl-2-arachidonoyl-sn-3-glycero-phosphorylcholine (OxPAPC) on chemokine expression and leukocyte recruitment in a facsimile synovium in vivo using the murine air-pouch model.
Methods
Air pouches were raised by 2 injections of sterile air, and inflammation was induced by injecting either lipopolysaccharide (LPS) or OxPAPC into the pouch lumen. Inflammation was assessed by analysis of inflammatory gene expression using reverse transcription–polymerase chain reaction or immunohistochemical analysis, and leukocytes were quantified in the lavage fluid and in the pouch wall after staining with Giemsa or after enzymatic digestion followed by fluorescence-activated cell sorter analysis.
Results
Application of OxPAPC resulted in selective recruitment of monocyte/macrophages into the air-pouch wall, but not in the lumen. In contrast, LPS induced both monocyte and neutrophil accumulation in the pouch lumen as well as in the wall. LPS, but not OxPAPC, induced the expression of adhesion molecules E-selectin, P-selectin, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1. OxPAPC increased the expression of the CCR2 ligands monocyte chemotactic protein 1 (MCP-1), MCP-3, and MCP-5, as well as RANTES and growth-related oncogene α (GROα), while it down-regulated the expression of CCR2 on macrophages. Moreover, oxidized phospholipid–induced macrophage accumulation was abrogated in CCR2−/− mice.
Conclusion
These data demonstrate that oxidized phospholipids trigger a type of inflammatory response that leads to selective macrophage accumulation in vivo, a process relevant for the pathogenesis of chronic inflammatory rheumatic diseases.
Patients with rheumatic diseases are at increased risk of cardiovascular complications (1). In fact, cardiovascular disease accounts for nearly 40% of deaths in rheumatoid arthritis (RA) patients (2); however, the common pathogenic mechanisms remain unclear. We hypothesized that “atherogenic” lipid oxidation products, which have been shown to cause vascular complications, also contribute to synovial inflammation.
The chronic inflammatory response in the RA synovium shares many similarities with the inflamed vascular wall during atherogenesis (3). Accumulation of macrophages in the vascular wall is a hallmark of the development of atherosclerotic lesions (4). In arthritic joints, monocyte/macrophages accumulate in the synovial tissue (5), and neutrophils migrate into the synovial cavity. In RA joints and in atherosclerotic lesions, accumulating macrophages take up oxidized lipoproteins and develop into foam cells (6). Genetic deletions of monocyte chemotactic protein 1 (MCP-1) or its receptor CCR2 have been shown to affect macrophage accumulation and joint destruction in experimental arthritis (7) and to decrease monocyte accumulation and lesion formation in mice susceptible to atherosclerosis (8,9). The factors that trigger monocyte recruitment and, thus, propagate chronic inflammatory processes are still poorly understood.
Due to persistent increased production of free-radical species, lipid oxidation products accumulate at sites of inflammation and exert a variety of biologic activities. Increased concentrations of lipid oxidation products have been found in synovial fluid (10) and synovial membranes (6) from RA patients as well as in atherosclerotic lesions (11). Biologically active oxidized phospholipids that are present in oxidized lipoproteins (12) and in the membranes of apoptotic cells (13) induce inflammatory gene expression (14) that may contribute to monocyte/macrophage recruitment and, thus, to the progression of chronic inflammation.
Considerable advances have been made in dissecting the molecular structures of oxidized phospholipids, allowing for the experimental use of structurally defined compounds rather than complex lipoproteins. In vitro oxidation of the common phospholipid 1-palmitoyl-2-arachidonoyl-sn-3-glycero-phosphorylcholine (PAPC) yields a series of structurally identified oxidation products (OxPAPC) that have been shown to accumulate in atherosclerotic lesions (11). The atherogenic potential of OxPAPC has been demonstrated in cell culture studies, as demonstrated by enhanced monocyte, but not neutrophil, binding to OxPAPC-stimulated endothelial cells, as well as induction of MCP-1 and interleukin-8 (IL-8) (15). Moreover, OxPAPC has been shown to induce inflammatory gene expression in vivo when applied intravenously or periadventitially in mice (14).
Whether oxidized phospholipids can induce and/or propagate chronic inflammation in vivo remains elusive due to the lack of data demonstrating oxidized phospholipid–induced leukocyte accumulation in adequate animal models. In the present study, we used the murine air-pouch model (16) to study the ability of oxidized phospholipids to induce leukocyte recruitment in vivo. Since the CCR2 chemokine receptor regulates monocyte recruitment and has been shown to be involved in macrophage-dependent inflammatory responses in various chronic inflammatory diseases (7,9, 17-19), we also examined the involvement of CCR2 in oxidized phospholipid–induced monocyte recruitment.
MATERIALS AND METHODS
Analysis of oxidized phospholipids
PAPC was purchased from Avanti Polar Lipids (Alabaster, AL) and oxidized by exposure of dry lipid to air for 72 hours. The extent of oxidation was monitored by positive ion–electrospray mass spectrometry as described previously (11). Analysis of OxPAPC was performed by mass spectrometry using a Finnigan LCQ Classic mass spectrometer (Thermo Electron Corporation, San Jose, CA) connected to an HP HPLC 1100 series system (Hewlett-Packard, McMinnville, OR). Phospholipids were introduced to the ion source of the mass spectrometer by flow injection, using a solvent consisting of acetonitrile/water/formic acid (50:50:0.1 volume/volume/volume). Lipids were stored at −70°C in chloroform and were used within 1 month after testing. OxPAPC preparations were tested for endotoxin by the Limulus amebocyte assay (BioWhittaker, Walkersville, MD).
Preparation of the air-pouch model
The air-pouch model was originally developed as a facsimile synovium for the study of inflammatory processes that occur in RA. The model allows the differential quantification of leukocyte species that accumulate in the air-pouch wall (tissue) as well as those that transmigrate into the air-pouch cavity (lavage), and it allows the characterization of the chemokines and adhesion molecules responsible for diapedesis induced by a variety of inflammatory stimuli. Another advantage of this model over systemic application is that it allows for local application of oxidized phospholipids, thus avoiding rapid uptake by the liver and degradation by serum enzymes.
Female CCR2−/− mice (B6.129S4-Ccr2tm1Ifc/J) as well as background control C57BL/6 mice (8−12 weeks old) were obtained from The Jackson Laboratory (Bar Harbor, ME). All animal experiments were approved by the Animal Care and Use Committee of the University of Virginia. Air pouches were raised by injection of 5 ml of sterile air into the skin on the dorsum of each mouse on day 1 and were maintained by reinjecting 3 ml of sterile air on day 4. Before the injection of air, mice were briefly anesthetized with isoflurane. To avoid pain after the creation of the pouch, animals received an intraperitoneal injection of buprenorphine (2.0 mg/kg). On day 7, inflammation was induced by injecting into the air pouch either 50 μg of LPS or 250 μg of OxPAPC, each of which was dissolved in 1 ml of sterile 0.9% saline solution. Control animals were injected with 1 ml of 0.9% saline solution. To avoid stretching of the lining tissue, 1 ml of air was withdrawn from the pouch prior to the injection of the test agent. Animals were killed by CO2 asphyxiation and perfused with phosphate buffered saline (PBS) containing 2% heparin via the right ventricle. By extensive perfusion and microdissection, we ensured that the tissues we collected were not contaminated by blood and nonadherent leukocytes.
Assessment of inflammation and inflammatory gene expression
Inflammation was assessed by the following 4 methods: analyzing cells in the lavage fluid; counting the leukocytes in the pouch wall after en face sections were prepared and stained with Giemsa; counting the leukocytes in the membrane after enzymatic digestion followed by fluorescence-activated cell sorting (FACS); and analyzing inflammatory gene expression using reverse transcription–polymerase chain reaction (RT-PCR) and immunohistochemistry. For the lavage, a shielded intravenous catheter (Becton Dickinson, Mountain View, CA) was inserted into the air-pouch lumen, which was then washed 3 times with 1 ml of 0.9% saline solution while the catheter remained in place. The total fluid was collected and used for cell counts.
To identify differences between LPS- and oxidized phospholipid–induced inflammatory gene expression, the lining tissue from the air pouch was isolated, and initial screenings for gene expression were performed using a Mouse Chemokines and Receptors Array (SuperArray Biosciences, Frederick, MD), which allows screening for 67 genes that encode chemokines and chemokine receptors (data not shown). For time course experiments, OxPAPC or LPS was injected into the air pouches, and animals were killed 1, 3, 6, 12, and 24 hours later. Messenger RNA (mRNA) was isolated from the pouch tissues and analyzed by real-time PCR.
Tissue preparation and FACS analysis
For flow analysis, air-pouch tissue was enzymatically digested for 1 hour at 37°C with 125 units/ml of type XI collagenase, 60 units/ml of type I-s hyaluronidase, 60 units/ml of DNase I, and 450 units/ml of type I collagenase (all from Sigma, St. Louis, MO) in PBS containing 20 mM HEPES and then mashed through a 70-μm strainer. This procedure yielded ∼107 cells. For determination of the different leukocyte subtypes, the cell suspension was processed for FACS analysis using a FACSCalibur (BD Immunocytometry Systems, San Jose, CA) or CyanADP (Dako, Fort Collins, CO) system. Data were analyzed using FlowJo software (Tree Star, Ashland, OR). Antibodies against CD45, CD11b, Gr-1, CD3, and CD19 were obtained from PharMingen (San Diego, CA), anti-CD68 from Serotec (Raleigh, NC), and anti-F4/80 from Caltag (South San Francisco, CA). Cells expressing high levels of Gr-1 and CD11b and being negative for F4/80 or I-Ab were characterized as neutrophils (20).
Monocyte/macrophages were defined as cells positive for CD11b and expressing intermediate or low levels of Gr-1 (21,22). Cells that were negative for Gr-1 and CD11b were defined as lymphocytes and were further characterized as T cells (CD3+) or B cells (CD19+). We used spleen tissue to confirm that the antigens were not degraded by the enzymes used for tissue preparation (data not shown). Briefly, spleen was cut into pieces and incubated separately either in enzyme cocktail or in PBS. After 1 hour, cell suspensions from splenic tissue were made and stained with antibodies. The expression of antigens from the enzyme-treated cell suspensions was compared with the antigen expression from untreated samples (23).
RNA isolation and quantitative RT-PCR
Freshly harvested tissue was immediately immersed in ice-cold RNAlater (Ambion, Austin, TX) and stored at −70°C until the time of analysis. For each determination, 100 ng of total RNA isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) was reverse transcribed to complementary DNA (cDNA) using a Gene-Amp RNA PCR core kit (Applied Biosystems, Foster City, CA). Messenger RNA sequences of the investigated genes were obtained from GenBank. PCR primers were designed using Primer3 software from the Whitehead Institute for Biomedical Research (Cambridge, MA). Amplified cDNA regions were chosen to span 1 or more large introns in the genomic sequence to avoid coamplification of genomic DNA. Melting point analysis, agarose gel electrophoresis, and DNA sequencing of the PCR products confirmed primer specificity. (A list of the primer sequences used can be obtained from the authors upon request.) Quantitative real-time PCR was performed using SYBR Green. Porphobilinogen deaminase or β2-microglobulin was used as an endogenous control. PCR efficiency was determined for each primer pair from dilution series of a typical sample of cDNA. Relative quantification of gene expression was performed as described previously (24).
Immunohistochemistry
Immunostaining was performed on 5-μm transverse cryosections. Sections were incubated with antibodies against F4/80 (eBioscience, San Diego, CA), myeloperoxidase, or heme oxygenase 1 (HO-1; Stress-Gen, San Diego, CA). Antigens were visualized with Alexa 488–conjugated secondary antibodies (Invitrogen). Sections were counterstained with 4′,6-diamidino-2-phenylindole and analyzed by epifluorescence microscopy (Olympus, Lake Success, NY).
Bone marrow–derived macrophages
Femurs and tibias from female C57BL/6 mice were harvested and flushed with ice-cold PBS containing 20 units/ml of heparin. Bone marrow cells were collected and incubated at room temperature with sterile 0.843% ammonium chloride solution for 10 minutes to lyse erythrocytes. A single-cell suspension of bone marrow cells was obtained by straining the suspension through a 70-μm filter. Cells were seeded into 24-well culture dishes at a density of 5 × 105/ml and maintained for 7 days in RPMI 1640 medium (Sigma, St. Louis, MO) supplemented with 1% antibiotics, 10% fetal bovine serum (Gibco-BRL, Grand Island, NY), and 10% L929 cell supernatant as the source of macrophage colony-stimulating factor (M-CSF). Nonadherent cells were removed, and adherent cells were maintained for 12 hours in medium without M-CSF. The cells were then stimulated with OxPAPC (50 μg/ml) for 18 hours.
RESULTS
OxPAPC-induced selective monocyte recruitment into the air pouch
Previous studies have characterized the air-pouch model as a reliable experimental approach to the study of inflammatory mechanisms that occur in the synovium (25-28) as well as the accumulation of blood cells in general (16,29). Here, we used a murine air-pouch model to investigate the effects of OxPAPC on leukocyte accumulation in vivo.
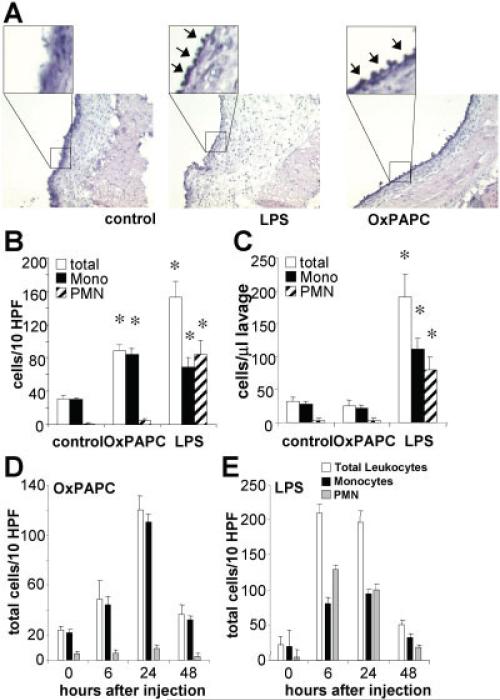
Injection of 50 μg of LPS or 250 μg of OxPAPC into the air-pouch cavity (5-ml volume) resulted in accumulation of inflammatory cells in the surrounding pouch lining tissue, or pouch wall. Transverse sections of the pouch wall revealed that leukocytes also adhered to the pouch wall on the luminal side (Figure 1A, arrows). Treatment with LPS, but not OxPAPC, also caused substantial edema (Figure 1A).
Figure 1.
Induction of mononuclear cell accumulation in air-pouch tissue by injection of oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC) into the air-pouch lumen. Air pouches were raised in the dorsal skin of mice and then injected with 1 ml of 0.9% saline (control) or with 1 ml of 0.9% saline containing either 250 μg of OxPAPC or 50 μg of lipopolysaccharide (LPS). After 24 hours, animals were euthanized, and the air-pouch tissue was analyzed. A, Representative cross-sections of the air-pouch wall show leukocytes adhering to the luminal side of the pouch membrane (arrows in insets) after injection of OxPAPC or LPS (original magnification × 4; × 20 in insets). B, Adherent cells were counted in en face preparations of air-pouch tissues from the 3 groups of mice. Cells were differentiated by morphologic characteristics. Shown are the numbers of total cells, monocytes (mono), and polymorphonuclear neutrophils (PMNs). C, Lavage was performed on air pouches in the 3 groups of mice, and the numbers of total cells, monocytes, and PMNs accumulating in the lumen were counted in the lavage fluid. D and E, Time course analyses of adherent cells in air pouches injected with OxPAPC (D) or LPS (E) were performed. Shown are the numbers of total cells, monocytes, and PMNs. Values in B–E are the mean and SEM of 4 mice per group. * = P < 0.01 versus control in B; P < 0.05 versus control in C, by analysis of variance. HPF = high-power fields. Color figure can be viewed in the online issue, which is available at http://www.arthritisrheum.org.
In order to assess cell counts microscopically, we produced en face preparations of the air-pouch wall after carefully dissecting the lining tissue from the overlying skin. Staining with Giemsa facilitated counting and enabled us to distinguish mononuclear (including monocytes and T cells) from polymorphonuclear (PMN) cells. We found that LPS induced the accumulation of PMNs and mononuclear cells, whereas OxPAPC predominantly induced mononuclear cell accumulation (>95%) (Figure 1B). Injection of nonoxidized PAPC did not lead to recruitment of inflammatory cells (data not shown). Analysis of air-pouch lavage fluid after 24 hours showed that LPS treatment also resulted in increased numbers of leukocytes in the pouch lumen, whereas OxPAPC treatment did not result in accumulation of leukocytes in the lumen (Figure 1C).
Moreover, we found that the kinetics of accumulation of leukocytes in the air-pouch wall in response to OxPAPC treatment differed from that induced by LPS. Time course experiments showed that monocyte recruitment induced by OxPAPC reached a maximum at 24 hours after application (Figure 1D), whereas LPS-induced accumulation of leukocytes was more rapid and peaked earlier (Figure 1E). Inflammation induced by both agonists resolved at 48 hours.
Next, we wanted to further characterize the leukocyte subsets in the OxPAPC-treated pouch wall. Immunostaining for inflammatory cells in transverse sections of the air-pouch wall revealed that OxPAPC caused the accumulation of F4/80-positive cells (Figure 2A). Myeloperoxidase-positive cells were present in LPS-treated air-pouch walls (Figure 2B), some of which were in the process of migrating into the pouch lumen (Figure 2C, arrows).
Figure 2.
Selective induction of the recruitment of CD11b+/Gr-1low cells by injection of oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC). Leukocytes accumulating in the air-pouch wall were analyzed in tissues collected 24 hours after injection with OxPAPC, lipopolysaccharide (LPS), or saline alone (control). A–C, Cross-sections of the air-pouch wall were stained for antibodies against F4/80 (A) or myeloperoxidase (B and C). Some myeloperoxidase-positive cells in the LPS-treated air-pouch wall are in the process of migrating into the pouch lumen (arrows). D, Tissue was weighed and enzymatically digested to obtain a single-cell suspension, which was stained with leukocyte markers and subjected to flow cytometry. The CD45+ cells were then analyzed for CD11b and Gr-1 expression. Polymorphonuclear neutrophils (PMNs) were defined as Gr-1high/CD11b+ cells and monocyte/macrophages (MN/MΦs) as Gr-1low/CD11b+. The remaining CD11b–/Gr-1– cells were defined as lymphocytes, which stained positive for CD3 (T cells) or CD19 (B cells). SSC = side scatter; FSC = forward scatter. E, Absolute numbers of leukocytes, monocyte/macrophages, PMNs, B cells, and T cells were obtained as the products of flow cytometry percentages and total cell counts in samples from the 3 experimental groups. Values are the mean and SD of 4 mice per group. * = P < 0.01 by analysis of variance. OxPL = oxidized phospholipids (i.e., OxPAPC).
We enzymatically digested the air-pouch wall to obtain single-cell suspensions (23) and then further characterized the accumulated leukocytes by FACS analysis. Prior to FACS, cells were stained for CD45, CD11b, Gr-1, CD19, and CD3 to differentiate leukocyte subtypes (Figure 2D). Treatment with either OxPAPC or LPS significantly increased the accumulation of CD45+ cells in the air-pouch wall (Figure 2E). In the CD45+ population, OxPAPC specifically induced the accumulation of CD11b+/Gr-1low cells, indicating monocyte/macrophages, whereas LPS induced the accumulation of CD11b+/Gr-1low cells as well as CD11b+/Gr-1high cells, indicating monocyte/macrophages and PMNs, respectively. No significant differences in the numbers of CD19+ (B cells) or CD3+ (T cells) cells were seen, indicating that the mononuclear cells that were counted above (Figure 1) predominantly consisted of monocytes.
Inflammatory genes differentially regulated by LPS and oxidized phospholipids
To study possible differences in inflammatory gene expression that could account for the differences in leukocyte subset recruitment induced by OxPAPC versus LPS, we examined the expression of endothelial adhesion molecules, which are known to control extravasation of inflammatory cells. For time course experiments, OxPAPC or LPS was injected into the air pouches, and animals were killed 1, 3, 6, 12, or 24 hours later. Studies using RNA isolated from the air-pouch wall demonstrated that expression of vascular cell adhesion molecule 1 (VCAM-1), E-selectin, intercellular adhesion molecule 1 (ICAM-1), and P-selectin were rapidly and potently induced by LPS. In contrast, OxPAPC treatment did not result in up-regulation of these genes (Figures 3A–D). These findings are consistent with our previously published results obtained in vitro (30).
Figure 3.
Induction of heme oxygenase 1 (HO-1) expression, but no increase in the expression of endothelial adhesion molecules, following injection of oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC). A–D, In contrast to lipopolysaccharide (LPS; 50 μg), OxPAPC (250 μg) failed to induce the expression of vascular cell adhesion molecule 1 (VCAM-1) (A), E-selectin (B), intercellular adhesion molecule 1 (ICAM-1) (C), or P-selectin (D) in air-pouch tissue analyzed at the indicated time points after injection, as determined by reverse transcription–polymerase chain reaction analyses. E, Cross-sections of the air-pouch wall 24 hours after saline (control) or OxPAPC injection show HO-1 protein in the OxPAPC-injected tissue (original magnification × 40). F, Time course analysis of the expression of mRNA for HO-1. Values in A–D and F are the mean and SD of 4 mice per group. Color figure can be viewed in the online issue, which is available at http://www.arthritisrheum.org.
Our previous study also demonstrated that HO-1 is differentially regulated by LPS and OxPAPC (31). In the present study, we demonstrated that in contrast to LPS, OxPAPC induced the expression of HO-1 protein and mRNA in the air-pouch wall (Figures 3E and F). Time course experiments showed that OxPAPC-induced HO-1 mRNA expression peaked at 6 hours after stimulation (Figure 3F).
Different kinetics and potency of OxPAPC-induced versus LPS-induced chemokine expression in air-pouch tissue
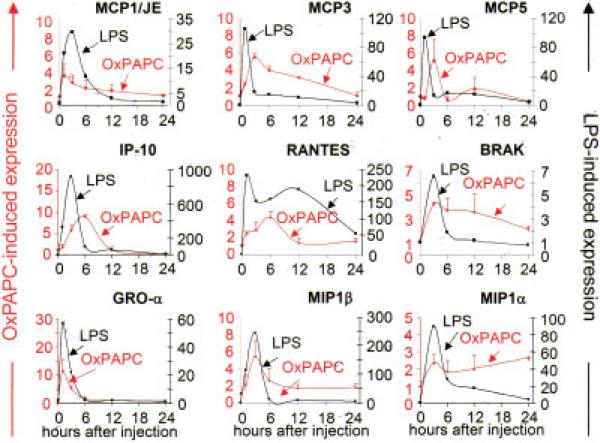
Treatment with OxPAPC induced the expression of the CCR2 ligands MCP-1/JE, MCP-3, and MCP-5 in the air-pouch wall. Interestingly, the expression of MCP-3 and MCP-5 induced by OxPAPC was delayed and sustained as compared with LPS treatment, with MCP-5 showing a biphasic induction (Figure 4). Similarly, OxPAPC induced the expression of interferon-γ–inducible 10-kd protein (CXCL10), RANTES (CCL5), and BRAK (CXCL14). Expression of these 3 chemokines was significantly delayed and sustained as compared with LPS-induced expression (Figure 4). The expression kinetics of OxPAPC-induced MCP-1/JE, growth-related oncogeneα (CXCL1), MIP-1α (CCL3), and MIP-1β (CCL4) overlapped with LPS-induced expression. In all cases, LPS induced the expression of these genes to a much greater extent than did OxPAPC (Figure 4).
Figure 4.
Induction of chemokine expression following injection of oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC). Animals were injected with either 1 ml of 0.9% saline (control) or 1 ml of 0.9% saline containing 250 μg of OxPAPC or 50 μg of lipopolysaccharide (LPS) into the air pouch and were euthanized at the indicated time points. RNA was isolated from air-pouch tissue, and the expression of monocyte chemotactic protein 1 (MCP-1)/JE, MCP-3, MCP-5, interferon-γ–inducible 10-kd protein (IP-10), RANTES, BRAK, growth-related oncogene α (GROα), macrophage inflammatory protein 1β (MIP-1β), and MIP-1α was analyzed by reverse transcription–polymerase chain reaction. Values are the mean and SD fold increase over controls (n = 4 mice per group). Color figure can be viewed in the online issue, which is available at http://www.arthritisrheum.org.
Mediation of oxidized phospholipid–induced monocyte recruitment by CCR2
MCPs 1, 3, and 5 are all ligands for CCR2. To investigate the role of CCR2 in OxPAPC-induced monocyte recruitment, we treated wild-type or CCR2−/− mice with OxPAPC and examined monocyte accumulation in the air-pouch tissue by quantitative FACS analysis. It was previously shown that CCR2−/− mice have lower numbers of circulating monocytes as a result of diminished egress from the bone marrow (18). Accordingly, we found that basal levels of resident macrophages were lower in CCR2−/− mice than in wild-type mice (2 × 105 versus 8 × 105) (Figure 5A). Total leukocyte accumulation, as characterized by the presence of CD45+ cells, was increased by 50% after OxPAPC treatment of wild-type mice. In contrast, in CCR2−/− mice, the OxPAPC-induced increase in accumulation of CD45+ cells was abrogated (Figure 5A). In addition, accumulation of F4/80+/CD68+ cells was significantly increased by OxPAPC treatment in wild-type mice, but not in CCR2−/− mice (Figure 5A).
Figure 5.
Requirement of CCR2 for oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC)–induced monocyte recruitment. A, F4/80-positive and CD68-positive cells in the air-pouch wall from wild-type (W/T) mice (top) and from CCR2-deficient (CCR2−/−) mice (bottom) injected with saline (control) or OxPAPC were analyzed by flow cytometry. B and C, Expression of CCR2 and CCR5 in the air-pouch tissue after treatment with OxPAPC was analyzed by reverse transcription–polymerase chain reaction (B), and a time course analysis of CCR2 expression (C) was performed. D, Bone marrow–derived macrophages were stimulated with OxPAPC in vitro, and CCR2 expression was analyzed by flow cytometry. Values in A–C are the mean and SD of 4 mice per group. * = P < 0.05 versus control in A and versus 0 hours in B and C, by analysis of variance.
Moreover, we found that during the course of OxPAPC-induced inflammation, the expression of CCR2 in the pouch tissue was significantly decreased, whereas CCR5 was not appreciably changed (Figure 5B). CCR2 mRNA reached a low at 12 hours and returned to basal levels after 24 hours (Figure 5C). To investigate the possibility that the down-regulation of CCR2 expression was due to a direct effect of oxidized phospholipids on macrophages, we isolated and cultured murine bone marrow–derived macrophages. Treatment of these macrophages with OxPAPC in vitro resulted in a significant decrease in CCR2 protein surface expression (Figure 5D).
DISCUSSION
The factors that trigger monocyte recruitment and, thus, propagate chronic inflammatory processes in RA are still poorly understood. A large body of data obtained in vitro indicates that oxidized phospholipids can be regarded as triggers of the inflammatory response in the setting of chronic inflammation (15). Increased concentrations of lipid peroxidation products and antibodies against oxidized lipoproteins have been found in the synovial fluid of RA patients (32,33); however, whether oxidized phospholipids contribute to synovial inflammation and subsequent leukocyte recruitment has not previously been demonstrated. This study of the air-pouch model is the first to show that oxidized phospholipids can be regarded as propagators of macrophage accumulation in a facsimile synovial tissue.
Chemokines serve a vital role in supporting the inflammatory response in chronically inflamed tissues, including rheumatoid synovium and atherosclerotic vessels (34,35). Monocytes that express CCR2 respond to MCP-1 (CCL2), as well as MCP-3 (CCL7) and MCP-5 (CCL12), which are produced by a variety of cells during inflammation (36). Consequently, CCR2−/− mice have profound defects in monocyte recruitment (18,37). In our study, oxidized phospholipids were shown to induce the expression of MCP-1/JE (CCL2) in various cell types in vitro. Our data demonstrate that OxPAPC induced the expression of a set of chemokines in the air-pouch wall, including the CCR2 ligands CCL2 (MCP-1/JE), CCL7 (MCP-3), and CCL12 (MCP-5). Although from our results, we cannot deduce which cell type in the air-pouch lining tissue contributed to the expression of these chemokines, we hypothesized that their production would lead to CCR2-dependent monocyte recruitment. Indeed, we found that monocyte/macrophage recruitment was abrogated in CCR2-deficient mice. These results demonstrate an important role of CCR2 in oxidized phospholipid–induced inflammation, consistent with a role of CCR2 ligands in monocyte recruitment in RA (7,17).
Of the 2 distinct subpopulations of circulating monocytes in mice, Gr-1–/CCR2–/CX3CR1high monocytes can be found in normal tissues, while Gr-1+/CCR2+/CX3CR1low cells represent “inflammatory” monocytes that accumulate in inflamed tissues (38). Interestingly, Gr-1high monocytes were not found in the pouch wall after treatment with OxPAPC. It is possible that either OxPAPC does not induce the accumulation of these cells or that Gr-1high monocytes quickly convert to Gr-1low cells due to the oxidized phospholipid–rich microenvironment in the pouch wall. Future experiments will address the question of whether oxidized phospholipids induce a preferential recruitment of Ly6Chigh or Ly6Cintermediate/low monocytes.
A major difference between oxidized phospholipid–induced and LPS-induced inflammation was that inflammatory cells were absent in the air-pouch lumen in animals treated with OxPAPC. In fact, macrophages accumulated in the air-pouch wall but did not migrate into the pouch lumen. This prolonged residence of inflammatory macrophages in the tissue has important implications for the progression of chronic inflammatory tissue damage. The reasons for decreased emigration of macrophages from inflamed tissue have not yet been fully elucidated, but down-regulation of the expression of chemokine receptors such as CCR2 by microenvironmental factors may cause prolonged arrest of macrophages (39). In this context, we showed that in macrophages, the expression of CCR2, but not CCR5, was down-regulated by the direct action of oxidized phospholipids. We conclude that down-regulation of CCR2 expression on macrophages by oxidized phospholipids is at least partly responsible for the prolonged arrest of inflammatory macrophages in damaged tissue, which would contribute to the propagation of chronic inflammation.
We and other investigators have previously shown that OxPAPC selectively induces monocyte–endothelial cell interactions in vitro (12,30,40-44). This is in sharp contrast to LPS-induced endothelial cell activation, since LPS caused monocyte adhesion as well as neutrophil adhesion (45,46). There are striking differences in endothelial cell activation between oxidized phospholipids and other proinflammatory mediators, such as IL-1, tumor necrosis factor, or LPS. The latter activate the classic NF-κB pathway that leads to the expression of the endothelial adhesion molecules E-selectin, VCAM-1, or ICAM-1, resulting in adhesion of monocytes as well as neutrophils. In contrast, oxidized phospholipids stimulate endothelial cells to specifically bind monocytes, but not neutrophils, a hallmark of chronic inflammation (12,43). Recent studies indicate that activation of MAP kinases, rather than the NF-κB pathway, by oxidized phospholipids mediates the expression of inflammatory genes that leads to specific monocyte adhesion (30). Furthermore, we have also shown that OxPAPC potently inhibits LPS-induced neutrophil accumulation (46), pointing to a potential mechanism by which oxidized phospholipids may determine monocyte specificity. Indeed, when LPS and OxPAPC were coinjected into the air pouch, leukocyte accumulation in the lumen was abrogated, whereas there was still significant macrophage accumulation in the pouch wall (data not shown).
In the present study, we confirmed major differences between oxidized phospholipid–induced and LPS-induced inflammation in vivo, including differences in adhesion molecule and HO-1 expression. The relative expression of these inflammatory genes determines the type of inflammatory cell that infiltrates inflamed tissues. Our results showed that monocyte recruitment can occur in the absence of increased expression of endothelial adhesion molecules. This indicates that the constitutive expression of certain adhesion molecules (e.g., ICAM-1) and increased expression of chemokines is sufficient to promote monocyte emigration. Neutrophils, in contrast, were shown to require increased expression of endothelial adhesion molecules. In this context, it has been shown that recruitment of inflammatory monocytes does not require the prior influx of neutrophils (20).
Our results indicate that monocyte selectivity induced by oxidized phospholipids cannot solely be explained by the chemokine expression pattern, since we found that chemokines with known neutrophil-activating capacity were also induced by oxidized phospholipids. Comparing the effects of LPS and OxPAPC, selectivity for monocyte recruitment induced by OxPAPC could have been brought about by the differential expression of inflammatory genes, by differences in potency, and/or by differences in the kinetics of inflammatory gene expression during the course of the inflammatory response. While both OxPAPC and LPS induced the expression of an overlapping set of chemokines in the air-pouch tissue, we found major differences in the extent and time course of expression. Whether these differences account for selectivity in leukocyte recruitment remains to be determined.
The enzymatic activity of HO-1 has been shown to limit overshooting inflammation and to contribute to the resolution of acute inflammatory reactions (46-48). It is tempting to speculate that HO-1 contributes to the monocyte selectivity in oxidized phospholipid–induced inflammation by actively inhibiting neutrophil accumulation. Moreover, it has been reported that resistance of macrophages to apoptosis is essential for the development of RA (5). Our finding that oxidized phospholipids up-regulate the antiapoptotic enzyme HO-1 supports the concept of prolonged survival of macrophages that accumulate in inflamed synovium.
Our findings have important implications for various chronic inflammatory diseases. Atherosclerotic lesions as well as inflamed synovial tissue reflect chronic inflammatory states, which are characterized by oxidative tissue damage and specific infiltration of monocytic cells. Synovial inflammation is accompanied by angiogenesis, and macrophages and their products seem to play an important role in this process as well (49). We have recently shown that oxidized phospholipids may increase the propensity of atherosclerotic lesions to rupture by inducing angiogenesis (50). Whether oxidized phospholipids can induce angiogenesis in inflamed synovium remains to be demonstrated.
The microenvironmental factors that induce the inflammatory reactions that cause specific monocyte/macrophage accumulation in chronically inflamed tissues have not been described. Based on our data, we propose a model in which the formation of a defined subset of oxidized phospholipids in tissues leads to a persistent inflammatory response. Regulation of the expression of chemokines and their receptors, in particular of the CCL2/CCR2 axis, mediates the selective monocyte/macrophage accumulation and, thus, the propagation of chronic inflammation. To fully understand the mechanisms that are involved in oxidized phospholipid–induced chronic inflammatory processes, structure–function relationships need to be further investigated. Identification of structures as well as receptors that recognize oxidized phospholipids will provide a foundation for the development of novel therapeutic strategies for the treatment of chronic inflammatory disorders, including RA.
Acknowledgments
Dr. Kadl's work was supported by a Postdoctoral Fellowship from the Max Kade Foundation. Dr. Galkina's work was supported by a Scientist Development grant from the American Heart Association. Dr. Leitinger's work was supported by NIH grant R01-HL-084422-01.
REFERENCES
- 1.Chung CP, Oeser A, Solus J, Avalos I, Gebretsadik T, Shintani A, et al. Inflammatory mechanisms affecting the lipid profile in patients with systemic lupus erythematosus. J Rheumatol. 2007;34:1849–54. [PubMed] [Google Scholar]
- 2.Dhawan SS, Quyyumi AA. Rheumatoid arthritis and cardiovascular disease. Curr Atheroscler Rep. 2008;10:128–33. doi: 10.1007/s11883-008-0019-x. [DOI] [PubMed] [Google Scholar]
- 3.Chung CP, Avalos I, Raggi P, Stein CM. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin Rheumatol. 2007;26:1228–33. doi: 10.1007/s10067-007-0548-7. [DOI] [PubMed] [Google Scholar]
- 4.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–95. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 6.Winyard PG, Tatzber F, Esterbauer H, Kus ML, Blake DR, Morris CJ. Presence of foam cells containing oxidised low density lipoprotein in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1993;52:677–80. doi: 10.1136/ard.52.9.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quinones MP, Estrada CA, Kalkonde Y, Ahuja SK, Kuziel WA, Mack M, et al. The complex role of the chemokine receptor CCR2 in collagen-induced arthritis: implications for therapeutic targeting of CCR2 in rheumatoid arthritis. J Mol Med. 2005;83:672–81. doi: 10.1007/s00109-005-0637-5. [DOI] [PubMed] [Google Scholar]
- 8.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–81. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 9.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–7. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 10.Dai L, Lamb DJ, Leake DS, Kus ML, Jones HW, Morris CJ, et al. Evidence for oxidised low density lipoprotein in synovial fluid from rheumatoid arthritis patients. Free Radic Res. 2000;32:479–86. doi: 10.1080/10715760000300481. [DOI] [PubMed] [Google Scholar]
- 11.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 12.Berliner JA, Territo MC, Sevanian A, Ramin S, Kim JA, Bamshad B, et al. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990;85:1260–6. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadl A, Bochkov VN, Huber J, Leitinger N. Apoptotic cells as sources for biologically active oxidized phospholipids. Antioxid Redox Signal. 2004;6:311–20. doi: 10.1089/152308604322899378. [DOI] [PubMed] [Google Scholar]
- 14.Furnkranz A, Schober A, Bochkov VN, Bashtrykov P, Kronke G, Kadl A, et al. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler Thromb Vasc Biol. 2005;25:633–8. doi: 10.1161/01.ATV.0000153106.03644.a0. [DOI] [PubMed] [Google Scholar]
- 15.Bochkov VN. Inflammatory profile of oxidized phospholipids. Thromb Haemost. 2007;97:348–54. [PubMed] [Google Scholar]
- 16.Willoughby DA, Sedgwick AD, Giroud JP, Al Duaij AY, de Brito F. The use of the air pouch to study experimental synovitis and cartilage breakdown. Biomed Pharmacother. 1986;40:45–9. [PubMed] [Google Scholar]
- 17.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–61. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–9. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–35. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 21.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–7. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 22.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–18. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–82. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal–induced inflammation. Arthritis Rheum. 2005;52:2936–46. doi: 10.1002/art.21238. [DOI] [PubMed] [Google Scholar]
- 26.Scott P, Ma H, Viriyakosol S, Terkeltaub R, Liu-Bryan R. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol. 2006;177:6370–8. doi: 10.4049/jimmunol.177.9.6370. [DOI] [PubMed] [Google Scholar]
- 27.Delano DL, Montesinos MC, D'Eustachio P, Wiltshire T, Cronstein BN. An interaction between genetic factors and gender determines the magnitude of the inflammatory response in the mouse air pouch model of acute inflammation. Inflammation. 2005;29:1–7. doi: 10.1007/s10753-006-8962-6. [DOI] [PubMed] [Google Scholar]
- 28.Delano DL, Montesinos MC, Desai A, Wilder T, Fernandez P, D'Eustachio P, et al. Genetically based resistance to the anti-inflammatory effects of methotrexate in the air-pouch model of acute inflammation. Arthritis Rheum. 2005;52:2567–75. doi: 10.1002/art.21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-κB in the resolution of inflammation. Nat Med. 2001;7:1291–7. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 30.Huber J, Furnkranz A, Bochkov VN, Patricia MK, Lee H, Hedrick CC, et al. Specific monocyte adhesion to endothelial cells induced by oxidized phospholipids involves activation of cPLA2 and lipoxygenase. J Lipid Res. 2006;47:1054–62. doi: 10.1194/jlr.M500555-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Kronke G, Bochkov VN, Huber J, Gruber F, Bluml S, Furnkranz A, et al. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J Biol Chem. 2003;278:51006–14. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 32.Lourida ES, Georgiadis AN, Papavasiliou EC, Papathanasiou AI, Drosos AA, Tselepis AD. Patients with early rheumatoid arthritis exhibit elevated autoantibody titers against mildly oxidized low-density lipoprotein and exhibit decreased activity of the lipoprotein-associated phospholipase A2. Arthritis Res Ther. 2007;9:R19. doi: 10.1186/ar2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamanli A, Naziroglu M, Aydilek N, Hacievliyagil C. Plasma lipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochem Funct. 2004;22:53–7. doi: 10.1002/cbf.1055. [DOI] [PubMed] [Google Scholar]
- 34.Szekanecz Z, Kim J, Koch AE. Chemokines and chemokine receptors in rheumatoid arthritis. Semin Immunol. 2003;15:15–21. doi: 10.1016/s1044-5323(02)00124-0. [DOI] [PubMed] [Google Scholar]
- 35.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 36.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–8. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci U S A. 1997;94:12053–8. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 39.Han KH, Chang MK, Boullier A, Green SR, Li A, Glass CK, et al. Oxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptor γ. J Clin Invest. 2000;106:793–802. doi: 10.1172/JCI10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berliner J, Leitinger N, Watson A, Huber J, Fogelman A, Navab M. Oxidized lipids in atherogenesis: formation, destruction and action. Thromb Haemost. 1997;78:195–9. [PubMed] [Google Scholar]
- 41.Berliner JA, Watson AD. A role for oxidized phospholipids in atherosclerosis. N Engl J Med. 2005;353:9–11. doi: 10.1056/NEJMp058118. [DOI] [PubMed] [Google Scholar]
- 42.Berliner JA, Territo M, Almada L, Carter A, Shafonsky E, Fogelman AM. Monocyte chemotactic factor produced by large vessel endothelial cells in vitro. Arteriosclerosis. 1986;6:254–8. doi: 10.1161/01.atv.6.3.254. [DOI] [PubMed] [Google Scholar]
- 43.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, et al. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci U S A. 1999;96:12010–5. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leitinger N. Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr Opin Lipidol. 2003;14:421–30. doi: 10.1097/00041433-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Kadl A, Huber J, Gruber F, Bochkov VN, Binder BR, Leitinger N. Analysis of inflammatory gene induction by oxidized phospholipids in vivo by quantitative real-time RT-PCR in comparison with effects of LPS. Vascul Pharmacol. 2002;38:219–27. doi: 10.1016/s1537-1891(02)00172-6. [DOI] [PubMed] [Google Scholar]
- 46.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 47.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–63. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 48.Kadl A, Leitinger N. The role of endothelial cells in the resolution of acute inflammation. Antioxid Redox Signal. 2005;7:1744–54. doi: 10.1089/ars.2005.7.1744. [DOI] [PubMed] [Google Scholar]
- 49.Szekanecz Z, Gaspar L, Koch AE. Angiogenesis in rheumatoid arthritis. Front Biosci. 2005;10:1739–53. doi: 10.2741/1657. [DOI] [PubMed] [Google Scholar]
- 50.Bochkov VN, Philippova M, Oskolkova O, Kadl A, Furnkranz A, Karabeg E, et al. Oxidized phospholipids stimulate angiogenesis via autocrine mechanisms, implicating a novel role for lipid oxidation in the evolution of atherosclerotic lesions. Circ Res. 2006;99:900–8. doi: 10.1161/01.RES.0000245485.04489.ee. [DOI] [PubMed] [Google Scholar]