Platelets are formed from megakaryocytes, where they are released into the blood stream via proplatelet extensions into the blood vessel lumen.1 Being the most numerous “white blood cell” and despite lacking a nucleus, they are highly complex cells containing a surface connected canalicular and tubular system, mitochondria, granules and bioactive mediators. Platelets are well-known for their seminal role in hemostasis and thrombosis.2 Aside from these physiologic functions, new roles for platelets are being identified in unwanted thrombosis that occurs in diabetes and cancer.
Platelets have been thought of as a kind of “cellular island” owing to their traditional role as mere hemostatic plugs. However, recent studies show that platelets are much more than simple cellular elements involved in sealing wounds. They are now recognized as highly interactive cells that influence both bone marrow-derived and non-bone marrow-derived cells.2 They accomplish this by their ability to release and synthesize a host of mediators that influence other cell types. For example, platelets produce a variety of important cytokines such as TGFβ and lipid mediators such as prostaglandins that stimulate many types of cells. Platelets also express and release CD40 ligand (CD154), which can quickly activate CD40 bearing cells such as B lymphocytes, neutrophils, macrophages, dendritic cells and platelets themselves.3-5 These CD40 bearing cells then become stimulated to produce antibody in the case of B cells 6 or become activated to increase their ability to present antigen 7 (dendritic cells) or kill invading microorganisms (neutrophils).8 Thus, the “new view” of platelets is that they are much more than simple hemostatic plugs and are important inducers of immunity and inflammation.
Platelets lack a nucleus, therefore why would they have need for transcription factors? Recently, our laboratory discovered that platelets do indeed contain transcription factors, in particular peroxisome proliferator activated receptor gamma (PPARγ) 9 and its binding partner retinoic × receptor (RXR).10 There was existing clinical data showing that the use of PPARγ ligands, mainly in type-2 diabetics, lowered their blood levels of proinflammatory and prothrombotic mediators including CD40L and others.11, 12 The discovery that human platelets expressed PPARγ and that PPARγ ligands attenuated platelet activation, showed that the platelet was a previously unknown target of the thiazolidinediones (such as rosiglitazone (Avandia) and pioglitazone (Actos)). Attenuating platelet activation would then blunt the release of CD40L and other mediators. Recently, platelets were also discovered to express PPARβ.13 The fact that human platelets express PPARs and that these factors are active (but not as traditional transcription factors), opens up the possibility that other small molecules could influence platelets through PPARs.
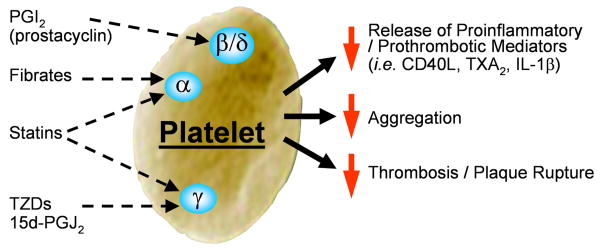
In this issue of ATVB Ali et al., uncover another facet of platelet regulation though PPARs.14 Statins are widely prescribed drugs owing to their ability to lower cholesterol levels. They also reduce the incidence of heart attack and stroke.15 Another class of drugs called fibrates, reduce triglycerides and increase high density lipoprotein cholesterol.16, 17 Both of these classes of drugs, namely statins and fibrates, appear to have more biologic effects than those originally targeted. In fact, both statins and fibrates have inhibitory activity on platelets.18, 19 Ali and colleagues reveal that statins and fibrates activate the PPAR system in platelets and this new finding helps reveal some of the pleiotropic effects of these drugs. The ability of statins, and in some cases fibrates, to lower blood levels of inflammatory mediators such as IL-6, IL-8, etc., is likely due to their influence on platelets. If, in low level inflammatory states, platelet activation is reduced, they release less CD40L, one of the major cellular stimuli capable of activating many cell types to produce proinflammatory and prothrombotic mediators.6 The figure (see diagram) shows a summary of how statins, fibrates and other molecules influence the platelet PPAR pathway and attenuate platelet activity.
Figure.
Diagram showing the PPAR targets in platelets and the consequences of engaging them with ligand. Platelets express PPAR α, β/δ and γ. Engagement of the various PPARs dampens platelet function such as release of proinflammatory/prothrombotic mediators and aggregation. The net effect is that there may be a reduction in heart attack and stroke in some patients.
The Ali and colleagues paper demonstrates that statins engage platelet PPARγ and PPARα and that the dampening activity of statins appears to be PPAR dependent.14 They also show that fibrates, through engagement of PPARα attenuate platelet activation. Interestingly, fibrates increased the bleeding time in normal, but not PPARα-/- mice. In addition to the knockout mouse approach, they also performed a small scale human study, where normal volunteers were treated with fluvastatin for 7 days and found that platelet PPARγ and PPARα were activated. Platelets from these statin treated volunteers had a reduced ability to aggregate when provoked. Some of these effects were due to activated PPAR being associated with (and possibly sequestering) PKCα, thus blunting (but not eliminating) platelet activation.
Identifying the mechanism by which statins and fibrates down-regulate platelet function further reveals the complexity of the “simple” platelet. The study also raises many questions. For example, should one expect bleeding problems in patients taking low dose aspirin plus statins and other anti-thrombotic agents such as clopidogrel (Plavix)? What about those patients also taking PPARγ ligands such as rosiglitazone (Avandia) or pioglitazone (Actos)? What might be the consequences of statin and/or fibrate use and implications for blood transfusions, which may predispose to inflammation and thrombosis? 20 Do these agents influence the ability of platelets to become subtly activated while being processed for transfusion? 4, 21 The number of drugs that attenuate platelet function is growing, which is useful, as it adds to the armamentarium of agents that can be used to treat patients.
In conclusion, the new data on statins and fibrates from the Warner laboratory is very exciting. It further reveals that traditional transcription factors such as the PPARs have complex non-genomic effects as evidenced by their function in platelets. The work also further supports the concept that the non-transcriptional roles of PPARs represent potential new therapeutic targets.
Acknowledgments
Sources of Funding: This work was supported by grants from the USPHS/NIH, HL078603, HL086367 DE011390 and ES01247.
Abbreviations
- PG
prostaglandin
- TZD
thiazolidinedione
- IL
interleukin
Footnotes
Disclosures: None
References
- 1.Geddis AE, Kaushansky K. Immunology. The root of platelet production. Science. 2007;317:1689–1691. doi: 10.1126/science.1148946. [DOI] [PubMed] [Google Scholar]
- 2.McNicol A, Israels SJ. Beyond hemostasis: the role of platelets in inflammation, malignancy and infection. Cardiovasc Hematol Disord Drug Targets. 2008;8:99–117. doi: 10.2174/187152908784533739. [DOI] [PubMed] [Google Scholar]
- 3.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 4.Phipps RP, Kaufman J, Blumberg N. Platelet derived CD154 (CD40 ligand) and febrile responses to transfusion. Lancet. 2001;357:2023–2024. doi: 10.1016/s0140-6736(00)05108-4. [DOI] [PubMed] [Google Scholar]
- 5.Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci U S A. 2003;100:12367–12371. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kehry MR. CD40-mediated signaling in B cells. Balancing cell survival, growth, and death. J Immunol. 1996;156:2345–2348. [PubMed] [Google Scholar]
- 7.van Kooten C, Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol. 1997;9:330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- 8.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;08:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akbiyik F, Ray DM, Gettings KF, Blumberg N, Francis CW, Phipps RP. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104:1361–1368. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]
- 10.Ray DM, Spinelli SL, Pollock SJ, Murant TI, O'Brien JJ, Blumberg N, Francis CW, Taubman MB, Phipps RP. Peroxisome proliferator-activated receptor gamma and retinoid × receptor transcription factors are released from activated human platelets and shed in microparticles. Thromb Haemost. 2008;99:86–95. doi: 10.1160/TH07-05-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marx N, Imhof A, Froehlich J, Siam L, Ittner J, Wierse G, Schmidt A, Maerz W, Hombach V, Koenig W. Effect of rosiglitazone treatment on soluble CD40L in patients with type 2 diabetes and coronary artery disease. Circulation. 2003;107:1954–1957. doi: 10.1161/01.CIR.0000069272.06194.91. [DOI] [PubMed] [Google Scholar]
- 12.Pfutzner A, Marx N, Lubben G, Langenfeld M, Walcher D, Konrad T, Forst T. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the pioneer study. J Am Coll Cardiol. 2005;45:1925–1931. doi: 10.1016/j.jacc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Ali FY, Davidson SJ, Moraes LA, Traves SL, Paul-Clark M, Bishop-Bailey D, Warner TD, Mitchell JA. Role of nuclear receptor signaling in platelets: antithrombotic effects of PPARbeta. Faseb J. 2006;20:326–328. doi: 10.1096/fj.05-4395fje. [DOI] [PubMed] [Google Scholar]
- 14.Ali FY, Armstrong PCJ, Dhanji AA, Tucker AT, Paul-Clark M, Mitchell JA, Warner TD. Anti-platelet actions of statins and fibrates are mediated by PPARs. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009 doi: 10.1161/ATVBAHA.108.183160. In Press. [DOI] [PubMed] [Google Scholar]
- 15.Rezaie-Majd A, Maca T, Bucek RA, Valent P, Muller MR, Husslein P, Kashanipour A, Minar E, Baghestanian M. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2002;22:1194–1199. doi: 10.1161/01.atv.0000022694.16328.cc. [DOI] [PubMed] [Google Scholar]
- 16.Staels B, Maes M, Zambon A. Fibrates and future PPARalpha agonists in the treatment of cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2008;5:542–553. doi: 10.1038/ncpcardio1278. [DOI] [PubMed] [Google Scholar]
- 17.Paumelle R, Staels B. Cross-talk between statins and PPARalpha in cardiovascular diseases: clinical evidence and basic mechanisms. Trends Cardiovasc Med. 2008;18:73–78. doi: 10.1016/j.tcm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Damas JK, Puccetti L, Aukrust P. Early anti-thrombotic and anti-inflammatory actions of statins and fibrates--time for adjuvant therapy in acute coronary syndromes? Thromb Haemost. 2005;94:1–3. doi: 10.1160/TH05-05-0317. [DOI] [PubMed] [Google Scholar]
- 19.Barter PJ, Rye KA. Cardioprotective properties of fibrates: which fibrate, which patients, what mechanism? Circulation. 2006;113:1553–1555. doi: 10.1161/CIRCULATIONAHA.105.620450. [DOI] [PubMed] [Google Scholar]
- 20.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–2381. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman J, Spinelli SL, Schultz E, Blumberg N, Phipps RP. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5:788–796. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]