Abstract
Objective
Efforts to understand specific effects of prenatal methamphetamine exposure on cognitive processing are hampered by high rates of concomitant alcohol use during pregnancy. We examined whether neurocognitive systems differed among children with differing prenatal teratogenic exposures when they engaged in a verbal memory task.
Patients and Methods
Participants (7-15 years old) engaged in a verbal paired associate learning task while undergoing functional magnetic resonance imaging. The MA group included 14 children with prenatal methamphetamine exposure, 12 of whom had concomitant alcohol exposure. They were compared to 9 children with prenatal alcohol but not methamphetamine exposure (ALC) and 20 unexposed controls (CON). Groups did not differ in age, gender, or socioeconomic status. Participants’ IQ and verbal learning performance were measured using standardized instruments.
Results
The MA group activated more diffuse brain regions, including bilateral medial temporal structures known to be important for memory, than both the ALC and the CON groups. These group differences remained after IQ was covaried. More activation in medial temporal structures by the MA group compared to the ALC group cannot be explained by performance differences because both groups performed at similar levels on the verbal memory task.
Conclusions
More diffuse activation in the MA group during verbal memory may reflect recruitment of compensatory systems to support a weak verbal memory network. Differences in activation patterns between the MA and ALC groups suggest that prenatal MA exposure influences the development of the verbal memory system above and beyond effects of prenatal alcohol exposure.
Keywords: Teratogen, cognitive, development, imaging, neurobehavioral
The effects of prenatal exposure to methamphetamine on neurocognitive skills are not well understood. Chang and colleagues documented lower scores on measures of verbal memory, spatial memory, attention, and visual motor integration in methamphetamine-exposed children compared to unexposed participants (1). However, the largest reported sample of prenatal MA exposure reported that 49% of participating methamphetamine users also used alcohol during pregnancy, and only 10% used methamphetamine alone (2). These high rates of co-exposure to alcohol during pregnancy render it difficult to separate effects of methamphetamine exposure from alcohol exposure.
Heavy prenatal alcohol exposure is known to detrimentally affect verbal memory (3). Verbal memory encompasses learning and recall of verbal information from long-term storage. Verbal learning is an important cognitive skill because it underlies basic academic skills such as vocabulary acquisition. The verbal nature of classroom instruction implicates that deficits in verbal learning may detrimentally influence achievement in other areas such as math and science. Three independent research groups have shown that children with prenatal exposure to alcohol perform more poorly on both immediate and delayed recall of verbal information compared to unexposed participants (4-6). All three studies concluded that problems of verbal memory were specific to encoding or learning of new information, and retention of information was not different between exposed and unexposed groups after accounting for initial encoding differences (4-6). Consistent with these behavioral findings, we have recently shown that unexposed control subjects activate left medial temporal structures important for verbal memory more than children with prenatal alcohol exposure during a verbal memory task using functional magnetic resonance imaging (fMRI) (7).
Examination of prenatal methamphetamine exposure presents significant challenges given that a significant number of individuals with methamphetamine exposure have concomitant alcohol exposure. For this reason, we have recently attempted to address the specificity of methamphetamine exposure on neurocognitive performance by comparing a methamphetamine plus alcohol-exposed group of children to an alcohol-exposed only group and an unexposed control group. Both exposed groups performed more poorly than the unexposed control group on measures of verbal memory; however, there was no difference between our methamphetamine plus alcohol-exposed and our alcohol exposure only groups (8). These behavioral data suggested that decreased verbal memory performance in children exposed to both methamphetamine and alcohol may be due to the effects of alcohol rather than to the effects of methamphetamine.
A limitation with behavioral performance data is that they cannot reveal whether neurocognitive systems engaged to achieve task completion are similar in children with different exposure histories. A similar level of performance can be achieved via engagement of different neurocognitive systems. For example, Brown and colleagues showed that children and adults engage different brain regions on word generation tasks despite equivalent levels of performance (9). Among atypically developing populations (e.g., dyslexia), different patterns of activation between the studied population and normally developing controls have been interpreted as effortful compensatory mechanisms and/or functional disruptions in the studied population (10, 11). To date, no studies have evaluated the effects of prenatal methamphetamine exposure on brain activation patterns.
In the present exploratory study, we asked whether children with histories of prenatal exposure to methamphetamine plus alcohol engaged different neurocognitive systems during a verbal memory task in comparison to unexposed controls and children with exposure to alcohol only. The alcohol-exposed group is a necessary control given that the majority of our methamphetamine-exposed participants also have concomitant alcohol exposure. The term Fetal Alcohol Spectrum Disorders (FASD) has been proposed to capture the continuum of effects attributable to prenatal alcohol exposure (12, 13). We followed the Washington diagnostic system (12), under which the FASD umbrella term includes Fetal Alcohol Syndrome (FAS) and Partial Fetal Alcohol Syndrome (PFAS) on the more severe end of the spectrum where facial dysmorphology is prominent. Those who do not meet criteria for FAS or PFAS have less prominent facial dysmorphology and physical evidence of brain damage, and have been referred to as Alcohol-Related Neurobehavioral Disorder (ARND). Individuals in the ARND category also show impaired cognitive performance across multiple domains, suggesting that alcohol teratogenesis on brain development exists in the absence of facial dysmorphology (14). In order to minimize the possibility that heavy alcohol exposure effects may mask more subtle effects of methamphetamine exposure, we excluded from both the methamphetamine-exposed and the alcohol-exposed only groups those who met criteria for FAS and PFAS, the most severe manifestations of prenatal alcohol exposure. If methamphetamine has a specific effect on the development of the verbal memory system or interacts with alcohol in influencing the development of this system, then the methamphetamine-exposed children are expected to show a different pattern of activation during our verbal memory task compared to both the unexposed and the alcohol-exposed only groups.
METHODS
Participants
Study participants were categorized into three groups based on exposure history, which was obtained from a detailed interview with the biological or adoptive parent, medical record, or adoption records. Participants were included in the methamphetamine group (MA) if they had prenatal exposure to methamphetamine based on positive toxicology or parent report (n=14). Twelve of the 14 children in the MA group also had reported prenatal alcohol exposure comparable to the alcohol-exposed only group (ALC). Exclusion criteria for the study included: 1) concomitant diagnosis of FAS or PFAS, which are the most severe manifestations of heavy alcohol exposure and may mask more subtle effects of methamphetamine exposure; 2) known prenatal exposure to cocaine or opiates; 3) age less than 7 years; 4) IQ less than 70, which may preclude understanding of instructions for the imaging task; 5) head injury with loss of consciousness over 20 minutes; 6) physical (e.g., hemiparesis), psychiatric illness, or developmental disability (e.g., autism) that would preclude participation; 7) other potential known causes of mental deficiency (e.g., chromosomal disorders); 8) significant maternal illness that has increased risk for fetal hypoxia (e.g., sickle cell disease); and 9) presence of implanted metal in the body which poses a risk for MRI.
Participants in the ALC group (n = 9) had exposure to four or more drinks per occasion at least once per week or 14 drinks or more per week, and were not exposed to methamphetamine during gestation. Other exclusionary criteria were exactly the same as the MA group, including the criterion that there was no concomitant diagnosis of FAS or PFAS. Non-exposed controls (CON; n=20) were excluded if they had exposure to more than two drinks on any occasion or to an average of one drink or more per week. Other exclusionary criteria were exactly the same as the other groups.
The MA group was recruited from several sources: 1) Older children of women referred from a rehabilitation program (serving women with infants born positive for methamphetamine, n=4); 2) a social skills training group at UCLA serving children with prenatal alcohol spectrum disorders (n=7); and 3) self-referred via advertisement and word-of-mouth (n=3). The ALC group was recruited primarily from the same social skills training group as described above. The CON group was recruited from the same Los Angeles communities as the exposed groups. Efforts to recruit CON participants from lower socioeconomic levels to match that of the exposed groups included recruiting participants’ playmates/classmates, bulk mail advertisements, magazine advertisements, and flyers distributed to local libraries. A total of 68 subjects were recruited. Reasons for subsequent exclusion included: concomitant diagnosis of PFAS or FAS (n=11), lack of fetal alcohol spectrum disorder diagnosis in methamphetamine-exposed participants (n=3), prenatal exposure to cocaine in addition to methamphetamine (n=1), no imaging data for the PAL task (MA n=3; CON n=2), poor image quality due to movement (MA n=2, CON n=2), and subsequent diagnosis of a CON participant with Attention-Deficit/Hyperactivity Disorder.
Procedures
All participants and their parents gave informed assent/consent according to procedures approved by the UCLA Institutional Review Board. Children underwent individual neuropsychological testing including assessments of general intellectual functioning (Wechsler Intelligence Scale for Children, 4th Edition (WISC-4)(15)) and verbal memory abilities (California Verbal Learning Test for Children (CVLT-C)(16)). In preparation for the functional imaging task (described below), participants were administered a pre-scanning paired associate learning task composed of word-pair sets at three levels of difficulty (easy, medium and hard) based on published word association rankings (17). The median score from typically developing children was chosen as the cutoff score to determine whether the easier or more difficult version of the task were administered in the scanner. This cutoff score was applied to the present study participants’ based on pre-scanning paired associate learning performance. This was done to minimize the potential confound that group differences in activation may be due to group differences in task performance. 78% of the ALC, 71% of the MA, and 45% of the CON received the easy-medium version, while remaining subjects received the medium-hard version during scanning. Parents underwent a detailed interview regarding the participant’s prenatal exposure to substances, developmental milestones, neurological and psychiatric history, and family history. Parent intelligence (IQ) was estimated with the Wechsler Abbreviated Scale of Intelligence (WASI)(18)
Functional imaging task: Paired Associate Learning (PAL)
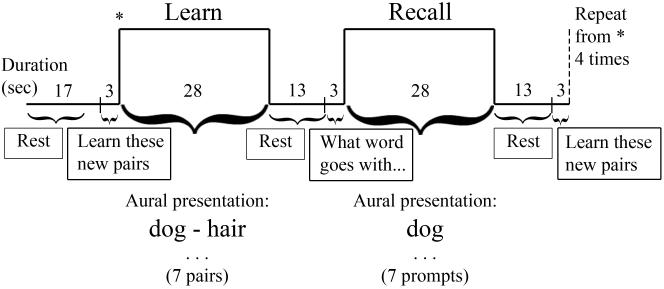
Figure 1 depicts our Paired Associate Learning task. Participants listened to seven novel pairs of words during each learning block. Word pairs were presented four seconds apart, with approximately two seconds of silence between pairs. During the recall block, the first word of each word pair (prompt) was presented at four-second intervals, and participants were instructed to think of the associated word after each prompt. The order of the two word pair sets (easy-medium or medium-hard) was counterbalanced across participants and repeated once for a total of four learning-recall block sets. The task lasted 6 minutes 12 seconds. Participants were informed that they would be asked to recall the word pairs after scanning, and verbal memory performance was tested post-scanning by presenting the prompts and asking participants to recall the word associated with each prompt.
Figure 1.
Paired Associate Learning (PAL) task.
Image Acquisition
fMRI data were collected on a 3 Tesla Siemens Allegra head-only magnet. Multi-slice echo-planar imaging (EPI) was used with a gradient echo EPI sequence. We used TR=2s, TE=25ms, 3mm slice thickness with 1mm skip, 36 slices, 64×64 pixels yielding 3.1mm in-plane resolution with whole-brain acquisition. A high resolution T2-weighted EPI volume was collected in the anterior commissure-posterior commissure plane coplanar with the functional scan to allow for spatial registration of each subject’s data into a standard coordinate space (TR=5000ms, TE=33ms, flip angle=90°, 3mm slice thickness with 1mm skip, 36 axial slices covering the entire brain, matrix size=128×128 with 1.6×1.6mm in-plane resolution).
Image Analysis
Functional imaging data were evaluated using FSL (FMRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl/index.html) (19). Preprocessing included the following: Motion correction (MCFLIRT tool in FSL (20)), slice timing correction for interleaved acquisition, smoothing (6 mm FWHM Gaussian kernel), and high pass filtering (60s). Single subject functional data was registered to his/her own structural data using a 6-parameter transformation, then to the MNI-152 standard space template with a 12-parameter transformation (20, 21).
Single subject image data were carried out using FMRIB’s fMRI Expert Analysis Tool (FEAT, version 5.63). Learning and recall conditions of each difficulty level were modeled separately and convolved with a canonical hemodynamic response function. Volumes that required more than 2 mm correction for motion were modeled as a covariate of no interest. Statistical analysis of the time series data was carried out using FMRIB’s Improved Linear Model, which applies a voxel-wise general linear model so that each voxel’s time course was individually fitted to the model with local autocorrelation correction (22). Verbal learning activation was derived by collapsing all learning and recall block activation compared against activation during the rest period. Higher-level group analysis was carried out using FMRIB’s Local Analysis of Mixed Effects. Z statistic images for group differences in the (Learning+Recall)-Rest contrast were thresholded using clusters determined by Z > 1.7 and a corrected cluster significance threshold of p = 0.05 (see (23, 24) for details of this method for correcting for multiple comparisons).
Statistical Analysis of Behavioral Data
Analyses were conducted on age-corrected standardized scores based on procedures provided by each publisher (WISC-4, CVLT-C, WASI). Raw scores were evaluated for the Paired Associate Learning task. Group differences for integer variables (e.g., age) were evaluated with analysis of variance (ANOVA). Only if this omnibus group difference was significant were independent samples t statistics applied. Group differences in categorical (e.g., gender) and rank data (e.g., income) were assessed with Kruskal-Willis ANOVA. Group differences for categorical variables (e.g., adoptive family) were assessed with Kolmogorov-Smirnov test. While no functional MRI studies of prenatal methamphetamine exposure have been reported in the literature from which to estimate effect sizes, expected effect size of verbal learning difference between the alcohol-exposed and nonexposed participants was large (4, 5), suggesting relatively small samples would yield significant group effects.
RESULTS
Demographic descriptors and behavioral performance are reported in Table 1. Groups did not differ from each other in age, gender distribution, handedness, or socioeconomic status as estimated from parental IQ, parental years of education, and family annual income. The groups differed in Full Scale IQ (F(2,40)=8.04, p<0.01), with the CON group scoring higher than the MA and ALC groups, but there was no difference between the MA and ALC groups. Despite efforts to equate verbal learning performance during imaging, group differences were still present on the post-scan recall task (F(2,39)=4.80, p=0.01). The CON group learned more word pairs than the MA group, but the MA and ALC groups’ recall of word pairs learned in the scanner did not differ from each other. This pattern was similar to the pattern of performance on the CVLT-C (16). Group differences (F(2,40)=7.54, p<0.01) were characterized by the CON group recalling more words compared to the two exposed groups, but the MA and ALC groups did not differ from one another.
Table 1.
Demographics and performance for each group (mean and standard deviation)
|
MA (n=14) |
ALC (n=9) |
CON (n=20) |
Group differences | |
|---|---|---|---|---|
| Age (standard deviation; range) | 9.50 (1.91; 7-15) |
11.33 (2.65; 7-13) |
10.30 (2.56; 7-15) |
None |
| M:F ratio | 10:4 | 5:4 | 9:11 | None |
| Handedness (right [100] to left [-100]) | 65.38 (54.87) |
57.50 (63.64) |
69.65 (34.37) |
None |
| FSIQ | 95.00 (14.95) |
86.67 (14.42) |
110.10 (16.79) |
CON>MA (t(32)=2.70, p=0.01) CON>ALC (t(27)=3.62, p<0.01) |
| Parent IQ | 111.71 (13.22) |
113.33 (8.69) |
108.83 (14.87) |
None |
| Parent Education (years)* | 15.93 (2.84) |
17.00 (1.73) |
15.80 (3.19) |
None |
| Family Annual Income** | 7.21 (2.12) |
6.67 (2.40) |
6.67 (2.87) |
None |
| % Adopted | 85 | 89 | 0 | MA>CON (D=0.86, p<0.01) ALC>CON (D=0.89, p<0.01) |
| % Nicotine-exposed (% unknown) | 43 (50) | 22 (78) | 0 (0) | MA>CON (D=0.86, p<0.01) ALC>CON (D=1.0, p<0.01) |
| PAL post-scan recall score† (0-14) | 4.71 (4.34) |
7.25 (4.65) |
9.40 (4.22) |
CON>MA (t(32)=3.15, p<0.01) |
| CVLT-C SDFR Z score*** (mean=0, SD=1) | -0.71 (1.12) |
-0.33 (1.22) |
0.60 (0.80) |
CON>MA (t(32)=3.99, p<0.01) CON>ALC (t(27)=2.45, p=0.02) |
More than 18 years of education were coded as 19.
Categorical variable: 1=<$5,000, 2=$5,000-9,999, 3=$10,000-19,999, 4=$20,000-29,999, 5=$30,000-39,999, 6=$40,000-49,999, 7=$50,000-74,999, 8=$75,000-100,000, 9=>$100,000.
PAL=Paired Associate Learning.
CVLT-C SDFR=California Verbal Learning Test, Children’s Version, Short Delay Free Recall.
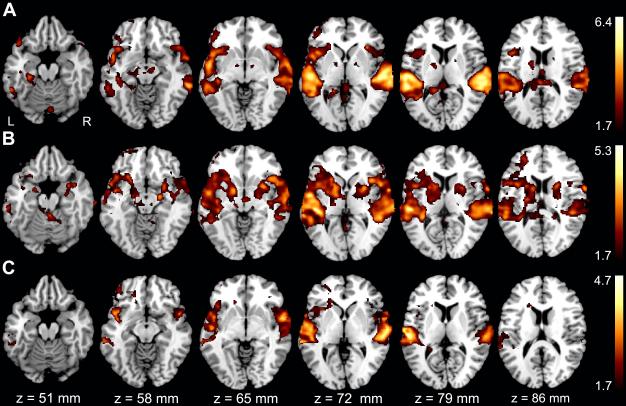
Figure 2 depicts each group’s activation during the verbal memory task ((Learning+Recall)-Rest contrast). Qualitative comparison shows that all groups activated bilateral superior temporal and inferior frontal regions consistent with the processing of aural verbal stimuli. The MA group showed bilateral activation of the medial temporal structures important for memory, and only the CON group showed lateralized activation of the left medial temporal region for our verbal memory task.
Figure 2.
Group activation for the (Learning+Recall)-Rest contrast. A CON group. B MA group. C ALC group. The color bars on the right code for Z statistics of significant clusters thresholded at p≤0.05. Z axis values on the bottom correspond to MNI standard space coordinates.
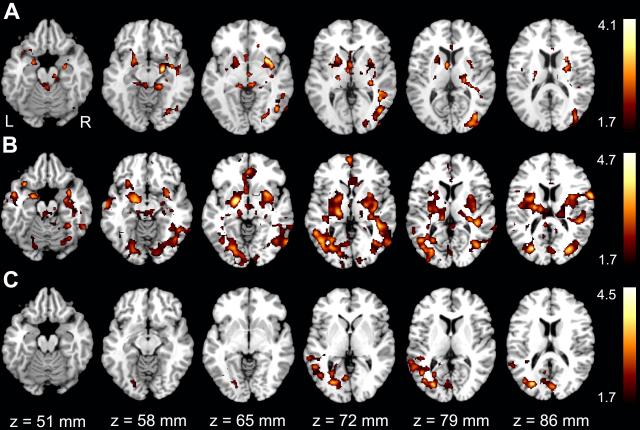
Results of group differences in activation are shown in Figure 3. To account for IQ differences between the CON and exposed groups, Full Scale IQ was entered as a covariate in the group contrasts. After controlling for IQ, the MA group showed greater activation than the CON group in bilateral medial temporal, bilateral basal ganglia, and right occipito-temporal regions during verbal learning and recall (Fig 3a). The ALC group showed less activation than unexposed individuals in the left occipito-temporal region (Fig 3c).
Figure 3.
Group differences in the (Learning+Recall)-Rest contrast with IQ regressed out. A MA>CON, B MA>ALC, C CON>ALC. The color bars on the right code for Z statistics of significant clusters thresholded at p≤0.05. Z axis values on the bottom correspond to MNI standard space coordinates.
The group difference of primary interest was between the MA and ALC groups. Compared to the ALC group, the MA group showed greater activation in diffuse brain regions including bilateral medial temporal structures, basal ganglia, occipito-temporal, parietal, and frontal regions (Fig 3b). Methamphetamine-exposed participants recruited more diffuse brain regions to achieve a similar level of performance on a verbal memory task relative to participants with prenatal alcohol exposure alone. These results remained virtually identical even after excluding the two participants with only MA exposure to rule out the possibility that participants with only MA exposure have very discrepant activation patterns from those with concomitant MA and alcohol exposure (data not shown). We also covaried for post-scan recall, which yielded similar results to Figure 3b (data not shown), which gave us confidence that activation differences between the MA and ALC groups seen in Figure 3b were not completely explained by potential differences in verbal learning performance.
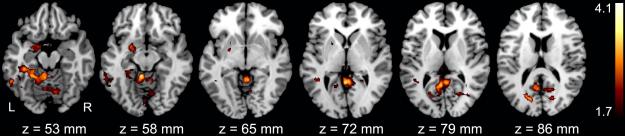
To better understand what activation patterns are associated with enhanced performance, we correlated activation ((Learning+Recall)-Rest)) with verbal memory performance (CVLT-C Short Delay Free Recall (SDFR) raw score) in the CON group. Results showed that better recall was associated with greater activation in the left medial temporal structures in unexposed participants (Fig 4). Activation of the medial temporal structures was not correlated with performance in the exposed groups.
Figure 4.
Correlation between performance (CVLT-C SDFR raw score) and activation ((Learning+Recall)-Rest contrast) was significant in left medial temporal structures for the CON group only. The color bar codes for Z statistics of significant clusters thresholded at p≤0.05. Z axis values on the bottom correspond to MNI standard space coordinates.
Because substantial development occurs between ages 7 and 15, and activation patterns change with age (9, 26-31), we evaluated for group differences in activation pattern while covarying for age. Results were similar to those in Figure 3, suggesting that age differences within groups did not completely account for activation differences seen between groups (data not shown). We also covaried for gender in a separate analysis to evaluate the possibility that a disproportionately higher number of males in the MA group may have influenced the results of the activation differences between groups. Results were very similar to those reported in Figure 3 (data not shown), suggesting that group differences in activation exist above and beyond effects of gender.
DISCUSSION
We examined whether prenatal exposure to methamphetamine affects the development of neurocognitive systems important for verbal memory relative to prenatal exposure to alcohol alone. Our exposed groups performed similarly to each other on verbal memory tasks, and were both impaired relative to their non-exposed counterparts. Because verbal memory difficulty in alcohol-exposed children is well documented, one possible interpretation of this similarity in performance may be that methamphetamine does not affect neurocognitive system development above and beyond the effects of alcohol alone. However, we demonstrated that despite similarities in performance, different patterns of brain activation emerged between the MA and ALC groups. Participants with prenatal methamphetamine exposure showed greater activation of diffuse brain regions during a verbal memory task, including medial temporal structures known to be important for verbal memory. This difference in activation pattern suggests that prenatal exposure to methamphetamine (with or without alcohol exposure) appears to influence activation of the verbal memory system differently than alcohol exposure alone.
More diffuse activation during the verbal memory task in the MA group may be comparable to findings in normal development that activation patterns change as cognitive skills mature (9, 27, 28). It has been proposed that the diffuse to local shift in activation from childhood to adulthood (26, 29-31) may reflect fine tuning of relevant neural systems (27, 28). That is, better performance is associated with focalization of activation as neural efficiency increases with maturation. In this view, activation in the methamphetamine-exposed participants may reflect an immature neural system where more diffuse networks are recruited to compensate for an inefficient verbal memory system. This interpretation is consistent with the observation that among the unexposed participants, greater activation of the left mesial temporal structures is positively correlated with better verbal memory performance; better learning is linked to greater activation of verbal memory structures in normally developing children. Methamphetamine-exposed participants may need to allocate more diffuse resources to achieve the same level of medial temporal lobe function and to compensate for a less efficient verbal memory network.
The difference in activation patterns between the MA and ALC groups highlights that functional imaging may yield more information about the integrity of neurocognitive networks than evaluations at the behavioral level alone. The MA group activated medial temporal structures important for verbal memory more than the ALC group, even though performance and IQ between these groups did not differ. A lack of medial temporal activation during our verbal memory task characterizes the ALC group, which replicates our previous finding (7), while diffuse activation characterizes the MA group. We have no reason to suspect a lack of effort in the ALC group as a factor in explaining their lack of activation, especially given that their performance did not reach floor levels on verbal memory tasks tested inside and outside the scanner. More likely, prenatal exposure to alcohol affected the verbal memory system in ways that render it more difficult to become engaged in an effective manner. Prenatal exposure to MA influenced this system differently, such that although engaged, it was not effective. Although the explanation for activation differences remains speculative, evidence of different patterns of neurocognitive engagement during verbal memory suggest that methamphetamine exposure during the in utero period influences development in ways that differ from alcohol alone.
Clinically, it may be tempting to consider prenatal methamphetamine and alcohol exposure as not different from prenatal alcohol exposure alone, given that these groups did not differ on behavioral measures of verbal memory performance, and both are likely to require more attention from educators and parents compared to nonexposed children to ensure that they benefit from traditional classroom instruction. However, the current data suggest that children with different exposure histories may have different physiological etiologies leading to a weakened verbal memory system, or that they may compensate differently. These differences may affect verbal learning under conditions of stress, difficulty level of the academic work, or distractions in the environment. Actual ability to benefit from classroom instruction requires interaction of the verbal learning system with other cognitive and emotional systems. Because children with prenatal methamphetamine and alcohol exposure may have different underlying verbal learning physiology than children with alcohol exposure alone, actual ability to learn in real-life situations may differ depending on exposure history.
There are several limitations to the current study; therefore, these results should be considered preliminary and interpreted with caution. First, quantities and frequencies of drug exposure are difficult to accurately recall years after the drug use. Recall issues are further compounded by guilt about substance use during pregnancy among biological mothers, and uncertain quantities used when women receive methamphetamine from their partners. These issues among biological mothers are of sufficient concern that reports from adoptive mothers (based on observation of biological mother’s behavior or from social services reports) may have similar levels of validity to that of biological mothers. Second, the majority of our MA-exposed participants also had concomitant alcohol exposure. The possibility that the activation pattern observed in the MA group is related to an interaction of methamphetamine with alcohol rather than to methamphetamine alone cannot be ruled out. However, this reflects a more realistic sample of methamphetamine users as most pregnant users co-expose the fetus to alcohol. A related issue is concomitant nicotine exposure. Based on known patterns of drug use in the general population, it is suspected that many participants in our exposed groups were also exposed to tobacco and marijuana. Nicotine exposure is of particular concern because in animal models, it has been shown to disrupt the timing of cell replication and differentiation, and such effects are longer lasting than effects from fetal cocaine exposure (32). Unfortunately, unavailability of nicotine-exposure information from a significant proportion of our exposed groups renders it difficult to evaluate if the rate of nicotine exposure in the MA and ALC groups differ. Third, our sample sizes in the exposed groups were relatively small. Estimated power to detect difference in verbal learning performance between groups ranged from 0.4 to 0.78 (the range is due to different number of subjects in each group). Clearly, our sample sizes were sufficient to detect the group differences in activation reported here, but power varies by brain region and cognitive task evaluated. It is possible that we did not have enough power to detect difference between groups on verbal learning performance. We covaried for verbal learning performance and have confidence that performance does not completely account for activation differences between the MA and ALC group. However, it remained possible that insufficient power precluded us from detecting subthreshold activation in the ALC group. Finally, we did not assess for drug or alcohol use in the participants, nor did we measure pubertal status biologically. Thus, we cannot rule out the possibility that brain activation of some subjects may be influenced by drug use.
In conclusion, we showed that differences in activation during verbal memory are evident despite similar levels of performance and IQ between methamphetamine-exposed and alcohol-exposed children. Effects of in utero methamphetamine exposure on neurocognitive development in humans have been difficult to establish due to high rates of concomitant alcohol use. By comparing methamphetamine-exposed participants (with concomitant alcohol exposure) to an alcohol-only exposed group as well as an unexposed group, we were able to demonstrate that methamphetamine affects the verbal memory system differently than prenatal alcohol exposure alone.
Acknowledgment
This work was supported by National Institute of Drug Abuse Grants R21 DA15878 and R01 DA017831 and the March of Dimes ((5FY03-12) awarded to ERS. Additional support was provided by the National Center on Research Resources, General Clinical Research Center (3 M01 RR00425) awarded to LMS and National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 RR021813 entitled Center for Computational Biology (CCB; http://nihroadmap.nih.gov/bioinformatics).
Abbreviations
- (MA)
Methamphetamine
- (FAS)
Fetal Alchohol Syndrome
- (PFAS)
Partial Fetal Alcohol Syndrome
- (FSIQ)
Full Scale Intelligence Quotient
Footnotes
Financial Disclosure: None
Conflict of Interest: None
References
- 1.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132(2):95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Derauf C, LaGasse LL, Smith LM, Grant P, Shah R, Arria A, et al. Demographic and psychosocial characteristics of mothers using methamphetamine during pregnancy: preliminary results of the infant development, environment, and lifestyle study (IDEAL) Am J Drug Alcohol Abuse. 2007;33(2):281–9. doi: 10.1080/00952990601175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230(6):357–65. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 4.Kaemingk KL, Mulvaney S, Halverson PT. Learning following prenatal alcohol exposure: performance on verbal and visual multitrial tasks. Arch Clin Neuropsychol. 2003;18(1):33–47. [PubMed] [Google Scholar]
- 5.Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26(6):875–82. [PubMed] [Google Scholar]
- 6.Willford JA, Richardson GA, Leech SL, Day NL. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcohol Clin Exp Res. 2004;28(3):497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]
- 7.Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ, et al. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. Neuroreport. 2007;18(7):635–639. doi: 10.1097/WNR.0b013e3280bad8dc. [DOI] [PubMed] [Google Scholar]
- 8.Lu LH, Houston SM, Nunez C, O’Hare ED, Bookheimer SY, Smith LM, et al. Preliminary neuropsychological effects of prenatal methamphetamine and alcohol exposure in children. under submission. [Google Scholar]
- 9.Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15(3):275–90. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- 10.Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke’s Wortschatz? Brain. 1999;122(Pt 10):1901–17. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- 11.Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci U S A. 1998;95(5):2636–41. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol Alcohol. 2000;35(4):400–10. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- 13.Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12(1):146–53. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- 15.Wechsler D. Wechsler Intelligence Scale for Children. Fourth ed. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- 16.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, Children’s Version. The Psychological Corporation; San Antonio, TX: 1994. [Google Scholar]
- 17.Palermo DS, Jenkins JJ. Word Association Norms: Grade School Through College. University of Minnesota Press; Minnesota: 1964. [Google Scholar]
- 18.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- 19.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 22.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 23.Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 24.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second ed. Lawrence Erlbaum Associates, Publishers; Hillsdale, NJ: 1988. The Analysis of Variance and Covariance. [Google Scholar]
- 26.Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, et al. Neural development of selective attention and response inhibition. Neuroimage. 2003;20(2):737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- 27.Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44:2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 29.Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54(1):180–5. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- 30.Sherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. Journal of Cognitive Neuroscience. 2006;18(7):1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- 31.Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, et al. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10(3 Pt 1):327–38. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- 32.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285(3):931–45. [PubMed] [Google Scholar]