Abstract
Microtubule-binding drugs (MBDs) are widely used in cancer chemotherapy and also have clinically relevant anti-angiogenic and vascular-disrupting properties. These anti-vascular actions are due in part to direct effects on endothelial cells, and all MBDs (i.e. both microtubule-stabilizing and -destabilizing) inhibit endothelial cell proliferation, migration, and tube formation in vitro, actions which are thought to correspond to therapeutic anti-angiogenic actions. In addition, the microtubule destabilizing agents cause prominent changes in endothelial cell morphology, an action associated with rapid vascular collapse in vivo. The effects on endothelial cells occur in vitro at low drug concentrations which do not affect microtubule gross morphology, do not cause microtubule bundling or microtubule loss, and do not induce cell cycle arrest, apoptosis, or cell death. Rather it has been hypothesized that at low concentrations, MBDs produce more subtle effects on microtubule dynamics, block critical cell signaling pathways, and prevent the microtubules from properly interacting with transient sub-cellular assemblies (focal adhesions and adherens junctions) whose subsequent stabilization and/or maturation are required for cell motility and cell-cell interactions. This review will focus on recent studies to define the molecular mechanisms for the anti-vascular actions of the microtubule-binding drugs, information which could be useful in the identification or design of agents whose actions more selectively target the tumor vasculature.
Microtubule-binding drugs have antitumor and anti-vascular actions
Microtubule-binding drugs (MBDs) have documented anti-tumor activity and are widely used in curative and palliative cancer chemotherapeutic regimens. Some MBDs are well established clinically and are active against solid tumors, including breast, lung, prostate and ovarian cancers (taxol and taxotere), and leukemias and lymphomas (vinblastine and vincristine) (1). Newer MBDs are in phase II and III clinical trials, where they have shown activity against refractory or advanced carcinomas of the breast, lung, prostate, ovary and thyroid (epothilones and combretastains) (1, 2). The large number of ongoing trials which include a MBD make it likely that their clinical spectrum of activity will continue to expand.
Microtubules are key components of the cytoskeleton, and are composed of heterodimers of α- and β-tubulin (proteins of approximately 50 kDa molecular weight) which assemble into linear, hollow, cytoplasmic filaments. MBDs can be broadly classified by their effect on microtubule polymer stability (i.e. they can promote polymerization or depolymerization), or based on their binding site on microtubules (i.e. to the Vinca, colchicine or taxane binding domains). All classes of MBDs, i.e. those which stabilize microtubules and stimulate their polymerization (e.g. taxanes, epothilones), and those which destabilize microtubules and cause their depolymerization (e.g. colchicine, vinca alkaloids, combretastatins), can interfere with mitotic spindle formation in tumor cells, block proliferation by cell cycle arrest, and cause cell death via the induction of apoptosis, actions which undoubtedly contribute to their clinical activity (1).
More recent studies have shown that most MBDs also have anti-angiogenic or vascular-disrupting activities or both; in this review these will be referred to collectively as anti-vascular effects (Table 1). Targeting of the tumor vasculature as a therapeutic approach has a compelling theoretical rationale, is strongly supported by pre-clinical studies, and has been validated by clinical trials (34). There is now data to suggest that MBDs would be a particularly useful class of drugs for this purpose, most notably, evidence that MBDs have multiple direct actions on endothelial cells (discussed in detail below), and that they can produce a much greater reduction in blood flow in tumors than in normal tissues (35). A critical unanswered question is whether the anti-vascular actions of the MBDs contribute to their clinical efficacy, relative to their direct cytotoxic actions against tumor cells. In this regard, there is data from mouse models to suggest that the anti-vascular actions of the MBDs are therapeutically important (36). In this study, taxotere was found to retain much of its antitumor activity against an ovarian cancer xenograft tumor that had been established by inoculating mice bearing taxotere-resistant ovarian cancer cells (36). Since these cells were partially or completely resistant to the cytotoxic actions of taxotere, the antitumor effect was attributed to the anti-angiogenic actions of taxotere that were observed in these tumors (36). This conclusion is in agreement with similar observations made in experiments done by treating mice with cyclophosphamide-resistant lung cancers with cyclophosphamide, using different doses and schedules (37). Indeed taxol, vinblastine and epothilone are prominent among the “cytotoxic” chemotherapeutic agents that have pronounced anti-angiogenic actions when administered by a metronomic schedule (38).
Table 1.
Microtubule-binding drugs with anti-vascular actions
| Agents which stimulate microtubule polymerization and increase their stability | Clinical Status | References |
|---|---|---|
| Paclitaxel (Taxol) | Approved | 3–8 |
| Docetaxel (Taxotere) | Approved | 5–6, 9–11 |
| Epothilones (Ixabepilone, Patupiline, KOS862) | Phase III | 6, 12–13 |
| Laulimalide | 14 | |
| IDN 5390 | 15 | |
| Agents which destabilize microtubules and cause their depolymerization | ||
| Colchicine | Non-neoplastic use | 6, 16–18 |
| Combretastatins (Zybrestat, AVE8062, OXI4503) | Phase II | 19–22 |
| 2-methoxyestradiol (Panzem) | Phase II | 4, 18, 23 |
| JG-03-14 | 24 | |
| N-Acetylcolchinol (ZD6126) | 25–27 | |
| Vinblastine (Velban) | Approved | 6, 17, 28–29 |
| Vincristine (Oncovin) | Approved | 29 |
| Vinflunine | Phase III | 30–31 |
| Tubulysin A | 32 | |
| XRP44X | 33 | |
Direct anti-proliferative effects of MBDs on endothelial cells are likely only partly responsible for their anti-vascular actions
It is not surprising that at appropriate concentrations, MBDs are cytotoxic toward endothelial cells and can inhibit their proliferation (3, 4, 9, 18, 19, 28). In fact, in some instances, endothelial cells are more sensitive to growth inhibition by MBDs than are cancer cell lines or other primary cells, an observation that may be due in part to the ability of endothelial cells to accumulate certain MBDs intracellularly at levels more than five-times higher than other cell types (28, 39 40). While the inhibition of proliferation and induction of apoptosis undoubtedly contribute to the anti-vascular effects of the MBDs, recent reports indicate that these drugs have other actions on endothelial cells that are more specifically related to the neovascularization process and the maintenance of the microvasculature. A number of the in vitro anti-vascular actions occur at MBD concentrations that are substantially lower than those required to block mitosis, produce cell cycle arrest or to induce apoptosis (9, 18, 28, 41). Similar observations have been made in vivo: a hallmark of vascular-disrupting MBDs such as the combretastatins is the induction of a rapid collapse in tumor blood flow, often first detected within five minutes of drug treatment in animal models and leading to complete vascular shutdown by 20 minutes (42). Tumor imaging studies have identified similar effects in phase I clinical trials of these agents (Table 2). Drug-induced effects on endothelial cell proliferation and/or endothelial cell apoptosis occur too slowly to account for these anti-vascular actions in vivo; rather morphological and functional changes in the endothelial cells are more likely to cause the tumor vasculature collapse (35, 40).
Table 2.
Vascular response measurements from phase I-pharmacodynamic clinical trials of combretastatin A4 phosphate (CA4P).
| CA4P Dose Range (10 min iv infusion) | Vascular Parameter | Measurement Timesa | Effects Observedb (p value) | Ref |
|---|---|---|---|---|
| 5–114 mg/m2 | Blood flowc | Pre, 0.5 & 24 hrs post | Mean changes: Tumor: −49% (0.001) Spleen: −35% (0.018) Kidney: −6% (0.026) |
43 |
| 5–114 mg/m2 | Blood volumed | Pre, 0.5 & 24 hrs post | Mean changes: Tumor: −15% (0.007) Spleen: −18% (0.022) Kidney: −6% (0.020) |
43 |
| 20–114 mg/m2 | Perfusione | Pre, 4 & 24 hrs post | Mean changes: Tumor: −37% (0.002) Muscle: ns Kidney: −2% (ns) |
44 |
| 52–75 mg/m2 | Perfusione | Pre and 6–8 hrs post | Tumor: Of 10 patients, 5 had modest and 3 had marked decreases Tumor: Of 21patients, 12 | 45 |
| 40–114 mg/m2 | Perfusione | Pre and 4 hrs post | had decreases ≥ 20%, of which 3 were significant | 46 |
Relative to CA4P administration.
Data for earliest post-CA4P measurement time shown. In all cases, the magnitude of change was reduced at later time points.
ml blood/ml tissue/min. 15O-water positron emission tomography (PET).
15O-carbon monoxide, PET.
Tumor perfusion is a measure of the tissue blood flow rate and the permeability of the vasculature wall. Measured by the transfer rate/min, Ktrans, using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI).
ns, not significant.
Microtubule-binding drugs inhibit multiple functions of endothelial cells
Tumor neovascularization occurs primarily (but not exclusively) by the sprouting of established capillaries. This process involves several steps, beginning with the cell-mediated proteolytic degradation of the surrounding basement membrane, the migration of endothelial cells out of an existing vessel into the surrounding extracellular matrix (ECM), the proliferation of the endothelial cells, and the organization and morphogenesis of the cells into tube-like structures (47). Aspects of these processes can be studied in tissue culture; thus, when plated on ECM, endothelial cells form structures which morphologically resemble capillary-like vessels, with lumens and an anastomosing and branching tubular network (48–49). MBDs potently inhibit vessel formation in vitro, and this is likely due to their ability to disrupt one or more of the individual components of the process, including endothelial cell adhesion, migration, and cell-cell interactions (3, 5–6, 9–11, 14–15, 21, 25, 28, 30, 41). MBDs have anti-vascular effects in vivo as well, blocking angiogenesis in assays utilizing subcutaneous matrigel plugs, chick embryo chorioallantoic membranes, and corneal micropockets; they also have anti-vascular effects in tumor xenografts (9–10, 19, 21, 26, 29, 41, 50). In vivo they can produce morphologic alterations of the neovasculature, increase tumor vascular permeability, and can cause near complete vascular shutdown (19–27). As noted above, many of these inhibitory effects are observed at MBD concentrations that may be 100-fold lower than those required to produce cell toxicity (3–5, 8–9 15, 18, 25, 28). For example, docetaxel inhibits endothelial cell proliferation in vitro with reported IC50s ranging from 5 to 21 nM, but inhibits endothelial cell tube formation at IC50s of 0.3 to 0.8 nM and cell migration with an IC50 of 0.01 nM (6, 10, 14). Similar results, although less pronounced, were observed with combretastatin, where the inhibition of endothelial cell migration and tube formation occurred at concentrations that were 8 to 16-fold lower than those which inhibited endothelial cell proliferation (41).
What actions of the MBDs might be responsible for these effects on endothelial cells? It has been known for some time that MBDs such as colchicine, nocodazole, vinblastine and paclitaxel can interfere with the migration of fibroblasts, monocytes, and carcinoma cells (50–55). These early reports, in which the MBDs caused near complete microtubule breakdown, concluded that the drugs’ actions were due to the loss of the ability of cells to polarize their actin activity, thereby preventing cell motion (54, 56). As microtubules play a critical role in regulating the organization of actin into stress fibers, agents which interfere with microtubule function can also cause the loss of cell polarity, interfere with the formation of cell protrusions such as lamellipodium, and interfere with cell contractility (54). However, the high concentrations of MBDs used in these early studies make it unclear whether these mechanisms are relevant to the actions of the drugs at concentrations that occur clinically. More recent studies have identified novel additional actions of the MBDs that occur at lower concentrations, and therefore could contribute to any clinical anti-vascular actions of the MBDs. These include the blockade of critical cell signaling pathways (including from the VEGF receptor) and the interference with the proper functioning of transient sub-cellular assemblies (focal adhesions and adherens junctions) whose subsequent stabilization and/or maturation are required for cell adhesion, cell motility and cell-cell interactions (11, 14, 20, 21).
MBDs target focal adhesions and adherens junctions in endothelial cells
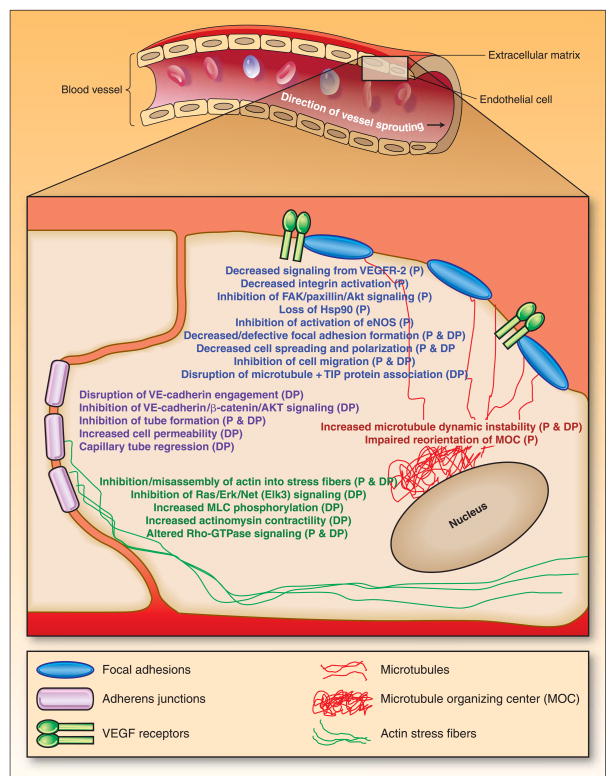
MBDs have been reported to have effects on focal adhesions and adherens junctions in endothelial cells, as well as on related pathways (Figure 1). When stimulated to migrate, endothelial cells become elongated and polarized (they develop a front and back), with lamellipodium at their leading edge and a trailing cell body (57). The highly dynamic lamellipodium form new contacts with the underlying extracellular matrix (ECM), a process mediated by focal adhesions and more specifically, by the binding of cell surface integrins to the ECM (57). Focal adhesions are transient self-assembling protein complexes that are located at the cell surface and which serve as links to the intracellular cytoskeleton. The microtubule-stabilizing and destabilizing MBDs caused decreased focal adhesion formation or defective focal adhesion assembly, respectively, in endothelial cells (11, 14, 20, 31). The microtubule-stabilizing MBDs blocked integrin activation and inhibited downstream signaling from the integrins along the FAK-paxillin-Akt pathway (11, 14). Inhibition of multiple Hsp90-dependent signaling pathways, including reduced activation of endothelial nitric oxide synthase (eNOS), was also observed. Interestingly, the microtubule-destabilizing MBDs appear not to affect FAK activation (24). They have been shown, however, to inhibit of Ras-Erk-Net signaling, and to increase myosin light chain phosphorylation and actinomysin contractility (20, 33).
Figure 1. Effects of microtubule-binding drugs on endothelial cells which could contribute to their anti-angiogenic and vascular-disrupting actions.
The effects of drugs which enhance polymerization (P) (e.g. paclitaxel, docetaxel, epothilones) or cause depolymerization (DP) (colchicine, cobretastatins, vincristine) of the microtubules are listed. Abbreviations used are: eNOS, endothelial nitric oxide synthetase; MOC, microtubule organizing center; MLC, myosin light chain.
The homotypic interaction of endothelial cells is critical for both vessel formation in sprouting angiogenesis, and for the maintenance of vasculature integrity in established capillaries (58). Endothelial cell engagement with other endothelial cells is mediated in part by adherens junctions, which like the focal adhesions, are cell surface protein complexes. Adherens junctions are primarily formed by the cadherin adhesion proteins (predominantly VE-cadherin in endothelial cells), and other proteins which contribute to their function, including α- and β-catenins, Arp2/3, afadin, p120, plakoglobin, α-actinin, and vinculin (58). Recent reports suggest that the microtubule-destabilizing agents can disrupt VE-cadherin engagement and inhibit signaling along the VE-cadherin/β-catenin/AKT pathway (21, 24). Disruption of adherens junctions contributes to the rounding-up of endothelial cells, which in vivo would lead to a direct increase in the geometric resistance to blood flow in capillaries and an increase in vasculature permeability. This would cause an acute leakage of plasma proteins from the vasculature, reducing the oncotic pressure difference between the inside and outside of the vessel and increasing the interstitial fluid pressure, subsequently contributing to vascular shutdown (26, 59).
Is there a unifying mechanism for these cellular effects of the MBDs? As described above, the integrins and VE-cadherin play central roles in endothelial cell attachment, migration, and capillary tube formation and maintenance, and they share a number of similar properties: they provide a direct connection to the actin cytoskeleton, the driving force for cell motility and which also help to stabilize adherens junctions; they mediate signaling across the cell membrane and activate downstream signaling pathways; and they link these processes to the actions of cell surface receptors which regulate angiogenesis, notably those for VEGF and FGF (57, 58, 60). In this regard it is noteworthy that both classes of MBDs are able to block signaling from the VEGF receptors, although in the case of microtubule-stabilizing agents, this may be mainly directed toward VEGF-mediated effects on focal adhesions, while the adherens junctions may be the predominant target of the microtubule-destabilizing drugs. The actions of VEGF receptors are interconnected with those of the integrins and VE-cadherin, and the formation of complexes between VEGF receptor-2 and either an integrin or VE-cadherin appears to be important for the optimal outside-in and/or inside-out signaling of all three proteins. Thus it is possible that MBDs have one or more cellular actions which ultimately prevent the endothelial cell from responding to and integrating multiple extracellular signals, whether they emanate from, or lead to the activation of, a VEGF receptor, an integrin, or VE-cadherin.
Can the disruption of microtubule functions account for the non-cytotoxic, anti-vascular effects of MBDs?
As discussed above, microtubules play a critical role in mitotic spindle function and mitosis, providing an attractive target for drug development for proliferating cells. These agents also affect the microtubule network in non-proliferating and interphase cells. The microtubules in endothelial cells in interphase are dynamic polarized structures, and during cell migration, the fast growing plus ends of the microtubule polymer is targeted to and captured by the forming focal adhesions, while the stable minus ends are localized to the microtubule organizing center (MOC, also known as the centrosome), a structure typically found at a perinuclear position in cells (61). While the role of microtubules in focal adhesion function is not fully understood, they have been shown to participate in the control of integrin clustering and avidity, actions associated with integrin activation and which allow their high affinity binding to ECM ligands (62). Less is known about the role of the microtubules in adherens junctions, although it has been suggested that protein regulators of the microtubule cytoskeleton might localize at adherens junctions (63). In this regard, a recent study found that low non-cytotoxic concentrations of vinflunine disrupted the localization of the protein EB1 at the microtubule plus ends (31). EB1 belongs to the family of microtubule (+) end-tracking proteins (+TIPs) which mediate many microtubule-regulated actions, including cell migration, the targeting and capture of microtubules at adhesion sites, and the stabilization of microtubules at the cell cortex (64, 65). Further studies are needed to confirm this observation, and to assess whether the function of other +(TIPS) regulatory proteins are altered by changes in microtubule dynamics due to MBDs.
An early event in directed cell migration that is dependent on the maintenance of microtubule plasticity is the reorientation of the MOC towards the side of the nucleus in the intended direction of movement (66). Extension of a new lamellipod in a migrating cell precedes MOC reorientation to the new leading edge of the cell. If MOC reorientation does not occur, the new lamellipod is retracted (66). Thus, although MOC reorientation does not direct cell motion, it has been postulated to be required for the maintenance of leading lamellipodia and for stabilizing a chosen direction of movement. Low concentrations of docetaxel have been reported to impair MOC reorientation in endothelial cells stimulated to undergo migration (9).
As noted above, the plus ends of microtubules exhibit dynamic instability (random changes between periods of growth and shortening), and this dynamic instability is essential for migration of some cell types. It may also contribute to the interactions between the microtubule and actin microfilament systems that are critical for cell migration (1, 67). The reorganization of actin into stress fibers and many aspects of cell locomotion are regulated by members of the Rho family of small GTPases. Thus Cdc42 and Rac1 induce formation of filopodia and lammelipodia, respectively, RhoA has been shown to regulate the formation of actin stress fibers and focal adhesions, and the reorientation of the MOC has been reported to depend upon the activation of Cdc42 (68). While the Rho proteins are regulated by a number of different upstream factors, direct evidence for microtubule-dependent regulation of Rho GTPases has come from biochemical studies which found that the depolymerization of microtubules resulted in an increase in the level of GTP-bound RhoA, whereas polymerization of microtubules resulted in activation of Rac1 (67–68).
The linkage between microtubule dynamic instability, and Rho GTPase regulation and actin reorganization is likely central to understanding how specific MBDs affect endothelial cell function. The predominant cellular actions of both classes (i.e. stabilizing and destabilizing) of MBDs can vary dramatically depending upon the concentration at which they are studied. While at high micromolar concentrations, the microtubule stabilizing and destabilizing agents have quite different phenotypic effects on microtubules and on cells in vitro, at lower anti-proliferative concentrations, both classes inhibit microtubule dynamics; this ultimately interferes with mitotic spindle function and leads to cell cycle arrest and/or apoptosis (1). Paradoxically, it has been reported that at even lower concentrations, MBDs can actually increase microtubule dynamics (7, 30, 31). These intriguing studies had several noteworthy aspects: increased microtubule dynamic instability was observed at drug concentrations that were 50 to 100-fold lower than those which inhibited endothelial cell proliferation, the effect was selective for endothelial cells and was not observed in tumor cells, and it was observed with both microtubule-stabilizing (taxol) and destabilizing (vinflunine) agents (7, 30). Consistent with these observations, both polymerizing and depolymerizing MBDs have been reported to cause altered Rho-GTPase signaling, including activation of RhoA and inhibition of Rac1 and cdc42, and to produce an inhibition, stimulation, and/or misassembly of actin into stress fibers and focal adhesions (6, 20, 69). These observations raise the possibility that at some level, the microtubule stabilizing and destabilizing classes of agents share similar or overlapping molecular mechanisms of action. It should be noted that different MBDs varied in the nature of their specific effects on the Rho-signaling pathway. Interestingly, direct disruption of actin microfilaments in endothelial cells by cytocholasin B had a much weaker effect on microvessel structure than did the MBDs (70).
In addition to being required for cell signaling and migration, microtubules are also involved in the intracellular transport of proteins and vesicles, in the maintenance of the composition of the plasma membrane, in the regulation of cell shape, and in the development of cell polarization (9, 20, 21, 25, 69, 71). The disruption of one or more of these by MBDs could interfere with the formation and maintenance of the tumor vasculature.
Do differences in the location and consequences of the binding of a specific agent to tubulin influence their effect on endothelial cell function?
Despite the similarities in cellular actions noted above, studies to date have tended to differentiate the two classes of MBDs based on their predominant anti-vascular actions, with the microtubule-stabilizing agents having anti-angiogenic actions that occur at concentrations well below their cytotoxic IC50s, versus the microtubule destabilizing drugs which more prominently cause vascular collapse in vivo, generally at concentrations that are closer to those that at which they are cytotoxic. As noted above, however, the microtubule destabilizing drugs are also potent inhibitors of endothelial cell migration and tube formation, and thus are likely to also be anti-angiogenic. Thus while the classification of an agent as either anti-angiogenic or vascular-disrupting is somewhat arbitrary, these distinctions may be helpful in discerning either broad patterns of anti-vascular effects, or as a way of categorizing therapeutically-relevant drug actions on endothelial cells.
A related question is whether differences in the nature of the binding of the MBDs can be exploited in future drug development to design agents which have the specific desired anti-vascular actions. As noted above, both classes of MBDs can cause an increase in microtubule dynamics; however differences between them have been noted. Thus while both the stabilizing agent taxol and the destabilizing agent vinflunine were associated with increased microtubule growth, increased shortening rates, and decreased pause times, only taxol also altered the time-based microtubule transition frequency (7, 30). Differences in the microtubule binding site can also influence selected molecular actions on endothelial cells. The phosphorylation of Net (a transcription factor involved in angiogenesis) was strongly inhibited in endothelial cells by combretastatin-A4, but only weakly blocked by vincristine and not at all by taxotere (33). Laulimalide, which stabilizes microtubules in manner indistinguishable from docetaxel but binds to a site distinct from that of the taxanes, was much more potent in blocking the phosphorylation of paxillin than was taxotere in endothelial cells (14). Whether these and other potential subtle differences impact the broader anti-vascular actions of these agents is not known.
Future microtubule-binding drug development
If the salient mechanistic aspects of the anti-vascular actions of the MBDs could be determined with some degree of confidence, these could then be used in structure-activity analyses to search for links to the way in which different MBDs interact with microtubules in endothelial cells. A practical consideration in the identification of therapeutically-useful compounds would be the selection of appropriate and progressively biologically-complex drug screening approaches. Most of the studies discussed in this review have examined the effects of the MBDs on cultured endothelial cells, either primary HUVEC or HMVEC (human microvascular endothelial cells) or immortalized lines derived from them. There is little data to indicate which, if any, of these cell types would be the most appropriate for evaluating the actions of the MBDs on the tumor vasculature. Interestingly, endothelial precursor cells (EPC) derived in vitro from human AC133+/CD34+ bone marrow progenitor cells, have been found to express genes that were more similar to those expressed by endothelial cells isolated from fresh surgical specimens of human tumors, when compared to HUVEC or HMVEC, suggesting that these cells could be valuable models (72). Assessment of drug effects on the architecture of vessel formation and maintenance, utilizing for example in vitro and in vivo Matrigel tubule assays, are likely to be particularly important for evaluating the effects of the MBDs. Nascent tumor vessels are often deficient in pericyte coverage, and these vessels have been found to be more sensitive to the disrupting effects of combretastatin than were more mature, pericyte-ensheathed, vessels (pericytes themselves were found to be resistant to combretastatin, relative to endothelial cells) (21). This study suggests that MBDs could have some selectivity for tumor vs. normal vasculature. Ultimately this hypothesis will have to be tested using pharmacodynamic measurements of tumor vascular parameters in pre-clinical and clinical studies.
Hundreds of analogs of MBDs have been synthesized and studied in detail in cell-free and cellular assays, and as would be expected, varying chemical substitutions affect the nature of the interaction of the agents with tubulin and microtubules, leading to wide variations of potencies in tubulin polymerization and depolymerization assays (73–75). However, for both agents which stabilize and destabilize microtubules, attempts to correlate the effects on microtubule polymerization with inhibition of proliferation or cytotoxicity are often not successful (73–74). One might anticipate that the potencies to produce cytotoxicity, which presumably incorporates any differences in the uptake, metabolism, non-specific binding, etc., of the agents in intact cells, could be used as a surrogate for their anti-vascular effects. Limited data, however, would suggest that this is not the case. In a study of over fifty 2-aroylindole derivatives which bind to and destabilize microtubules, there seemed to be little relationship between the IC50 to inhibit the proliferation of multiple cancer cell lines, and the anti-vascular activity in a CAM assay (75). Thus while we are far from understanding the relationship between specific effects on microtubules and anti-vascular activity, this study does support the premise that the anti-vascular effects of the MBDs can be separated from their cytotoxic actions. Identification of microtubule-binding drugs with greater therapeutic anti-vascular selectivity, relative to their cancer cell cytotoxicity, would be an important objective for the next generation of MBDs.
Acknowledgments
Grant support: This work was supported by NIH grants RO1 CA 98456 and RO1 CA 89352 from the National Cancer Institute.
Footnotes
Translational Relevance
Pre-clinical and clinical studies have found that microtubule-binding drugs have therapeutically relevant anti-angiogenic and vascular-disrupting actions. While the cellular and molecular mechanisms for these actions are not fully understood, they likely include direct effects of the drugs on the endothelial cells which form tumor blood vessels. Identification of the salient cellular and molecular actions of the microtubule-binding drugs which are responsible for their anti-vascular actions would be an important objective of future studies. This information could be incorporated into screens to isolate or design microtubule-binding drugs whose actions more selectively target the tumor vasculature, relative to their anti-mitotic and pro-apoptotic effects on cancer cells. In addition to their potential clinical value as cancer chemotherapeutic drugs, such agents could be used to determine the extent to which the anti-vascular actions of the microtubule-binding drugs contribute to their anti-tumor efficacy.
References
- 1.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Reviews Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 2.Rowinsky EK, Calvo E. Novel agents that target tubulin and related elements. Semin Oncol. 2006;33:421–35. doi: 10.1053/j.seminoncol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Belotti D, Vergani V, Drudis T, et al. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res. 1996;2:1843–9. [PubMed] [Google Scholar]
- 4.Klauber N, Parangi S, Flynn E, Hamel E, D’Amato RJ. Inhibition of angiogenesis and breast cancer in mice by the microtubule inhibitors 2-methoxyestradiol and taxol. Cancer Res. 1997;57:81–6. [PubMed] [Google Scholar]
- 5.Grant DS, Williams TL, Zahaczewsky M, Dicker AP. Comparison of the antiangiogenic activities using paclitaxel (taxol) and docetaxel (taxotere) Int J Cancer. 2003;104:121–9. doi: 10.1002/ijc.10907. [DOI] [PubMed] [Google Scholar]
- 6.Bijman MNA, van Nieuw Amerongen GP, Laurens N, van Hinsbergh VWM, Boven E. Microtubule-targeting agents inhibit angiogenesis at subtoxic concentrations, a process associated with inhibition of Rac1 and Cdc42 activity and changes in the endothelial cell cytoskeleton. Molec Cancer Ther. 2006;5:2348–57. doi: 10.1158/1535-7163.MCT-06-0242. [DOI] [PubMed] [Google Scholar]
- 7.Pasquier E, Honore S, Pourroy B, et al. Antiangiogenic concentrations of paclitaxel induce an increase in microtubule dynamics in endothelial cells but not in cancer cells. Cancer Res. 2005;65:2433–40. doi: 10.1158/0008-5472.CAN-04-2624. [DOI] [PubMed] [Google Scholar]
- 8.Naumova E, Ubezio P, Garofalo A, et al. The vascular targeting property of paclitaxel is enhanced by SU6668, a receptor tyrosine kinase inhibitor, causing apoptosis of endothelial cells and inhibition of angiogenesis. Clin Cancer Res. 2006;12:1839–49. doi: 10.1158/1078-0432.CCR-05-1615. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss KA, Ashton AW, Mahmood R, Russell RG, Sparano JA, Schwartz EL. Inhibition of endothelial cell function in vitro and angiogenesis in vivo by docetaxel (taxotere): association with impaired repositioning of the microtubule organizing center. Molec Cancer Ther. 2005;1:1191–200. [PubMed] [Google Scholar]
- 10.Sweeny CJ, Miller KD, Sissons SE, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–72. [PubMed] [Google Scholar]
- 11.Murtagh J, Lu H, Schwartz EL. Taxotere-induced inhibition of human endothelial cell migration is a result of heat shock protein 90 degradation. Cancer Res. 2006;66:8192–9. doi: 10.1158/0008-5472.CAN-06-0748. [DOI] [PubMed] [Google Scholar]
- 12.Bocci G, Nicolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002;62:6938–43. [PubMed] [Google Scholar]
- 13.Lee FYF, Covello KL, Castaneda S, et al. Synergistic antitumor activity of ixabepilone (BMS-247550) plus bevacizumab in multiple in vivo tumor models. Clin Cancer Res. 2008;14:8123–31. doi: 10.1158/1078-0432.CCR-08-0025. [DOI] [PubMed] [Google Scholar]
- 14.Lu H, Murtagh J, Schwartz EL. The microtubule binding drug laulimalide inhibits vascular endothelial growth factor-induced human endothelial cell migration and is synergistic when combined with docetaxel (taxotere) Mol Pharmacol. 2006;69:1207–15. doi: 10.1124/mol.105.019075. [DOI] [PubMed] [Google Scholar]
- 15.Taraboletti G, Micheletti G, Rieppi M, et al. Antiangiogenic and antitumor activity of IDN 5390, a new taxane derivative. Clin Cancer Res. 2002;8:1182–8. [PubMed] [Google Scholar]
- 16.Ettenson DS, Gotlieb AI. Centrosomes, microtubules, and microfilaments in the reendothelialization and remodeling of double-sided in vitro wounds. Lab Invest. 1992;6:722–33. [PubMed] [Google Scholar]
- 17.Baguley BC, Holdaway KM, Thomsen LL, Zhuang L, Zwi LJ. Inhibition of growth of colon 38 adenocarcinoma by vinblastine and colchicine: evidence for a vascular mechanism. Eur J Cancer. 1991;27:482–7. doi: 10.1016/0277-5379(91)90391-p. [DOI] [PubMed] [Google Scholar]
- 18.Stafford SJ, Schwimer J, Anthony CT, Thomson JL, Wang YZ, Woltering EA. Colchicine and 2-methoxyestradiol inhibit human angiogenesis. J Surg Res. 2005;125:104–8. doi: 10.1016/j.jss.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Dark GG, Hill SA, Prise VE, Tozer GM, Pettit GR, Chaplin DJ. Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res. 1997;57:1829–34. [PubMed] [Google Scholar]
- 20.Kanthou C, Tozer GM. The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells. Blood. 2002;99:2060–9. doi: 10.1182/blood.v99.6.2060. [DOI] [PubMed] [Google Scholar]
- 21.Vincent L, Kermani P, Young LM, et al. Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J Clin Invest. 2005;115:2992–3006. doi: 10.1172/JCI24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hori K, Saito S. Microvascular mechanisms by which the combretastatin A-4 derivative AC7700 (AVE8062) induces tumour blood flow stasis. Br J Cancer. 2003;89:1334–44. doi: 10.1038/sj.bjc.6601261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mooberry SL. New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Current Opin Oncol. 2003;15:425–430. doi: 10.1097/00001622-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Dalyot-Herman H, Delagado-Lopez F, Gewirtz DA, Upton JG, Schwartz EL. The novel microtubule-binding substituted pyrrole, JG-03–14, is a potent anti-vascular agent. Submitted. [Google Scholar]
- 25.Micheletti G, Poli M, Borsotti P, Martinelli M, Imberti B, Taraboletti G, Giavazzi R. Vascular-targeting activity of ZD6126, a novel tubulin-binding agent. Cancer Res. 2003;63:1534–7. [PubMed] [Google Scholar]
- 26.Blakey DC, Westwood FR, Walker M, Hughes GD, Davis PD, Ashton SE, Ryan AJ. Antitumor activity of the novel vascular targeting agent ZD6126 in a panel of tumor models. Clin Cancer Res. 2002;8:1974–83. [PubMed] [Google Scholar]
- 27.Davis PD, Dougherty GJ, Blakey DC, et al. ZD6126: a novel vascular-targeting agent that causes selective destruction of tumor vasculature. Cancer Res. 2002;62:7247–53. [PubMed] [Google Scholar]
- 28.Vacca A, Iuralaro M, Ribatti D, et al. Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood. 1999;94:4143–55. [PubMed] [Google Scholar]
- 29.Hill SA, Lonergan SJ, Denekamp J, Chaplin DJ. Vinca alkaloids: anti-vascular effects in a murine tumour. Eur J Cancer. 1993;29A:1320–4. doi: 10.1016/0959-8049(93)90082-q. [DOI] [PubMed] [Google Scholar]
- 30.Pourroy B, Honoré S, Pasquier E, et al. Antiangiogenic concentrations of vinflunine increase the interphase microtubule dynamics and decrease the motility of endothelial cells. Cancer Res. 2006;66:3256–63. doi: 10.1158/0008-5472.CAN-05-3885. [DOI] [PubMed] [Google Scholar]
- 31.Honoré S, Pagano A, Gauthier G, et al. Antiangiogenic vinflunine affects EB1 localization and microtubule targeting to adhesion sites. Mol Cancer Ther. 2008;7:2080–9. doi: 10.1158/1535-7163.MCT-08-0156. [DOI] [PubMed] [Google Scholar]
- 32.Kaur G, Hollingshead M, Holbeck S, et al. Biological evaluation of tubulysin A: a potential anticancer and antiangiogenic natural product. Biochem J. 2006;396:235–42. doi: 10.1042/BJ20051735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasylyk C, Zheng H, Castell C, Debussche L, Multon MC, Wasylyk B. Inhibition of the Ras-Net (Elk-3) pathway by a novel pyrazole that affects microtubules. Cancer Res. 2008;68:1275–83. doi: 10.1158/0008-5472.CAN-07-2674. [DOI] [PubMed] [Google Scholar]
- 34.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature Reviews Cancer. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 35.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nature Reviews Cancer. 2005;5:423–35. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 36.Kamat AA, Kim TJ, Landen CN, Jr, et al. Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res. 2007;67:281–8. doi: 10.1158/0008-5472.CAN-06-3282. [DOI] [PubMed] [Google Scholar]
- 37.Browder T, Butterfield CE, Kräling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–86. [PubMed] [Google Scholar]
- 38.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 39.Merchan JR, Jayaram DR, Supko JG, He X, Bubley GJ, Sukhatme VP. Increased endothelial uptake of paclitaxel as a potential mechanism for its antiangiogenic effects: potentiation by Cox-2 inhibition. Int J Cancer. 2005;113:490–8. doi: 10.1002/ijc.20595. [DOI] [PubMed] [Google Scholar]
- 40.Tozer GM, Kanthou C, Parkins CS, Hill SA. The biology of the combretastatins as tumour vascular targeting agents. Int J Exp Pathol. 2002;83:21–38. doi: 10.1046/j.1365-2613.2002.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed B, Van Eijk LI, Bouma-Ter Steege JC, et al. Vascular targeting effect of combretastatin A-4 phosphate dominates the inherent angiogenesis inhibitory activity. Int J Cancer. 1993;105:20–5. doi: 10.1002/ijc.11010. [DOI] [PubMed] [Google Scholar]
- 42.Tozer GM, Prise VE, Wilson J, et al. Mechanisms associated with tumor vascular shutdown induced by combretastatin A-4 phosphate: intravital microscopy and measurement of vascular permeability. Cancer Res. 2001;61:6413–22. [PubMed] [Google Scholar]
- 43.Anderson HL, Yap JT, Miller MP, Robbins A, Jones T, Price PM. Assessment of pharmacodynamic vascular response in a phase I trial of combretastatin A4 phosphate. J Clin Oncol. 2003;21:2823–30. doi: 10.1200/JCO.2003.05.186. [DOI] [PubMed] [Google Scholar]
- 44.Galbraith SM, Maxwell RJ, Lodge MA, et al. Combretastatin A4 phosphate has tumor antivascular activity in rat and man as demonstrated by dynamic magnetic resonance imaging. J Clin Oncol. 2003;21:2831–42. doi: 10.1200/JCO.2003.05.187. [DOI] [PubMed] [Google Scholar]
- 45.Stevenson JP, Rosen M, Sun W, et al. Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol. 2003;21:4428–38. doi: 10.1200/JCO.2003.12.986. [DOI] [PubMed] [Google Scholar]
- 46.Gaya A, Daley F, Taylor NJ, et al. Relationship between human tumour angiogenic profile and combretastatin-induced vascular shutdown: an exploratory study. Br J Cancer. 2008;99:321–6. doi: 10.1038/sj.bjc.6604426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 48.Bach TL, Barsigian C, Chalupowicz DG, et al. VE-cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels. Exptl Cell Res. 1998;238:324–34. doi: 10.1006/excr.1997.3844. [DOI] [PubMed] [Google Scholar]
- 49.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–98. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldman RD. The role of three cytoplasmic fibers in BHK-12 cell motility. I. Microtubules and the effect of colchicine. J Cell Biol. 1971;51:752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasiliev JM, Gelfand IM, Domnina LV, Ivanova OY, Komm SG, Olshevkaya LV. Effect of colcemid on the locomotory behavior of fibroblasts. J Embryol Exp Morphol. 1970;24:625–640. [PubMed] [Google Scholar]
- 52.Schliwa M, Euteneuer U, Graf R, Ueda M. Centrosomes, microtubules and cell migration. Biochem Soc Symp. 1998;65:223–31. [PubMed] [Google Scholar]
- 53.Zakhireh SH, Malech H. The effect of colchicine and vinblastine on the chemotactic response of human monocytes. J Immunol. 1980;125:2143–2153. [PubMed] [Google Scholar]
- 54.Liao G, Nagasaki T, Gundersen GG. Low concentrations of nocodazole interfere with fibroblast locomotion without significantly affecting microtubule level: implications for the role of dynamic microtubules in cell locomotion. J Cell Sci. 1995;108:3473–83. doi: 10.1242/jcs.108.11.3473. [DOI] [PubMed] [Google Scholar]
- 55.Stearns ME, Wang M. Taxol blocks processes essential for prostate tumor cell invasion and metastases. Cancer Res. 1992;52:3776–3781. [PubMed] [Google Scholar]
- 56.Singer SJ, Kupfer A. The directed migration of eukaryotic cells. Ann Rev Cell Biol. 1986;2:337–65. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- 57.Lauffenberger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 58.Vesteber D. VE-cadherin. The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223–32. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- 59.Milosevic MF, Fyles AW, Hill RP. The relationship between elevated interstitial fluid pressure and blood flow in tumors: a bioengineering analysis. Int J Radiat Oncol Biol Phys. 1999;43:1111–23. doi: 10.1016/s0360-3016(98)00512-4. [DOI] [PubMed] [Google Scholar]
- 60.Byzova TV, Goldman CK, Pampori N, et al. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–60. [PubMed] [Google Scholar]
- 61.Schliwa M, Euteneuer U, Graf R, Ueda M. Centrosomes, microtubules and cell migration. Biochem Soc Symp. 1998;65:223–231. [PubMed] [Google Scholar]
- 62.Zhou X, Li J, Kucik DF. The microtubule cytoskeleton participates in control of β2 integrin avidity. J Biol Chem. 2001;276:44762–69. doi: 10.1074/jbc.M104029200. [DOI] [PubMed] [Google Scholar]
- 63.Gates J, Peifer M. Can 1000 reviews be wrong? Actin, α-catenin, and adherens junctions. Cell. 2005;123:769–72. doi: 10.1016/j.cell.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 64.Manna T, Honnappa S, Steinmetz MO, Wilson L. Suppression of microtubule dynamic instability by the +TIP protein EB1 and its modulation by the CAP-Gly domain of p150glued. Biochemistry. 2008;47:779–86. doi: 10.1021/bi701912g. [DOI] [PubMed] [Google Scholar]
- 65.Lansbergen G, Akhmanova A. Microtubule plus end: a hub of cellular activities. Traffic. 2006;7:499–507. doi: 10.1111/j.1600-0854.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 66.Ueda M, Graf R, MacWilliams HK, Schliwa M, Euteneuer U. Centrosome positioning and directionality of cell movements. Proc Natl Acad Sci (USA) 1997;94:9674–78. doi: 10.1073/pnas.94.18.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waterman-Storer CM, Salmon ED. Positive feedback interactions between microtubule and actin dynamics during cell motility. Current Opinion Cell Biology. 1999;11:61–7. doi: 10.1016/s0955-0674(99)80008-8. [DOI] [PubMed] [Google Scholar]
- 68.Wittmann T, Waterman-Storer CM. Cell motility: Can Rho GTPases and microtubules point the way? J Cell Science. 2001;114:3795–3803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- 69.Hamm-Alvarez SF, Alayof BE, Himmel HM, et al. Coordinate depression of bradykinin receptor recycling and microtubule-dependent transport by taxol. Proc Natl Acad Sci U S A. 1994;91:7812–6. doi: 10.1073/pnas.91.16.7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bayless KJ, Davis GE. Microtubule depolymerization rapidly collapses capillary tube networks in vitro and angiogenic vessels in vivo through the small GTPase Rho. J Biol Chem. 2004;79:11686–95. doi: 10.1074/jbc.M308373200. [DOI] [PubMed] [Google Scholar]
- 71.Liu SM, Magnusson KE, Sundqvist T. Microtubules are involved in transport of macromolecules by vesicles in cultured bovine aortic endothelial cells. J Cell Physiol. 1993;156:311–6. doi: 10.1002/jcp.1041560213. [DOI] [PubMed] [Google Scholar]
- 72.Bagley RG, Walter-Yohrling J, Cao X, et al. Endothelial precursor cells as a model of tumor endothelium: characterization and comparison with mature endothelial cells. Cancer Res. 2003;63:5866–73. [PubMed] [Google Scholar]
- 73.Ganesh T, Yang C, Norris A, et al. Evaluation of the tubulin-bound paclitaxel conformation: synthesis, biology, and SAR studies of C-4 to C-3′ bridged paclitaxel analogues. J Med Chem. 2007;50:713–25. doi: 10.1021/jm061071x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA. Medicinal chemistry of combretastatin A4: present and future directions. J Med Chem. 2006;49:3033–44. doi: 10.1021/jm0512903. [DOI] [PubMed] [Google Scholar]
- 75.Mahboobi S, Pongratz H, Hufsky H, et al. Synthetic 2-aroylindole derivatives as a new class of potent tubulin-inhibitory, antimitotic agents. J Med Chem. 2001;44:4535–53. doi: 10.1021/jm010940+. [DOI] [PubMed] [Google Scholar]