Abstract
Background
Renal damage caused by cold preservation and warm reperfusion has been well documented and involves tissue edema, cell swelling, ATP depletion, calcium toxicity, and oxidative stress. However, more common proximal mechanisms have not been identified, which may limit the development of effective clinical treatment strategies. Previous work indicates that many cytoskeletal structures are affected by cold preservation and reperfusion, including membrane rich ezrin associated complexes. The aim of this study was to investigate whether the sub-lamellar cytoskeletal protein ezrin is causally involved in cold preservation injury in renal tubule epithelial cells.
Methods
We created a stably transfected cell Line [LLC-EZ] using the pig kidney proximal tubular epithelial cell line [LLC-PK1], which constitutively over-expresses wild-type ezrin. These cells were cold stored in UW solution and reperfused in-vitro to model renal tubule preservation injury, which was assessed by biochemical, metabolic, functional, and structural end points.
Results
Over-expression of ezrin increased cell viability (LDH release), mitochondrial activity (ATP synthesis, dehydrogenase activity, and inner mitochondrial membrane potential), and protected the structure of cell membrane microvilli and mitochondria after cold storage preservation injury. Reperfusion-induced apoptosis was also significantly reduced in LLC-EZ cells over-expressing ezrin.
Conclusions
Enhanced ezrin expression protects tubule epithelial cells from cold storage preservation injury, possibly by membrane or mitochondrial mechanisms.
Keywords: Cytoskeletal System, ERM, Renal Transplantation, Hypothermia, Reperfusion Injury, Mitochondria, Membrane Morphology
INTRODUCTION
Renal transplantation is the treatment of choice for patients with end-stage renal disease. About 50% of renal transplants derive from cadaver donors so these grafts experience hypothermia, cold ischemia, and warm reperfusion injury at transplantation. In order to expand the donor pool, extended criteria donors with prior exposure to warm renal ischemia are increasingly being used, which further exacerbates the problem of preservation injury. Extended criteria donors with prior warm renal ischemia are typically Donation after Cardiac Death (DCD) or non-heart beating donors (NHBD) whose kidneys are recovered after cessation of oxygen delivery, unlike brain dead donors where the kidneys are recovered with full blood flow and oxygen delivery at the time of organ harvest. The accumulated preservation injury and prior warm ischemia in DCD donors typically causes primary non-function or delayed graft function after transplantation. This plays an important role in the development of chronic graft failure, late graft loss, and reduces the life expectancy of the graft, which puts further pressure on organ availability as these patients are re-transplanted or go back on dialysis (1–5).
While many factors are involved in preservation injury including tissue edema, cell swelling, ATP depletion, cytosolic calcium, oxidative stress, cellular inflammation, and mediators (6–10); intervening in these areas has not greatly improved clinical preservation injury. This suggests that a more proximal root mechanism may be important in understanding and controlling preservation injury.
Hypothermic preservation injury is characterized by severe membrane failure with blebbing, microvilli loss, calcium overload, and phospholipids catabolism (11–13). Membrane ultrastructural components, such as microvilli and the polarization of vital surface proteins, are important for the proper function of epithelial rich organs like the kidney, and these components are held in place by elements of the sub-lamellar cytoskeletal system. Ezrin, an ERM (Ezrin–Radixin–Moesin) protein, functions as a cross-linker between the actin cytoskeleton and the plasma membrane (14). ERM proteins are involved in microvilli formation and breakdown and are also necessary for cell–cell and cell–substrate adhesions (15,16). Membrane failure characterized by breakdown of microvilli, increased membrane permeability, and membrane bleb formation is a common early event in various types of apoptosis (17) and occurs during cold storage preservation (18–20). Therefore, it is reasonable to suggest that preservation-induced failure of the cell membrane and events distal to such failure may be caused by primary disengagement of the ezrin system supporting the plasma membrane ultrastructure.
Recently, evidence from our lab suggests that disruption of the sub-lamellar cell cytoskeletal system (ezrin and spectrin) occurs during cold preservation and may cause preservation injury. Renal tubules, tubular epithelial cells, and livers subjected to cold ischemia show biochemical and morphological signs of cytoskeletal disruption, which is attenuated by maneuvers that strengthen these systems (21,22). Cold ischemic storage of renal tubules caused an increase in the solubility of both free ezrin and free Na/K ATPase proteins extracted from the cell membranes (23). This suggests a disassociation of ezrin binding to both the actin cytoskeletal system and to the outer cell membrane (24–27). However, our preliminary study was not designed to adequately address whether preservation injury caused ezrin breakdown or whether ezrin breakdown causes preservation injury. Our central hypothesis is that hypothermic ischemia during kidney preservation results in ezrin failure of the tubular epithelium that is causally involved in renal preservation injury via ezrin based structural and signaling mechanisms. We have tested this hypothesis using the pig kidney proximal tubular epithelial cell line [LLC-PK1 and demonstrated that over-expression of ezrin has protective effects on cold preservation and warm reperfusion injury in this model, which supports our central hypothesis.
Materials and Methods
Materials
The expression plasmid, pEX_Ezrin-YFP, containing mouse wild-type Ezrin cDNA and the pig kidney proximal tubular epithelial cell line LLC-PK1 were purchased from American Type Culture Collection (ATCC). There is about a 94% sequence homology between porcine, human, dog, and mouse ezrin. CytoTox 96 non-radioactive cytoxicity assay kits were ordered from Promega Corp. (Madison, WI). MitoTracker® Red CMXRos (M7512) was purchased from Invitrogen (Carlsbad, CA). In-Situ Cell Death Detection Kit (TMR Red) was obtained from Roche Diagnostics (Indianapolis, IN). Cell proliferation reagent WST-1 was ordered from Cayman Chemical (Ann Arbor, Michigan). ATP assay kit SL was purchased from BioThema AB (Handen, Sweden).
Cell culture and cold storage system
LLC-PK1 cells were grown in Media 199 (GIBCO) supplemented with 5% FBS and 50 unit/ml of penicillin-streptomycin and maintained at 37°C in 5% CO2. LLC-EZ cells were grown in Media 199 supplemented with 5% FBS and 100 μg/ml neomycin and maintained at 37°C in 5% CO2. For cold preservation experiments, the cell culture plates were placed into a sealed 1 liter Tupperware® box filled with N2 gas and stored in a cold room (4°C) for 4–24 hrs.
Stably Transfected Cell Lines
Stably transfected LLC-EZ cells were created by transfecting LLC-PK1 cells with the pEX_Ezrin-YFP plasmid. The LLC-PK1 cells (5×105 cells/well) were seeded in 6-well plates the day before transfection. Transfection was carried out using Lipofectamine 2000 reagent (Invitrogen) with 2 μg plasmid pEX_Ezrin-YFP (containing the neomycin resistance gene) per well in Opti-MEM cell culture media. Six hours after transfection, the medium was changed. Media 199 supplemented with 5% FBS and 100μg/ml of neomycin were added to the cells and incubated for one week. The surviving cells were detached by trypsinization (Trypsin 0.25%, EDTA 0.02%, ~12 min) and analyzed on a MoFlo flow cytometer (Beckman Coulter Corporation, Miami, Florida), using the 488 nm argon line of the Enterprise laser and standard optical filters, which included an ND1 (Neutral Density 1), a 95/5 % beam splitter (for detection of 90-degree light scatter), a 555 nm DLP (Dichroic Long Pass), and a 530/40 nm Band Pass filter. YFP-positive cells were sorted into 96-well plates, one cell per well, using the manufacturer’s Cyclone cell deposition system. Discrete colonies were picked after 1 month, re-grown, and tested for expression of YFP by flow cytometry. A maintenance concentration of 100 μg/ml of neomycin was used for the growth of stably transfected clones.
Western blot
LLC-PK1 and LLC-EZ cells were seeded on 10 cm cell culture dishes one day before cold preservation and grown in Media 199 supplemented with 5% FBS. The complete media was replaced by UW solution and cells were subjected to cold storage for 24 hours. After cold preservation, the UW solution was replaced by warm complete media and culture at 37°C for 1 hour. At the end of one hour, the cells were washed with cold PBS and lysed for 1 h with a buffer containing 25 mM HEPES, 0.5 mM, Na3V04, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 10 mM sodium J3-glycerophosphate, 1 mM EGTA, 1 mM EDTA, 1 mM DTT, 1% Triton X-100, 0.1 mM PMSF, 1 mg aprotinin, 1 pM microcystin, 1.0 pg/ml pepstatin, and 1.0 pg/ml leupeptin. The cell lysates were centrifuged (1 × 104 rpm at 4°C for 15 rnin). The supernatant containing 20 μg total protein was loaded in each lane of 10% gels (Invitrogen), electrophoresed for 1 hr, transferred to PVDF membranes (Sigma). Ezrin was detected using an ezrin primary rabbit polyclonal antibody (Santa Cruz, CA, Catalog # Sc-20773, 1: 100 dilution), an HRP-linked secondary donkey anti-rabbit IgG antibody (Sigma, St. Louis, MO, 1: 1000 dilution), and ECL detection with luciferase reagent (Santa Cruz, CA). The bands were visualized on a Kodak ECL Image Analyzer (2000R) and the net intensities were calculated using Kodak molecular imaging software (Kodak ID 3.6, Eastman Kodak, New Haven, CT).
Assessment of cell viability
For determining Cell viability, LLC-PK1 and LLC-EZ cells were cultured at a density of 1×106 cells per well in 96-well microtiter plates. Twenty-four hours after attachment, cells were preserved at 4°C in UW solution or DMEM for 1, 3 and 5days. After cold storage, cell proliferation was assessed by adding WST-1 reagent (Cayman Chemical, Ann Arbor, Michigan) to each well followed by incubation at 37°C for 1 hr. The optical density (OD) was read at 450 nm by a micro-plate reader (Bio-Tek, Winooski, VT).
LDH Assay
Membrane permeability was determined by measuring the release of LDH into the culture medium. Cells were cultured at a density of 1 × 104 cells per well in 96-well micro-titer plates. After 24 h from attachment, cells were cold preserved in DMEM at 4°C for 4–24 hours followed by 1 hour warm reperfusion at 37°C. LDH activity was measured spectrophotometrically at 490 nm using the CytoTox 96® Non-Radioactive Cytotoxicity Assay Kit (Promega, Madison, WI). For LDH release, the cells were stored in their original cell culture media (DMEM) rather than UW solution. Storage in UW solution would require washing the cells before reperfusion to remove the high K+ preservative. This wash step would have also removed the LDH that was released during cold storage, thereby complicating the assay.
ATP Assay
Intracellular ATP was measured after 4–24 hrs of cold storage and 1 hr of warm reperfusion in DMEM using an ATP kit (BioThema AB, Handen, Sweden) in accordance with the manufacturer instructions. Luminosity, proportional to the amount of intracellular ATP present, was measured and calculated on a fluorescent micro-titer plate reader (Model FLX800TB, Bio-Tek, Winooski, VT).
Mitochondrial membrane potential
The mitochondrial membrane potential was estimated by using a voltage sensitive-mitochondrial specific fluorescent dye (MitoTracker® Red, CMXROS, # M7512, Invitrogen). Cells were grown on cover slips inside a Petri dish filled with complete M199 culture medium. When the cells reached 80% confluence, they were cold stored in UW preservation solution for 24 hrs at 4 °C, washed, and reperfused at 37°C with growth medium containing 25 nM of MitoTracker dye for 1 hour. Fluorescence of the cells, representing the relative inner mitochondrial membrane potential, was observed and documented at constant exposure time using a Nikon fluorescence microscope (Nikon Instruments, Melville, NY) equipped with a digital camera for documentation.
TUNEL Staining
TUNEL staining was done using the In Situ Cell Death Detection Kit (TMR Red, Roche). Briefly, 1 × 104 cells were growing on 12mm poly-L-lysine coated round cover slips. After 24h of cold storage preservation in UW solution at 4°C and 1h of warm DMEM reperfusion at 37°C, the cells were fixed with 2% paraformaldehyde, permeabilized (0.1% Triton X-100 and 0.1% citrate), and stained with 50 μl of TUNEL reaction mixture. To counter-stain the nuclei, the cover slips were mounted in SlowFade™ containing 2-(4-amidinophenyl)-6-indolecarbamidine (DAPI; Molecular Probes, Eugene, OR, USA). After washing with PBS, the samples were directly analyzed under a Nikon fluorescence microscope (Nikon Instruments, Melville, NY) equipped with DAPI, FITC, and Rhodamine-Red filters and a digital camera with 3x magnification. Multiple control experiments were carried out to exclude non-specific labeling and false-positive results. For each experiment group, we counted 2000 cells at 1200x magnification and calculated the percentage of positive cells.
Transmission Electron Microscopy
Electron Microscopy samples were processed according to the following procedure. The cells were grown in 60 mm Permanox Petri dishes (Electron Microscopy Sciences) and fixed for 6 h in a solution of 2% glutaraldehyde in 0.1 mol L−1 cacodylate buffer, pH 7.4. After four rinses in cacodylate-buffered solution containing 0.22 mol L−1 sucrose, the cells were post-fixed for 1 h in 1% OsO4 in 0.1 mol L−1 cacodylate buffer. After a rinse for 10 min in 0.1M cacodylate buffer, the cells were dehydrated through a graded ethanol series (50%, 70%, 80%, and 95% - 5 minutes each; 100% - 3 × 10 min). The cells were then infiltrated with 50/50 mix of 100% ethanol and EmBed 812 resin mix (1: 1) for 90 minutes, followed by a 3: 1 mixture of Embed 812: 100% Ethyl alcohol for 90 minutes. The cells were then infiltrated with pure Embed 812 for 90 minutes followed by a second change of pure Embed 812 for 2.5 hours. The cells were infiltrated with Embed 812 and polymerized overnight at 55°C. The resin containing cells were removed from the plastic dish and the area of interest was excised and mounted on a blank stub with super glue. Semi-thin sections (0.5–1.0 μm) were cut with the Leica EM UC6i Ultramicrotome (Leica Microsystems). These sections were stained with a solution of Toluidine Blue in 0.1 mol/L borate buffer and then observed under a light microscope. Thin sections (70–90 nm thick) of the selected areas were collected on copper mesh grids and stained with 5% uranyl acetate (7 min.) and Reynold’s lead citrate (5 min.). These sections were examined under a JEOL JEM-1230 electron microscope (JEOL USA, Inc.) and photographed with the Gatan Ultrascan 4000 digital camera (Gatan Inc, Pleasanton, CA).
Statistical Analysis
Results are presented as mean ± SEM. Means were obtained from three to four experiments performed at least in triplicate. Statistical Analysis was conducted using the GraphPad Prism version 4.00 program for windows (Graphpad Software, San Diego, California). The differences between the groups were determined by T-test and one-way ANOVA. A P value < 0.05 was considered statistically significant.
Results
Identification of LLC-EZ cells
Using MoFlo flow cytometer, YFP-positive cells were sorted into 96-well plates, one cell per well. Discrete colonies were picked after 1 month, re-grown, and tested for expression of YFP by flow cytometry. Single-colony isolated LLC-EZ cells show bright yellow-green fluorescence of the YFP reporter protein under a fluorescence microscope (Fig 1A), compared to the background signal from sham transfected control cells (LLC-PK, Fig 1B). As seen on Western blot (Fig 1C), there is strong expression of ezrin protein in fresh LLC-EZ cells. The expression of ezrin decreased to 15.3% during 24 hour cold preservation and recovered to 27.2% after 1 hour warm reperfusion. Comparatively, the expression of ezrin protein in LLC-PK cells was at a very low level.
Figure 1.
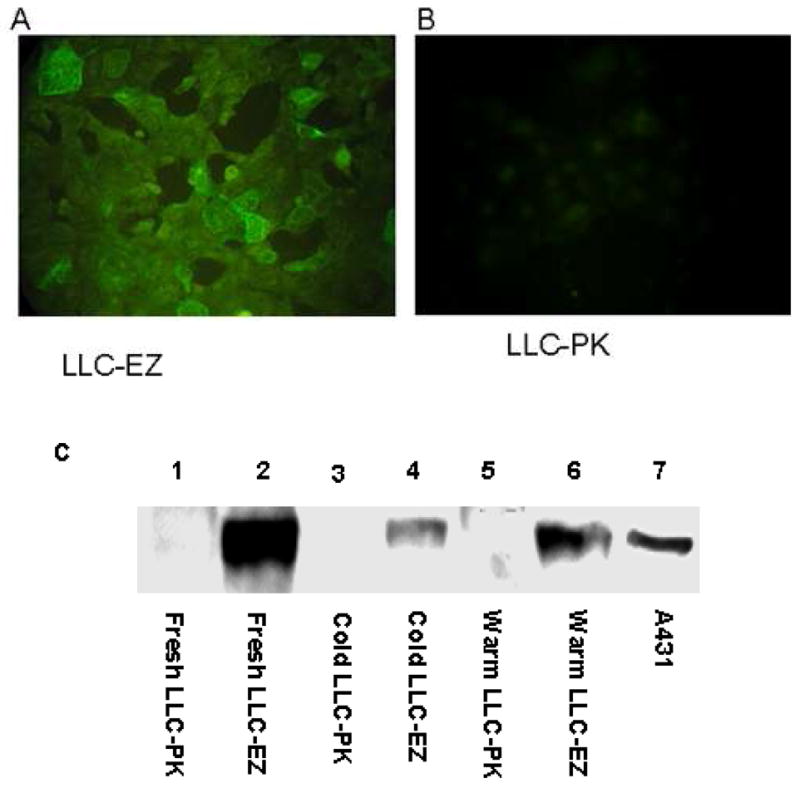
LLC-PK Cells transfected with the plasmid pEX Ezrin-YFP express YFP and ezrin. The transfected cell line LLC-EZ expressing YFP under fluorescence microscopy (A) and sham transfected LLC-PK1 cells showing only background autofluorescence (B). A representative Western blot shows the expression of Ezrin protein after cold storage and warm reperfusion (C). There is strong expression of Ezrin protein in fresh LLC-EZ cells. The expression of ezrin decreased to 15.3% of the baseline after 24 hours of cold preservation and recovered to 27.2% of the baseline after 1 hour of warm reperfusion. Comparatively little expression of Ezrin protein was observed in any of the LLC-PK cells.
Cell viability determinations
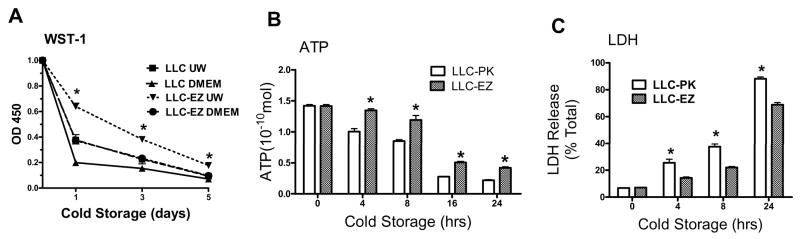
Progressive cold storage and reperfusion caused a storage time dependent loss of cell viability (WST-1 dye conversion), which was further dependent on the quality of the organ preservation solution used (Fig. 2A). Specifically, WST-1 conversion was significantly less in cells preserved with DMEM compared with the UW solution for any given storage time. Transfection of LLC-PK cells with the wild type Ezrin gene significantly increased WST-1 uptake after 1, 3, 5 days of cold storage in UW solution or DMEM, relative to the corresponding control cells. Cold storage and reperfusion in DMEM also caused a storage time dependent loss of intracellular ATP content (Fig. 2B). The intracellular content of ATP was significantly higher in the cells transfected with the ezrin plasmid compared with the control cells for all time periods. Finally, assessment of cell viability by LDH release demonstrated that progressive cold storage time caused significant and progressive increases in cell LDH release, which again was significantly attenuated in the ezrin transfected cell lines, relative to the corresponding values in the control cells (Fig 2C).
Figure 2.
Progressive cold storage and reperfusion caused a storage time dependent loss of cell viability, which was further dependent on the quality of the organ preservation solution used (UW Vs DMEM). Over expression of ezrin (LLC-EZ cell line) significantly increased cell viability as assessed by LDH release after reperfusion (A), by WST-1 dye conversion (B), and by intracellular ATP regeneration after reperfusion (C). Values are mean ± SD from 4 independent experiments. * P< 0.05 between the LLC-PK1 controls and the corresponding values from the LLC-EZ cell line.
Ezrin protects cells from apoptosis
We used a highly sensitive and specific fluorescent TUNEL assay to detect DNA cleavage during apoptosis in fresh, cold stored, and cold stored after warm reperfusion in both control and ezrin transfected cell lines. The percentage of cells staining positively with TUNEL was less than 0.2% in fresh LLC-PK and LLC-EZ cells (Fig. 3A). With cold storage for 24 hours, there was no significant increase in TUNEL positive cells in either cell line, indicating the absence of apoptosis with cold ischemia per se. However, after 1 hour of warm reperfusion, the percentage of TUNEL positive cells was higher in both cell lines, relative to their corresponding values in the cold storage or fresh treatments. Ezrin transfection significantly reduced this post-reperfusion apoptosis (9.6% positive in the LLC-PK cells Vs only 2.3% positive in the ezrin transfected LLC-EZ cell line).
Figure 3.
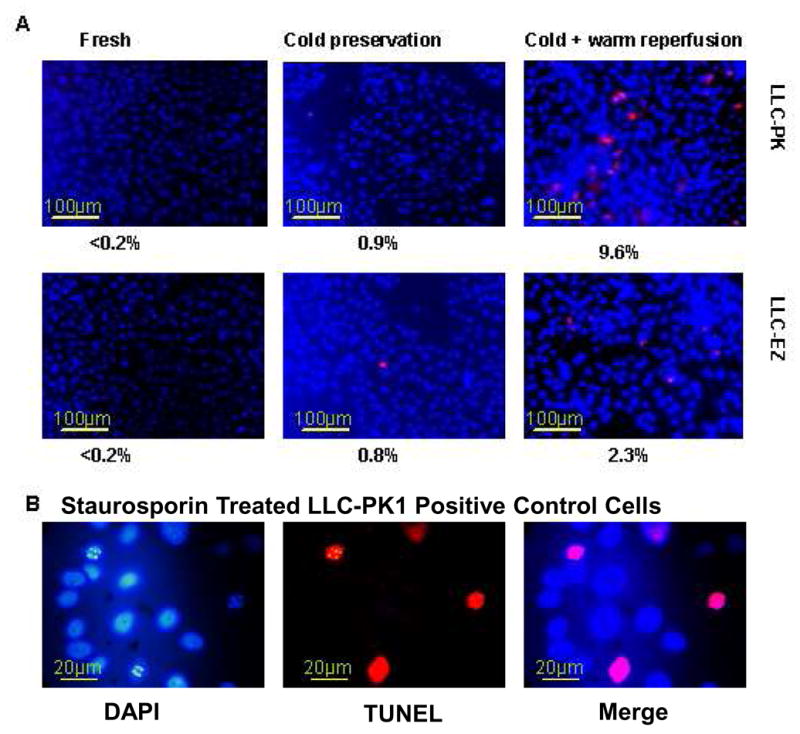
Over expression of Ezrin protein protects cells from apoptosis during cold preservation and warm reperfusion. Apoptosis rates of LLC-PK and LLC-EZ cells before storage (fresh), after 24 h of cold storage, and after 1 h of warm reperfusion (A, 120x). Positive control LLC-PK1 cells treated with Staurosporin (B, 480x). TUNEL positive labeling shows red color with blue DAPI staining of nuclei as a counter stain.
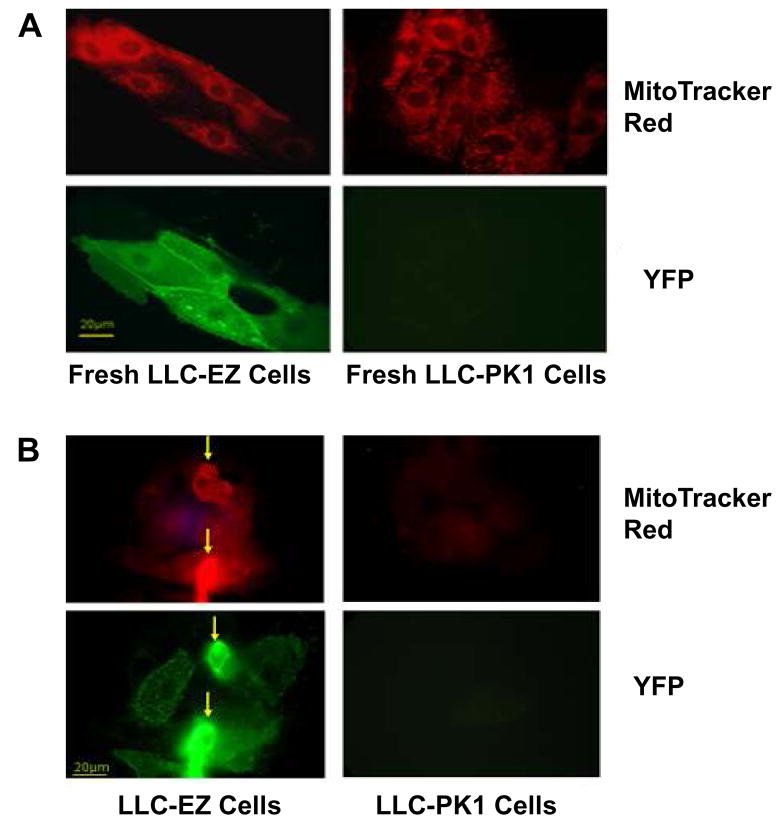
Mitochondria specific dye activity
MitoTracker® Red dye (CMXRos, M7512), a derivative of X-rosamine, does not fluoresce until it enters an actively respiring cell, where it is oxidized and then sequestered in the mitochondria. Figure 4 shows red emitting MitoTracker red dye and green-yellow staining of the YFP reporter in control LLC-PK and ezrin transfected LLC-EZ cells. The baseline signals for both cells before cold storage and reperfusion is shown in panel A and the signals generated after 24 hrs of cold storage and 1 hr of warm reperfusion is shown in panel B. Both cell lines stain strongly for MitoTracker red dye in fresh cells but only the ezrin transfected LLC-EZ cells show strong staining after 24 hrs of cold storage ischemia and 1 hr of warm reperfusion (Fig 4, block B). Also, the co-localization of the YFP reporter and MitoTracker signals (arrows) in the cold stored LLC-EZ cells (Fig 4B, left side) suggest a correlation between ezrin expression and mitochondrial function.
Figure 4.
MitoTracker Red dye (M7512, red) and YFP (green) fluorescence of fresh epithelial cells (A) and epithelial cells after 24 hrs of cold storage and 1 hr of reperfusion (B). The LLC-EZ cells show strong MitoTracker Red staining after cold storage and warm reperfusion, whereas the non-transfected cells show almost no signal. The co-localization and proportionality of the MitoTracker Red and the YFP fluorescent signals in the cold stored LLC-EZ cells (B-Left, arrows) suggests an ezrin-induced protection of the inner mitochondrial potential required for mitochondrial function and ATP synthesis.
TEM
Transmission Electron Microscopy (TEM) studies were undertaken in LLC-PK and LLC-EZ cells to discern the cell structure changes during cold preservation and the effects of ezrin expression on these changes. Cells that were not subjected to cold storage showed normal morphological appearance under electron microscope (Fig. 5A). The plasma membrane and microvilli were well defined and glycogen storage sites were homogeneously distributed in the cells. The organelles were also well delineated, particularly the inner mitochondrial membranes and the mitochondrial cristae (Fig. 5B). After 8 hr of cold storage, LLC-PK cells demonstrated swelling and loss of microvilli, reductions in visible glycogen storage sites, and mitochondrial damage characterized by smaller, pinched, and electron dense structures with evidence of cristolysis (Fig 5B). The LLC-EZ cells transfected with the ezrin plasmid showed much better structural morphology after 8 hours of cold storage ischemia cold (Fig 5B).
Figure 5.
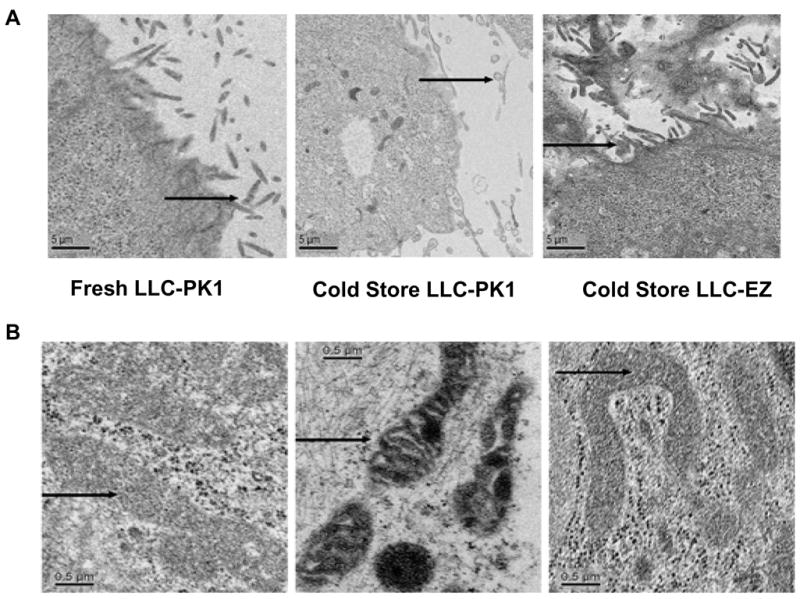
Transmission electron microscopy (TEM) of fresh LLC-PK cells before cold storage (left), LLC-PK cells after 8 hrs of cold storage (middle), and TEM of ezrin over expressing LLC-EZ cells after 8 hrs of cold storage preservation (right). Microvilli details of the plasma membrane are featured in panel-A and show loss of brush border after cold storage in LLC-PK cells that is largely prevented in cells that over express ezrin. Arrows show cell microvilli. Similarly, mitochondria (panel-B) are shrunken, electron dense, and some show signs of cristalysis after 8 hrs of cold storage in control LLC-PK1 cells (B, middle). However, the mitochondrial changes are much milder in the ezrin over expressing LLC-EZ cells after cold storage (B, right). Many of their mitochondria appear normal with fewer electron dense structures. The mitochondrial structural preservation associated with ezrin over expression is consistent with the changes observed in mitochondrial function in these cells. Arrows show mitochondria.
Discussion
ERM proteins are a family of cytoskeletal cross-linkers that form a bridge between the plasma membrane of the cell and cytoplasmic actin filaments. These structures are critical for maintaining normal kidney function since they are essential for supporting ion channels, adhesion molecules, and membrane ultrastructural components like microvilli (28). Ezrin is also involved in cell signaling and can modify the mitochondrial apoptotic pathway (29). Over expression of recombinant ERM proteins result in enhanced cell adhesion while suppression of expression produces the opposite effects (30,31). Suppressing the expression of all three ERM proteins in cell culture result in the loss of cell-cell and cell-substrate adhesion, disappearance of microvilli, and loss of the cell adhesion molecule E-cadherin (32). Microinjection of anti-ezrin antibodies led to the collapse of actin fibers and the formation of irregular cell edges (33). These studies clearly suggest that ERM proteins, and ezrin in particular, are essential for the maintenance of normal cell membrane ultrastructure, which may be crucial for normal cell and tissue function in epithelial cell rich organs like the kidney.
Disruption of the renal proximal tubule brush border is a prominent early event during warm ischemic injury to the kidney (34,35). Within the brush border, ezrin normally links the actin cytoskeleton to the cell plasma membrane (36) and structurally forms the microvillus. In renal proximal tubules, normothermic anoxia causes ezrin dephosphorylation and disassociation from the cytoskeleton, microvillar break down, and bleb formation (37–39). Similarly, we have recently demonstrated that hypothermic ischemia associated with organ preservation causes ezrin disassociation in isolated renal tubules, which results in the extraction of free ezrin and Na/K ATPase protein from membrane isolates (40). It is impossible to discern from these previous studies if ezrin disassociation during cold storage ischemia causes preservation injury or if ezrin disassociation is simply an epiphenomenon that co-occurs with preservation injury. Therefore, we designed specific experiments targeting ezrin expression to help determine the causality of these events in renal epithelial cells experiencing hypothermic preservation injury.
Establishing causation between cytoskeletal system changes and a biological response is difficult since specific and direct pharmacological tools are not commonly available that either augments or ablates their function or synthesis. Consequently, we derived a renal epithelial cell line that constituitively over expresses wild-type ezrin following stable transfection. We chose the LLC-PK1 epithelial cell line derived from porcine renal proximal tubules since it retains functional and morphological characteristics of it’s original primary cell precursor (41). Furthermore, this cell line experiences classic attributes of hypothermic preservation injury after cold storage in UW solution and rewarming that are remarkably similar to preservation injury induced in more physiological systems such as primary isolated renal proximal tubules or kidney tissue slices (42–44). Therefore, by directly comparing the LLC-PK1 (control) and the LLC-EZ (ezrin over expressing) cell lines in our models, we are able to unambiguously dissect out the over all role of ezrin in cold preservation injury. We are also confident that the results are largely translatable to clinical preservation injury in humans since the cell line behaves similarly to primary organ cultures. With these comparisons, we clearly see that ezrin is causally related to hypothermic preservation injury. Specifically, preservation injury was significantly attenuated in the LLC-EZ cell line, whose only known difference is the over expression of ezrin, relative to the control LLC-PK1 cells. This indicates that preservation injury, is in part, caused by ezrin failure, which is known to be associated with preservation injury (45).
The mechanisms of ezrin protection in hypothermic preservation injury in this model likely relates to the known functions of ezrin in epithelial cells. The involvement of ezrin in maintaining membrane structure is an important mechanism. The structural cross linking function of ezrin is dependent on its molecular configuration. The carboxy and amino terminals of ezrin bind to the actin filament and cytoplasmic domains of specific membrane associated proteins, respectively. The activated form of ezrin both holds molecules on the cell membrane in proper orientation to generate cell polarity and allows for the formation of microvilli. Both attributes are essential for the specialized epithelial cell functions that are characteristic of renal tubules. Ezrin typically is in a head-to-tail association in the cytoplasm, which blocks these binding sites and prevents cross linking functionality. Ezrin binding activation is controlled by site specific phosphorylation of threonine-567, which opens the molecule for binding. Phosphorylation of this site is controlled by agonist-induced Rho activation, the phosphorylation potential of the cell, and other factors, all of which may be altered during hypothermic ischemia during organ preservation. Our data clearly show that preservation injury in LLC-PK1 cells causes loss of membrane microvilli and brush border details with bleb formation, which is reversed with ezrin over expression. Ezrin over expression also significantly reversed LDH release after reperfusion and suggests that the membrane permeability defect that allows the loss of macromolecules like LDH is ezrin-dependent. This is further supported by the exacerbation of LDH release in cold stored primary canine renal tubule epithelial cells treated with a specific siRNA duplex that significantly blocked ezrin expression (46). Therefore, over expression of wild type ezrin may increase the likelihood of ezrin activation during preservation and retard membrane degradation in the cold or at reperfusion. Further testing of this hypothesis using site directed mutagenesis of the T567 control site to produce constituitively active or inactive binding mutants of ezrin are currently in progress.
Ezrin is also involved in cell signaling. Phosphorylation of ezrin at Y353 activates PI3K and Akt pathways to promote cell survival by suppressing mitochondrial apoptosis pathways (47) and phosphorylation of ezrin at T567 promotes FAS-mediated apoptosis (48). A significant amount of preservation injury in this study (10%) was due to apoptosis, which was almost completely reversed in the ezrin over expressing clones. These data indicate that ezrin is also involved in apoptosis signaling during hypothermic preservation in this model, probably via the inhibitory Y353/PI3K/Akt pathway. Once again, site directed mutagenesis of these active control points are necessary to conclusively dissect out these circuits.
Our data clearly show that ezrin over expression preserves renal epithelial cell mitochondrial function. Hypothermic preservation of renal epithelial cells caused loss of mitochondrial dehydrogenase activity (WST-1 data), collapse of the inner mitochondrial membrane potential (MitoTracker Dye data), and inhibition of ATP synthesis at reperfusion (ATP data). All of these effects were significantly reversed with ezrin over expression in the LLC-EZ cell line and strongly suggests that ezrin may be acting via protection of the mitochondria during cold preservation injury. Mitochondria become injured during hypothermic preservation by loss of calcium control and opening of the mitochondrial permeability transition pore (mPTP), which further results in calcium entry, osmotic overload, swelling and inner membrane rupture (49). Ezrin expression or activation in the cell may influence mitochondrial injury by protecting the structure, function, and permeability of the plasma membrane to the high extracellular calcium load or by direct actions on the mitochondria. Although there are no direct links between ezrin and mitochondria function so far, we believe that it is possible for ezrin to directly modulate mitochondria activity. Ezrin co-localizes with CD44 (50–52), which is a normally occurring mitochondrial protein necessary for mitochondrial function (53). Immuno-gold labeling and TEM analysis of J774 cells visualized ezrin binding to mitochondria (54) and we have preliminarily identified ezrin from purified mitochondria isolated from LLC-PK1 cells by Western blot, with greater amounts present in LLC-EZ cells (unpublished observations). Therefore, it is tempting to suggest that ezrin may protect mitochondria during preservation stress by directly interfering with the formation of the permeability transition pore or some other direct pathway. Although this suggestion remains purely speculative, we are testing the possibility. With the present data, it is more reasonable to suggest that ezrin protection of mitochondrial function during preservation injury in this model is secondary to the control of the permeability of the outer plasma membrane to mitochondrial activators like calcium.
In conclusion, this study has convincing evidence that causally links ezrin failure to cold storage preservation injury in renal tubular epithelial cells. Since these cells are an important target for renal preservation injury in the whole kidney, this mechanism may have physiological and clinical relevance in human renal preservation injury and DGF after transplantation. Pharmacological agents or other maneuvers that activate or strengthen ezrin, or other components of the sub-lamellar cytoskeletal system, may be effective therapeutic targets.
Acknowledgments
We thank Frances White and Julie Farnsworth for the flow cytometry assay. We also acknowledge the kind assistance of Judy Williamson in preparing the TEM samples.
This work was supported by a grant from the National Institutes of Health DK63309. The VCU Flow Cytometry facility is supported, in part, by NCI Grant P30 CA16059, awarded to the Massey Cancer Center. Microscopy was performed at the VCU Dept. of Neurobiology & Anatomy Microscopy Facility, supported, in part, with funding from NIH-NINDS Center core grant 5P30NS04746. There were no conflicts of interest during this study.
Abbreviations
- NHBD
Non-heart beating donor
- DCD
Donation after Cardiac Death
- DGF
Delayed Graft Function
- YFP
Yellow Fluorescent Protein
- LDH
Lactate Dehydrogenase
- UW
University of Wisconsin Solution, Viaspan®
- ERM
Ezrin, Radixin, Moesin
- TEM
Transmission Electron Microscopy
- mPTP
Mitochondrial Permeability Transition Pore
Reference List
- 1.Hauet T, Eugene M. A new approach in organ preservation: potential role of new polymers. Kidney Int. 2008;74:998–1003. doi: 10.1038/ki.2008.336. [DOI] [PubMed] [Google Scholar]
- 2.Peters TG, Shaver TR, Ames JE, Santiago-Delpin EA, Jones KW, Blanton JW. Cold ischemia and outcome in 17,937 cadaveric kidney transplants. Transplantation. 1995;59:191–196. [PubMed] [Google Scholar]
- 3.Pfaff WW, Howard RJ, Patton PR, Adams VR, Rosen CB, Reed AI. Delayed graft function after renal transplantation. Transplantation. 1998;65:219–223. doi: 10.1097/00007890-199801270-00013. [DOI] [PubMed] [Google Scholar]
- 4.Troppmann C, Gillingham KJ, Benedetti E, et al. Delayed graft function, acute rejection, and outcome after cadaver renal transplantation. The multivariate analysis. Transplantation. 1995;59:962–968. doi: 10.1097/00007890-199504150-00007. [DOI] [PubMed] [Google Scholar]
- 5.Lee CM, Carter JT, Alfrey EJ, Ascher NL, Roberts JP, Freise CE. Prolonged cold ischemia time obviates the benefits of 0 HLA mismatches in renal transplantation. Arch Surg. 2000;135:1016–1019. doi: 10.1001/archsurg.135.9.1016. [DOI] [PubMed] [Google Scholar]
- 6.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Mangino MJ, Jendrisak MD, Murphy MK, Halstead M, Anderson CB. Prostanoids and hypothermic renal preservation injury. Prostaglandins Leukot Essent Fatty Acids. 1990;41:173–179. doi: 10.1016/0952-3278(90)90086-z. [DOI] [PubMed] [Google Scholar]
- 8.Mangino MJ, Mangino JE, Kotadia B, Sielczak M. Effects of the 5-lipoxygenase inhibitor A-64077 on intestinal hypothermic organ preservation injury. J Pharmacol Exp Ther. 1997;281:950–956. [PubMed] [Google Scholar]
- 9.Schneeberger H, Illner WD, Abendroth D, et al. First clinical experiences with superoxide dismutase in kidney transplantation--results of a double-blind randomized study. Transplant Proc. 1989;21:1245–1246. [PubMed] [Google Scholar]
- 10.Rauen U, de GH. New insights into the cellular and molecular mechanisms of cold storage injury. J Investig Med. 2004;52:299–309. doi: 10.1136/jim-52-05-29. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Southard JH. Membrane stabilizing effects of calcium and taxol during the cold storage of isolated rat hepatocytes. Transplantation. 1999;68:938–943. doi: 10.1097/00007890-199910150-00007. [DOI] [PubMed] [Google Scholar]
- 12.Nowak G, Ungerstedt J, Wernerson A, Ungerstedt U, Ericzon BG. Hepatic cell membrane damage during cold preservation sensitizes liver grafts to rewarming injury. J Hepatobiliary Pancreat Surg. 2003;10:200–205. doi: 10.1007/s00534-002-0760-4. [DOI] [PubMed] [Google Scholar]
- 13.Hertl M, Hertl MC, Kluth D, Broelsch CE. Hydrophilic bile salts protect bile duct epithelium during cold preservation: a scanning electron microscopy study. Liver Transpl. 2000;6:207–212. doi: 10.1002/lt.500060201. [DOI] [PubMed] [Google Scholar]
- 14.Bretscher A, Reczek D, Berryman M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci. 1997;110 (Pt 24):3011–3018. doi: 10.1242/jcs.110.24.3011. [DOI] [PubMed] [Google Scholar]
- 15.Kondo T, Takeuchi K, Doi Y, Yonemura S, Nagata S, Tsukita S. ERM (ezrin/radixin/moesin)-based molecular mechanism of microvillar breakdown at an early stage of apoptosis. J Cell Biol. 1997;139:749–758. doi: 10.1083/jcb.139.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol. 1997;138:423–434. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo T, Takeuchi K, Doi Y, Yonemura S, Nagata S, Tsukita S. ERM (ezrin/radixin/moesin)-based molecular mechanism of microvillar breakdown at an early stage of apoptosis. J Cell Biol. 1997;139:749–758. doi: 10.1083/jcb.139.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neto JS, Nakao A, Kimizuka K, et al. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am J Physiol Renal Physiol. 2004;287:F979–F989. doi: 10.1152/ajprenal.00158.2004. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Southard JH. Membrane stabilizing effects of calcium and taxol during the cold storage of isolated rat hepatocytes. Transplantation. 1999;68:938–943. doi: 10.1097/00007890-199910150-00007. [DOI] [PubMed] [Google Scholar]
- 20.Mangino MJ, Tian T, Ametani M, Lindell S, Southard JH. Cytoskeletal involvement in hypothermic renal preservation injury. Transplantation. 2008;85:427–436. doi: 10.1097/TP.0b013e31815fed17. [DOI] [PubMed] [Google Scholar]
- 21.Mangino MJ, Tian T, Ametani M, Lindell S, Southard JH. Cytoskeletal involvement in hypothermic renal preservation injury. Transplantation. 2008;85:427–436. doi: 10.1097/TP.0b013e31815fed17. [DOI] [PubMed] [Google Scholar]
- 22.Kohli V, Gao W, Camargo CA, Jr, Clavien PA. Calpain is a mediator of preservation-reperfusion injury in rat liver transplantation. Proc Natl Acad Sci U S A. 1997;94:9354–9359. doi: 10.1073/pnas.94.17.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangino MJ, Tian T, Ametani M, Lindell S, Southard JH. Cytoskeletal involvement in hypothermic renal preservation injury. Transplantation. 2008;85:427–436. doi: 10.1097/TP.0b013e31815fed17. [DOI] [PubMed] [Google Scholar]
- 24.Aufricht C, Bidmon B, Ruffingshofer D, et al. Ischemic conditioning prevents Na, K-ATPase dissociation from the cytoskeletal cellular fraction after repeat renal ischemia in rats. Pediatr Res. 2002;51:722–727. doi: 10.1203/00006450-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Van Why SK, Mann AS, Ardito T, et al. Hsp27 associates with actin and limits injury in energy depleted renal epithelia. J Am Soc Nephrol. 2003;14:98–106. doi: 10.1097/01.asn.0000038687.24289.83. [DOI] [PubMed] [Google Scholar]
- 26.Van Why SK, Kim S, Geibel J, Seebach FA, Kashgarian M, Siegel NJ. Thresholds for cellular disruption and activation of the stress response in renal epithelia. Am J Physiol. 1999;277:F227–F234. doi: 10.1152/ajprenal.1999.277.2.F227. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Doctor RB, Mandel LJ. Cytoskeletal dissociation of ezrin during renal anoxia: role in microvillar injury. Am J Physiol. 1994;267:C784–C795. doi: 10.1152/ajpcell.1994.267.3.C784. [DOI] [PubMed] [Google Scholar]
- 28.Bach LA, Gallicchio MA, McRobert EA, Tikoo A, Cooper ME. Effects of advanced glycation end products on ezrin-dependent functions in LLC-PK1 proximal tubule cells. Ann N Y Acad Sci. 2005;1043:609–616. doi: 10.1196/annals.1338.069. [DOI] [PubMed] [Google Scholar]
- 29.Gautreau A, Poullet P, Louvard D, Arpin M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96:7300–7305. doi: 10.1073/pnas.96.13.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin M, Andreoli C, Sahuquet A, Montcourrier P, Algrain M, Mangeat P. Ezrin NH2-terminal domain inhibits the cell extension activity of the COOH-terminal domain. J Cell Biol. 1995;128:1081–1093. doi: 10.1083/jcb.128.6.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi K, Sato N, Kasahara H, et al. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol. 1994;125:1371–1384. doi: 10.1083/jcb.125.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi K, Sato N, Kasahara H, et al. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol. 1994;125:1371–1384. doi: 10.1083/jcb.125.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaul SC, Kawai R, Nomura H, Mitsui Y, Reddel RR, Wadhwa R. Identification of a 55-kDa ezrin-related protein that induces cytoskeletal changes and localizes to the nucleolus. Exp Cell Res. 1999;250:51–61. doi: 10.1006/excr.1999.4491. [DOI] [PubMed] [Google Scholar]
- 34.Molitoris BA. Actin cytoskeleton in ischemic acute renal failure. Kidney Int. 2004;66:871–883. doi: 10.1111/j.1523-1755.2004.00818.x. [DOI] [PubMed] [Google Scholar]
- 35.Bylander J, Li Q, Ramesh G, Zhang B, Reeves WB, Bond JS. Targeted disruption of the meprin metalloproteinase beta gene protects against renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2008;294:F480–F490. doi: 10.1152/ajprenal.00214.2007. [DOI] [PubMed] [Google Scholar]
- 36.Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Doctor RB, Mandel LJ. Cytoskeletal dissociation of ezrin during renal anoxia: role in microvillar injury. Am J Physiol. 1994;267:C784–C795. doi: 10.1152/ajpcell.1994.267.3.C784. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Cohn JA, Mandel LJ. Dephosphorylation of ezrin as an early event in renal microvillar breakdown and anoxic injury. Proc Natl Acad Sci U S A. 1995;92:7495–7499. doi: 10.1073/pnas.92.16.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spence HJ, Chen YJ, Batchelor CL, et al. Ezrin-dependent regulation of the actin cytoskeleton by beta-dystroglycan. Hum Mol Genet. 2004;13:1657–1668. doi: 10.1093/hmg/ddh170. [DOI] [PubMed] [Google Scholar]
- 40.Mangino MJ, Tian T, Ametani M, Lindell S, Southard JH. Cytoskeletal involvement in hypothermic renal preservation injury. Transplantation. 2008;85:427–436. doi: 10.1097/TP.0b013e31815fed17. [DOI] [PubMed] [Google Scholar]
- 41.Hull RN, Cherry WR, Weaver GW. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976;12:670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- 42.Mangino MJ, Tian T, Ametani M, Lindell S, Southard JH. Cytoskeletal involvement in hypothermic renal preservation injury. Transplantation. 2008;85:427–436. doi: 10.1097/TP.0b013e31815fed17. [DOI] [PubMed] [Google Scholar]
- 43.Mangino MJ, Ametani M, Szabo C, Southard JH. Poly(ADP-ribose) polymerase and renal hypothermic preservation injury. Am J Physiol Renal Physiol. 2004;286:F838–F847. doi: 10.1152/ajprenal.00230.2003. [DOI] [PubMed] [Google Scholar]
- 44.Mangino MJ, Ametani MS, Gilligan BJ, Szabo C, Brounts L, Southard JH. Role of peroxynitrite anion in renal hypothermic preservation injury. Transplantation. 2005;80:1455–1460. doi: 10.1097/01.tp.0000181099.20099.d1. [DOI] [PubMed] [Google Scholar]
- 45.Mangino MJ, Tian T, Ametani M, Lindell S, Southard JH. Cytoskeletal involvement in hypothermic renal preservation injury. Transplantation. 2008;85:427–436. doi: 10.1097/TP.0b013e31815fed17. [DOI] [PubMed] [Google Scholar]
- 46.Mangino MJ, Tian T, Ametani M, Lindell S, Southard JH. Cytoskeletal involvement in hypothermic renal preservation injury. Transplantation. 2008;85:427–436. doi: 10.1097/TP.0b013e31815fed17. [DOI] [PubMed] [Google Scholar]
- 47.Gautreau A, Poullet P, Louvard D, Arpin M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96:7300–7305. doi: 10.1073/pnas.96.13.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hebert M, Potin S, Sebbagh M, Bertoglio J, Breard J, Hamelin J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. J Immunol. 2008;181:5963–5973. doi: 10.4049/jimmunol.181.9.5963. [DOI] [PubMed] [Google Scholar]
- 49.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura H, Ozawa H. Immunolocalization of CD44 and the ezrin-radixin-moesin (ERM) family in the stratum intermedium and papillary layer of the mouse enamel organ. J Histochem Cytochem. 1997;45:1481–1492. doi: 10.1177/002215549704501105. [DOI] [PubMed] [Google Scholar]
- 51.Stapleton G, Malliri A, Ozanne BW. Downregulated AP-1 activity is associated with inhibition of Protein-Kinase-C-dependent CD44 and ezrin localisation and upregulation of PKC theta in A431 cells. J Cell Sci. 2002;115:2713–2724. doi: 10.1242/jcs.115.13.2713. [DOI] [PubMed] [Google Scholar]
- 52.Mielgo A, Brondani V, Landmann L, et al. The CD44 standard/ezrin complex regulates Fas-mediated apoptosis in Jurkat cells. Apoptosis. 2007;12:2051–2061. doi: 10.1007/s10495-007-0115-3. [DOI] [PubMed] [Google Scholar]
- 53.Lakshman M, Subramaniam V, Jothy S. CD44 negatively regulates apoptosis in murine colonic epithelium via the mitochondrial pathway. Exp Mol Pathol. 2004;76:196–204. doi: 10.1016/j.yexmp.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Defacque H, Egeberg M, Habermann A, et al. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J. 2000;19:199–212. doi: 10.1093/emboj/19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]