Abstract
Purpose
Endoscopic examination has proven effective in both detecting and preventing colorectal cancer; however, only about a quarter of eligible patients undergo screening. Even if the compliance rate increased, limited endoscopic capacity and cost would be prohibitive. There is a need for an accurate method to target colonoscopy to those most at risk of harboring colonic neoplasia. Exploiting field carcinogenesis seems to be a promising avenue. Our group recently reported that an early increase in blood supply (EIBS) is a reliable marker of field carcinogenesis in experimental models. We now investigate whether in situ detection of EIBS in the rectum can predict neoplasia elsewhere in the colon.
Experimental Design
We developed a novelpolarization-gated spectroscopy fiber-optic probe that allows depth-selective interrogation of microvascular blood content. Using the probe, we examined the blood content in vivo from the rectal mucosa of 216 patients undergoing screening colonoscopy.
Results
Microvascular blood content was increased by ~ 50% in the endoscopically normal rectal mucosa of patients harboring advanced adenomas when compared with neoplasia-free patients irrespective of lesion location. Demographic factors and nonneoplastic lesions did not confound this observation. Logistic regression using mucosal oxyhemoglobin concentration and patient age resulted in a sensitivity of 83%, a specificity of 82%, and an area under the receiver operating characteristic curve of 0.88 for the detection of advanced adenomas.
Conclusions
Increased microvascular blood supply in the normal rectal mucosa is associated with the presence of clinically significant neoplasia elsewhere in the colon, supporting the development of rectal EIBS as a colon cancer risk-stratification tool.
The lifetime incidence of colorectal cancer (CRC) for adults is 6%, which translates into 148,000 Americans expected to develop this malignancy in 2008 (1). CRC is generally curable if detected at a localized stage. However, because early-stage disease is generally asymptomatic, approximately two thirds of patients with CRC are diagnosed at a more advanced stage, resulting in CRC to be the second leading cause of cancer deaths among Americans. This underscores the need to screen the asymptomatic at-risk population (patients ≥50 years old), which is advocated by existing guidelines. Colonoscopy is considered the current gold standard in CRC screening because of its ability to both detect ~ 97% of advanced neoplastic lesions (advanced adenomas and carcinomas) and reduce CRC incidence by 65% to 90% by removal of precursor lesions (2). Despite the unequivocal benefit, only about one fourth of the eligible screening population undergoes endoscopic CRC screening (3). The reasons for the low compliance rate are numerous, but the major factor is patient aversion to the invasiveness of endoscopic procedures with the associated discomfort, expense, and risk for complications. Additionally, the inconvenience of bowel preparation represents a major hurdle for patients from undergoing a colonoscopy or alternative techniques such as CT colography (virtual colonoscopy). Even if compliance could be improved, it would be impractical to perform colonoscopy on the entire at-risk population (90 million Americans older than 50 years) given resource constraints and the potential complication rate. Moreover, given that only 20% to 30% of patients harbor neoplasia and only ~ 5% harbor clinically/biologically significant lesions, the majority of colonoscopies would retrospectively be deemed as unnecessary (4). Thus, finding a precolonoscopic means to identify patients more likely to harbor neoplasia (risk stratification) is of major importance in population screening.
Translational Relevance
This work embodies several technological, biological, and clinical innovations. The development of a polarization-gated fiber-optic probe has allowed us to accurately measure rectalmicrovascular content in vivo with depth selectivity. Using this novel technology, we have shown that rectal microvascular blood content is elevated in the presence of neoplastic lesions anywhere in the colon. Biologically, this study provides the first in vivo evidence that an early increase in blood supply (EIBS) is a promising marker of field carcinogenesis in the colon and shows that augmented blood supply precedes tumorigenesis supporting the emerging field of angioprevention. Finally, this work may open the feasibility of population screening for colon cancer by using EIBS as a risk-stratification tool. During an annual rectal exam, rectal blood content could be assessed with the probe and only those patients with a positive finding would be targeted for colonoscopy. Patients deemed to be at low risk by the EIBS test would not need to undergo uncomfortable and costly endoscopic procedures.
A number of risk stratification techniques exploit the field effect, the proposition that the genetic and environmental milieu that results in a neoplastic lesion at a particular site should be detectable, at least in some form, throughout the colonic mucosa. This concept lends a practical means for CRC screening. If an accurate marker of the field effect is available, it should be feasible to measure it from a normal-appearing and easily accessible portion of the colon and, by doing so, assess the likelihood of a patient harboring a neoplastic lesion elsewhere in the colon. The field effect is the basis for the widespread use of flexible sigmoidoscopy for cancer screening given that distal adenomas may serve as a marker for proximal neoplasia (5). The suboptimal performance characteristics (~ 40% in detecting advanced proximal lesions) and modest invasiveness represent important problems for utilization in population screening (6). An ideal marker of the field effect should be assessed in the rectum and have robust performance characteristics. Accordingly, a number of other markers of field carcinogenesis have been proposed, including rectal aberrant crypt foci (7), microarray proteomics (8), and tissue proteomics (9), but these and other biomarkers do not have the required sensitivity and practicality for clinical use. More accurate biomarkers of colon carcinogenesis that are easily measured from a clinical perspective are needed to allow the rectal screening approach to be practical for CRC risk stratification.
Increased blood supply (primarily through angiogenesis) is a ubiquitous marker of tumorigenesis. Recent data suggest that this may occur early in colon carcinogenesis at the small adenomatous polyp and aberrant crypt foci stage (10, 11). All these studies, however, focused on the blood supply to a given lesion. Little attention has been paid to whether increased blood supply could be a marker of the field effect (i.e., does it occur in the histologically normal mucosa of patients with neoplasia?). To investigate this issue, we previously developed a novel optics technology, four-dimensional elastic light-scattering fingerprinting (4D-ELF), which allows depth-selective quantification of the colonic mucosal microvasculature (12–14). Using this technique, microvascular blood content was found to be increased in the histologically normal mucosa before development of adenomas and aberrant crypt foci as shown by studies using the azoxymethane-treated rat and the MIN mouse model of CRC (14). The effect was primarily limited to the pericryptal microcirculation of the mucosa and was not present in the larger blood vessels located deeper in colonic tissue (e.g., submucosa). Because most conventional techniques used for blood supply analysis are better suited to interrogate larger blood vessel content, the observation of an early increase in blood supply (EIBS) requires a specialized depth-selective technique such as 4D-ELF. This EIBS was confirmed with a nonoptics technique, Western blot analysis for hemoglobin, which, as expected, was found to be less sensitive than the optical approach (14).
These studies with animal models support the role of EIBS as a marker of field carcinogenesis. The question remained as to whether EIBS could be observed in humans in vivo and if this effect could be used as an accurate and practical marker of the field effect. To answer this question, we first simplified the benchtop 4D-ELF instrument, which could only be used with ex vivo tissue specimens, into an endoscopically compatible polarization-gated fiber-optic probe capable of measurements in vivo. Using this probe, in the present study, we provide the proof-of-concept that optical interrogation of the visually normal rectal mucosa can be used to screen for colon cancer. Our studies show that rectal microvascular blood content is increased in patients who harbor significant colonic neoplasia.
Materials and Methods
Participants and acquisition of clinical data
All studies were approved and conducted under the supervision of the institutional review board at Evanston-Northwestern Healthcare, Evanston, IL. Subjects were eligible if they were scheduled for colonoscopy at Evanston Hospital for CRC screening or surveillance. Exclusion criteria included inability to give informed consent, colitis, poor preparation, and failure to intubate the cecum. A total of 216 patients were used in this study. At the end of the withdrawal (visualization) phase for colonoscopy, the probe was inserted into the accessory channel of the colonoscope and the probe tip was placed in gentle contact with the rectal surface. Ten endoscopically normal rectal sites were selected for probe measurements. For each tissue site, a total of five spectra, each taking 50 ms, were acquired with the probe in gentle contact with the tissue surface. For subsequent analysis, the five spectra were averaged together to form a single averaged spectrum per tissue site from which the hemoglobin from that site was determined. The per patient rectal hemoglobin was determined as the average of the 10 individual rectal readings. Any polyps found during the procedure were sent for pathologic analysis and classified as benign lesions (i.e., hyperplastic polyps), nonadvanced adenomas, or advanced adenomas. Advanced adenomas were defined as adenomas sized ≥10 mm, with >25% villous component, or having the presence of high-grade dysplasia.
Endoscopically compatible fiber-optic probe for measurement of microvascular blood supply
This has been described in detail in recent publications (15, 16). Briefly, we developed a polarization-gated fiber-optic probe to fit within the accessory channel of a standard or pediatric colonoscope. Previously, we observed EIBS using a 4D-ELF benchtop instrument, which could only be used with ex vivo tissue specimens and measured the angular, spectral, azimuthal, and polarization dependence of backscattered light. The polarization-gated probe, on the other hand, measures only the spectral and polarization dependence. This information was sufficient to allow for depth-selective quantification of blood concentration in vivo. As discussed below, polarization information was used to ensure optimal depth selectivity whereas spectral data were used to quantify blood content.
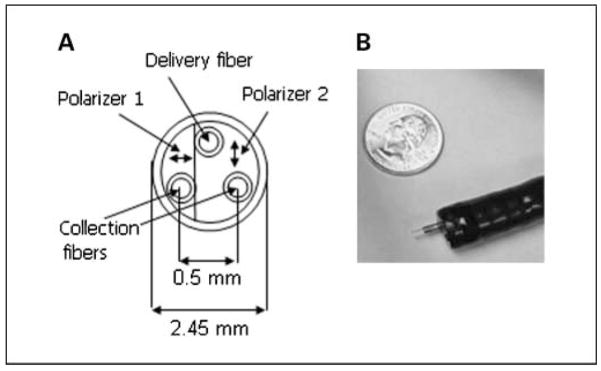
The probe was 2.45 mm and was able to fit easily through the accessory channels of a pediatric and adult colonoscope. The probe design has been previously published (15, 16). In essence, the probe is comprised of three 200-μm core diameter multimode fibers (numerical aperature, 0.22) arranged in an equilateral triangle with two polarization sheets with transmission axes orthogonal to each other cemented on the faces of the fibers. One of the fibers served as an illumination channel that coupled light from a 75-W xenon arc lamp onto the tissue surface, whereas the remaining two fibers collected backscattered light from the tissue. The two thin-film polarizer sheets polarized the incident light and allowed the independent collection of light polarized parallel to the incident beam (copolarized signal I ||) and light polarized perpendicular to the incident beam (cross-polarized signal I ⊥). A gradient refractive index lens was placed in front of the polarizing sheet to collimate the incident light (half-angle divergence of 3 degrees) and to focus backscattered light onto the two collection fibers. An integrated charge coupled device spectrometer recorded the spectra of I || and I ⊥ from collection fibers in the spectral range 280 to 780 nm. As can be seen from Fig. 1B, the probe easily fits within the accessory channel of the colonoscope.
Fig. 1.
A, schematic diagram (frontal view) of endoscopically compatible polarization-gated spectroscopy probe. B, photograph of the polarization-gated probe inserted through the biopsy port of the standard colonoscope.
A distinguishing feature of the probe design is that it enables depth-selective tissue analysis by using polarization gating (17–19). The differential polarization signal ΔI = I || − I ⊥, copolarized signal I ||, and cross-polarized signal I ⊥ probe progressively deeper tissue depths. For a given polarization signal, its depth of penetration is determined by the angle between incident and collected light and the sizes of the illumination and collection areas on the tissue surface. In turn, both variables can be controlled by selecting a proper focal length of the gradient refractive index lens, fiber diameters, and interfiber spacing. Therefore, sensitivity to a particular tissue depth can be tuned by both the physical design of the probe and the polarization signal analyzed. This is significant as the depth of the colonic microvasculature is ~ 100 μm, whereas light normally diffuses several millimeters into tissue. To selectively assess the mucosal microvasculature, the polarization-gated probe design ensured that light preferentially emerges within the penetration depth of ~ 100 μm. We tested several probe configurations and determined the design that had an optimized depth of penetration for colonic mucosa. Studies in tissue phantoms showed that the average penetration depths of the ΔI, I ||, and I ⊥ signals are ~ 95, 145, and 185 μm, respectively (16).
Blood content in colonic tissue was optically measured using Beer’s law and by taking advantage of the unique absorption spectra of oxygenated hemoglobin (OHb) and deoxygenated hemoglobin (DHb) in the visible wavelength range, as previously described (15, 16, 20). The collected backscattered light has an inverse exponential relationship with absorber concentration in tissue: , where I scattering (λ) represents the scattering signal from the sample if it were devoid of absorbers, A OHb(λ) and A DHb(λ) are the extinction spectra of the two primary absorbers in tissue: oxygenated and deoxygenated hemoglobin, and αOHb and αDHb are coefficients that are the products of light path length and the concentrations of the oxygenated and deoxygenated forms of hemoglobin, respectively. The individual spectra of extinction coefficients for OHb and DHb were compiled from published sources (21) and then corrected for the phenomenon known as hemoglobin packing described by Finlay and Foster (22). Published extinction spectra currently consider only homogeneous solutions of hemoglobin, but in vivo hemoglobin is distributed inhomogeneously in RBCs and blood vessels leading to hemoglobin molecules shielding each other from incident light. For a solution of RBCs, Finlay and Foster showed that the corrected extinction spectra, A(λ), can be found by multiplying the extinction spectrum in solution by a correction factor that depends on the absorption coefficient inside the RBC as well as the cellular radius R. When blood vessels are present, the packaging length scale R will be determined by the effective blood vessel diameter (23, 24). The effect of scattering on the measured spectrum was modeled using the fractal Born approximation with no upper bound on the fractal range of correlation lengths such that I scattering (λ) ∝ λ2β− 4 (25). The variables αOHb, αDHb, β, and R were chosen by a nonlinear optimization MATLAB routine such that the sum of square error between calculated and measured spectra in the range of 480 to 680 nm is minimized. Because the coefficients αOHb and αDHb have a linear relationship with oxyhemoglobin and deoxyhemoglobin concentration, the exact concentrations were extracted from the αOHb and αDHb coefficients by using a calibration curve.
Statistical analysis
Statistical analysis was done using Stata 9 (StataCorp) and Microsoft Excel. Markers were compared between patients with no neoplasia and patients with adenoma using a two-sided Welch’s t test. A two-sided P < 0.05 was considered statistically significant. Effects of benign diseases were assessed using a single-factor ANOVA analysis. The influence of demographic factors was found using a least squares multivariate linear regression. The difference in proportions of patients having a certain demographic factor between disease groups was tested using Fisher’s exact test.
Diagnostic performance for discrimination between patients without adenomas and those patients with advanced adenomas was assessed with logistic regression using two markers (mucosal oxygenated hemoglobin content and patient age). Using the logistic regression algorithm, the predicted probability of having an advanced adenoma is computed for each patient based on the mucosal oxygenated hemoglobin content and age marker values. A “cutoff” probability value is chosen to classify each patient as positive or negative. A receiver operating characteristic (ROC) curve was generated by plotting sensitivity and 1 - specificity for the whole range of possible cutoff values. The area under the ROC curve represents the probability that the logistic regression model will give a higher predicted probability to a randomly selected positive sample versus a randomly chosen negative sample. This metric is used to summarize the overall accuracy of the logistic regression algorithm. To test the stability of the logistic regression model, leave-one-out cross-validation (LOOCV) was done using MATLAB. LOOCV trains the algorithm on all samples except for one point that serves as the test set. The process is repeated until all samples have been tested. For each iteration, a logistic model was formed using marker values from the n − 1 training samples and a predicted probability was calculated for the test point. After all iterations were completed, another ROC curve was generated using the predicted probabilities generated by the LOOCV algorithm.
Results
Patient characteristics
We obtained readings from 216 patients undergoing colonoscopy. Of these 216 patients, 194 were Caucasian and 11 had polyps from previous colonoscopy. Grouped by pathologic findings, 165 patients were adenoma-free, 39 had nonadvanced adenomas with 9 of these harboring more than one nonadvanced adenoma, and 12 patients possessed advanced adenomas. Subjects with no neoplasia had a mean age of 56 ± 11 years and 69 (42%) were females; the subjects with adenomas had a mean age of 57 ± 9 years and 11 (28%) were females; and the subjects with advanced adenomas had a mean age of 64 ± 11 years and 5 (42%) were females. Table 1 indicates that the proportion of patients in the nonadvanced adenoma group having a family history of CRC was significantly different than the no neoplasia group (26% versus 12%, respectively). Advanced adenoma patients had significantly different proportions of patients having a smoking history and personal history of polyps when compared with the no neoplasia patients (33% versus 15% and 33% versus 7%, respectively). Patients with advanced adenomas also had a significantly increased age when compared with the no neoplasia subjects, with advanced adenoma patients having an age (mean ± SD) of 64 ± 11 years and the no neoplasia patients having an age of 56 ± 11 years (P = 0.03).
Table 1.
P values for test of two proportion analyses showing differences in demographic factor representation between no dysplasia and adenoma groups
| Adenoma (n = 39) | Advanced adenoma (n = 12) | |
|---|---|---|
| Gender | N.S. | N.S. |
| Age | N.S. | 0.034 |
| Race (non-white vs white) | N.S. | N.S. |
| Current smokers | N.S. | N.S. |
| Current alcohol intakers | N.S. | N.S. |
| Personal history of polyps | N.S. | 0.011 |
| Personal history of CRC | N.S. | N.S. |
| Family history of CRC | 0.038 | N.S. |
Abbreviation: N.S., insignificant values (P > 0.05).
Assessment of EIBS markers
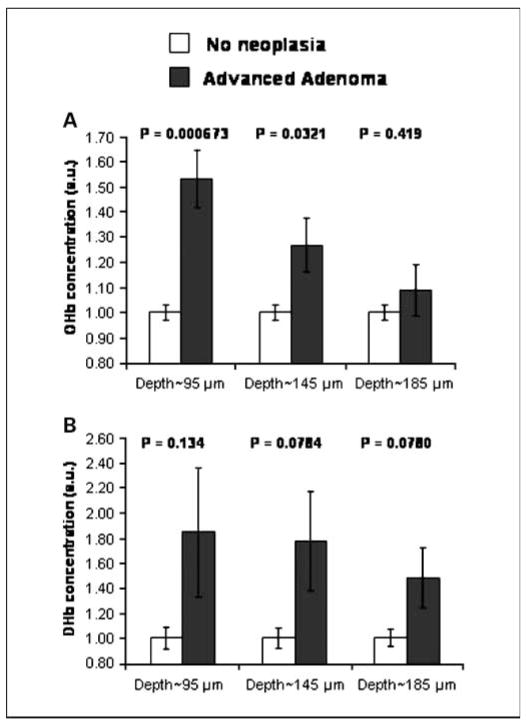
We analyzed rectal hemoglobin content from patients belonging to four categories: no neoplasia, patients having a single nonadvanced adenoma (size <1 cm, no high-grade dysplasia, and <25% villous features), patients harboring multiple nonadvanced adenomas, and patients harboring an advanced adenoma. Figure 2A and B shows an increase in OHb and DHb contents in patients with advanced adenomas. Three conclusions can be made from Fig. 2. First, the effect in advanced adenomas was statistically significant for OHb but not for DHb. Second, Fig. 2A shows that the OHb content from a depth of ~ 95 μm provides the greatest diagnostic separation between patients without neoplasia and those having an advanced adenoma. Indeed, OHb was markedly (54%) different (depth, ~ 95 μm) between the no neoplasia and advanced adenoma patients (P = 0.000673). Finally, Fig. 2A indicates that the effect size and hence the P values diminish as the penetration depth is increased. This suggests that EIBS in the uninvolved mucosa of patients harboring neoplasia is predominantly localized within the capillary network underlying the epithelium.
Fig. 2.
Depth-selective measurement of the microvascular blood content in the uninvolved rectal mucosa enables diagnosis of advanced colonic neoplasia. A, OHb concentration measured from three different penetration depths for advanced adenoma (n = 12) and no neoplasia patients (n = 165): OHb content as assessed from a depth of ~ 95 μm was significantly increased in patients with advanced adenomas (P = 0.000673). The P value gradually becomes insignificant as the penetration depth is increased, indicating that the EIBS effect is localized within the capillary network underlying the epithelium. B, DHb measured from three different penetration depths for advanced adenoma and no neoplasia patients: DHb content was increased in patients with advanced adenomas but was not statistically significant. Bars, SE.
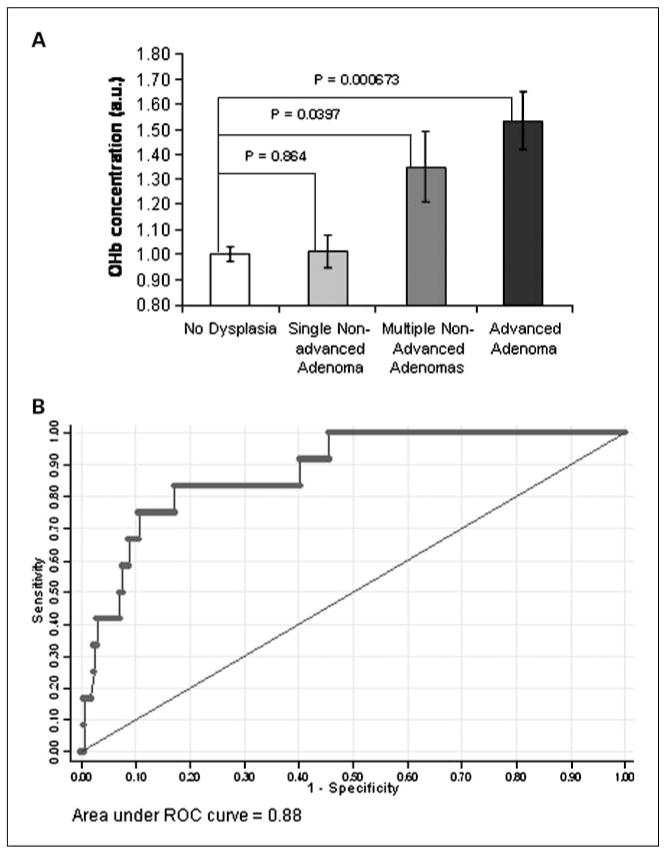
Having determined from Fig. 2 that OHb (depth, ~ 95 μm) was the most diagnostic marker, we proceeded to investigate whether neoplastic progression was associated with alterations in OHb content. Figure 3A shows that OHb content in the rectal mucosa mirrors the significance of neoplastic lesions harbored elsewhere in the colon (no neoplasia → advanced adenoma). There is an augmentation of OHb content in patients having multiple adenomas when compared with no neoplasia patients. The increase in OHb content is statistically significant with a P = 0.0397 and an effect size of 33%.
Fig. 3.
Mucosal blood supply increase parallels the significance of colon neoplasia and enables accurate diagnosis of advanced adenomas. A, the effect of the stage of colon neoplasia on OHb content (depth, ~ 95 μm): OHb is significantly increased for patients harboring advanced adenomas (n = 12, P = 0.000673) and multiple nonadvanced adenomas (n = 9, P = 0.0397) but not for single nonadvanced adenomas (n = 30, P = 0.864). Bars, SE. B, ROC for the logistic regression analysis using mucosal OHb content (depth, ~ 95 μm) and patient age to discriminate advanced adenoma patients from those patients without adenomas. The area under the ROC curve is 0.88 with an achievable 83% sensitivity and 82% specificity.
Performance characteristics of the EIBS markers
To characterize the diagnostic performance of the rectal EIBS for advanced adenomas, a logistic model was constructed using mucosal oxygenated hemoglobin content and patient age. Oxygenated hemoglobin content and patient age were found to be uncorrelated with each other in the no neoplasia group (Pearson r value = −0.00882). Therefore, mucosal oxygenated hemoglobin content and patient age were treated as independent predictors of advanced neoplasial risk. Application of the logistic model to the patient data set resulted in an area under the ROC curve of 0.88 with an achievable sensitivity and specificity of 83% and 82%, respectively, as shown in Fig. 3B. LOOCV was done to test the stability of the area under the ROC curve. After LOOCV, the area under the ROC curve remained high at 0.84, demonstrating the robustness of the logistic model.
Influence of location
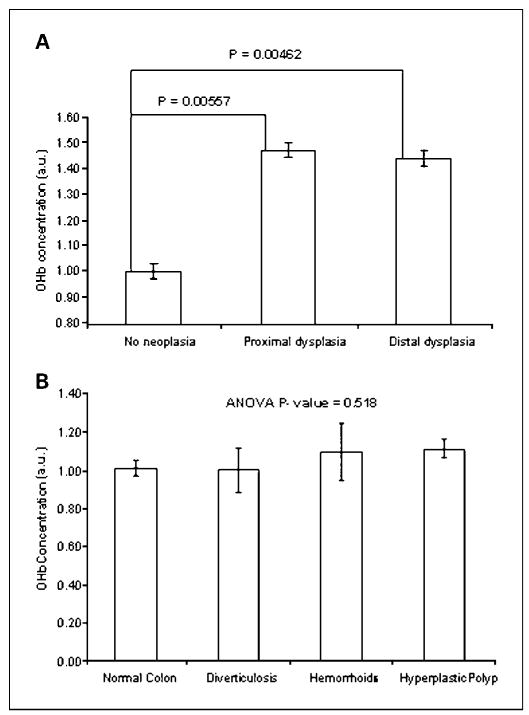
We next analyzed the increase in OHb content as a function of the adenoma location. Advanced and multiple nonadvanced adenomas were grouped into proximal and distal locations (defined at the level of the splenic flexure). Patients with both a distal and a proximal adenoma were grouped into the distal location category. Figure 4A shows that both proximal and distal neoplastic lesions gave rise to an ~ 40% increase in OHb content. Adenoma location did not influence the increase in OHb content in the uninvolved rectal mucosa.
Fig. 4.
A, proximal and distal lesions (advanced and multiple nonadvanced adenomas) give rise to approximately the same increase in mucosal OHb concentration (depth, ~ 100 μm). Patients with proximal lesions (n = 10) have OHb content increased by a statistically significant 47% (P = 0.00557) whereas patients with distal lesions (n = 11) have OHb content increased by a statistically significant 44% (P = 0.00462). B, the presence of benign disease was not associated with the alteration of the OHb content (depth, ~ 95 μm). Patients without neoplasia were stratified by the presence of benign diseases such as hyperplastic polyp (n = 31), hemorrhoids (n = 13), diverticulosis (n = 14), or no benign disease (n = 107). P = 0.518 for ANOVA. Bars, SE.
Potential confounding factors
The effect of benign diseases and demographic factors on OHb was assessed to prove that the observed EIBS represented the presence of neoplasia rather than any potential confounding factor. To characterize the effect of benign pathologies, patients were categorized into four groups: patients with no dysplasia and no benign lesions of any sort (as determined by colonoscopy and personal history), patients with hyperplastic polyps, patients with hemorrhoids, and patients with diverticulosis. The average OHb readings from these groups are presented in Fig. 4B. ANOVA was P = 0.518 for OHb. Any effect of these benign diseases must therefore be small and is unlikely to confound neoplasia identification.
In addition to benign diseases, the effect of demographic factors on the OHb content of no neoplasia patients was characterized by multivariate linear regression. The outcome of the regression analysis is summarized in Table 2. Gender was the only demographic factor to have a statistically significant effect (P = 0.006) on OHb. Males had an average of 17.1% greater mucosal OHb content compared with females. Because age is a known risk factor for neoplastic lesions, it was an important finding that age did not significantly affect OHb (P = 0.412). We therefore conclude that age and OHb are independent predictors of colonic neoplasia risk.
Table 2.
Multivariate linear regression ANOVA P values showing effect of demographic factors on measured OHb (depth, ~ 95 μm)
| ANOVA (P) | |
|---|---|
| Gender | 0.007 |
| Age (<55 vs ≥55 y) | 0.412 |
| Race (white vs non-white) | 0.079 |
| Current alcohol status | 0.667 |
| Current smoking status | 0.132 |
| Personal history of polyp | 0.512 |
| Personal history of CRC | 0.560 |
| Family history of CRC | 0.396 |
NOTE: Only no dysplasia patients (n = 165) were used in the analysis.
Next, we investigated the effect of bowel preparation on rectal hemoglobin concentration measurements given that phospho-soda–based preparations can commonly result in erythema/erosions in the distal colon (26). Rectal hemoglobin concentration readings from 48 patients with polyethylene glycol–based preparations (TriLyte, HalfLytely, and GoLytely) were compared with rectal hemoglobin concentration readings from 110 patients with Fleet’s phospho-soda preparation. There was no significant change in hemoglobin concentration between these two types of preparations (effect size = 1.4%, P = 0.83), indicating that the type of preparation used did not interfere with rectal concentration readings.
Finally, we investigated whether the duration of the colonoscopy or the removal of an adenoma had any effect on rectal measurements. To assess these potential confounding factors, rectal measurements were taken both at the beginning of colonoscopy and at the end of the withdrawal (visualization) phase for a subset of 54 patients, with 16 of these patients harboring adenomas. We found that there was no significant change in hemoglobin concentration from the rectal measurements before and after the withdrawal phase for the 16 patients harboring adenomas (effect size = 4%, P = 0.56). In addition, there was no significant correlation between the time of colonoscopy (defined as the time between the last rectal measurement at the beginning of colonoscopy and the first rectal measurement after the visualization phase) and the ratio of before and after visualization average rectal hemoglobin concentration readings for both patients without neoplasia (R2 = 0.0002) and patients harboring an adenoma (R2 = 0.046). Therefore, we conclude that both removal of an adenoma and duration of colonoscopy do not confound our rectal measurements.
Discussion
We provide the first demonstration that the blood supply in the endoscopically normal rectal mucosa is altered in subjects with concurrent biologically significant colonic neoplasia. Thus, increased mucosal blood content may serve as a potential marker of field carcinogenesis and hence might be used for neoplasia screening. This blood supply augmentation tended to be localized in the capillary network underlying the epithelium (depth, ~ 100 μm). The mucosal OHb (depth, ~ 95 μm) was significantly augmented in those patients harboring either multiple nonadvanced adenomas or advanced adenomas. Using the OHb and patient age markers in a logistic regression rule gave excellent discrimination between patients with no neoplasia and those with advanced adenomas with an area under the ROC curve of 0.88, a sensitivity of 83%, and a specificity of 82%. Depth-selective measurement of hemoglobin concentration from the uninvolved rectal mucosa using the endoscopically compatible polarization-gated probe has provided in vivo human validation of our previous reports on the EIBS phenomenon.
Our group initially described EIBS as a marker of field carcinogenesis. Although there has been emerging evidence of genetic/proteomic alterations in histologically normal mucosa of patients harboring neoplasia, no previous reports had assessed the blood supply. This was largely due to the inability of conventional techniques to discriminate and accurately assess a relatively small proportion of the total colonic blood supply—mucosal microcirculation including the pericryptal plexus. Polarization-gated spectroscopy, on the other hand, allows accurate quantification of this blood content through the depth selectivity of polarization gating. Given the field effect, patients having neoplastic lesions would be expected to have hyperproliferative and hypermetabolic mucosa and would therefore require an increased blood supply. Previous studies have shown that epithelial proliferation from the rectum is elevated in subjects harboring neoplastic lesions anywhere in the colon (27–29). Studies from human biopsies showed that the superficial blood supply from the midtransverse colonic mucosa was increased in patients who harbored advanced adenomas either in the rectum, sigmoid, or cecum, suggesting the presence of a diffuse field effect throughout the colon (14). Moreover, our initial in situ studies showed a gradient effect in which EIBS magnitude mirrored the distance to lesions (15), suggesting that both field carcinogenesis and tumor-elaborated factors seemed to be important. Our current report showing increased blood content in the uninvolved rectal mucosa of patients possessing neoplastic lesions provides in vivo evidence of the diffuse field effect component, which is consonant with our previous animal studies (14). Furthermore, the current report shows how this diffuse field effect may be leveraged for screening purposes.
Attempts to use the field effect for CRC screening have yielded poor performance to date. Flexible sigmoidoscopy is commonly used, with ~ 1.2 million exams done in 2002, but the sensitivity of a sentinel distal adenoma as a marker of advanced proximal neoplasia is remarkably low (~ 33% in women; ref. 5). Advanced technologies such as microarray and proteomic analyses have attempted to identify the genetic/epigenetic changes associated with field carcinogenesis (8, 9, 30). Although these techniques have validated the approach of detecting the field effect in the uninvolved rectal mucosa, their translation to a clinical setting has been stymied by low accuracy and technological hurdles.
Methods of CRC screening not based on the field effect concept have also been implemented but they too suffer from poor diagnostic performance or difficulty being applied in the clinic. The most frequently used method, guaiac-based fecal occult blood testing, has a sensitivity of 10.8% (31), whereas immunohistochemical fecal occult blood testing has a sensitivity of 27% (32). CT colography (virtual colonoscopy) represents one of the most promising new-generation tests and was recently sanctioned for average-risk screening (33). However, the reported large multicenter trial showed a sensitivity for advanced adenomas that were comparable to our rectal EIBS data (per lesion rate of 84% versus 83%). Moreover, there are many possible advantages of rectal EIBS to CT colography, including lack of need for colon purge, less pain and expense, and no radiation exposure.
Our results show that rectal mucosal blood content measurement is sensitive to advanced adenomas and multiple nonadvanced adenomas, but not to single nonadvanced adenomas. From a clinical perspective, this is acceptable as the advanced adenoma has been suggested by Winawer and Zauber to be the primary target of screening because it is associated with a relatively high risk of progressing to colon cancer (34). The insensitivity of our technique to single nonadvanced adenomas supports the notion that the magnitude of field carcinogenesis alterations mirrors the neoplastic outcomes. This may be clinically adequate given studies in which the vast majority of nonadvanced adenomas never progress to CRC. On the other hand, the presence of multiple nonadvanced adenomas has been clearly shown to be a marker of significant long-term risk for advanced neoplasia (35). Indeed, clinical guidelines for polyp surveillance frequently consider advanced adenomas and multiple nonadvanced adenomas equivalently (36). A patient with multiple adenomas may have a more robust field effect, which is consonant with our finding of augmented microvascular blood content. Thus, rectal OHb seems to be sensitive to clinically significant neoplasia (advanced adenomas and multiple nonadvanced lesions), supporting the potential viability of rectal EIBS measurement for screening purposes.
There are several limitations to this study that need to be acknowledged. In our study, patients were classified into diagnostic categories based on colonoscopic findings. Colonoscopy, however, is not perfect. Tandem colonoscopy studies reveal that approximately one quarter of adenomas may be missed (37). Advanced adenoma detection rates can vary 4-fold among endoscopists, indicating a sizable miss rate (38). Given these findings, it is plausible to assume that some of our controls actually harbored adenomatous lesions. If this were true, then actual baseline blood content levels from controls would likely be lower than observed in our study. This would actually improve our technique’s discrimination between patients harboring adenomas and those who do not. The estimation of adenoma size by endoscopy may be somewhat inaccurate, leading to the possibility of misclassification of some size-borderline advanced adenomas but this should not bias our results (39). Given patients were enrolled for screening, the cancer rate was very low as expected. Thus, it is not possible to comment on whether rectal EIBS engendered by carcinomas would be of greater magnitude. However, as Fig. 3 and previous reports show (15), larger adenomas give rise to more EIBS than smaller lesions, supporting the “dose dependence” of rectal EIBS. This will need to be confirmed in separate studies focusing on patients diagnosed with cancer.
The small number of “events” (advanced adenomas) always raises concerns about overfitting with regard to performance characteristics. However, given that only one variable was assessed (OHb concentration and patient age), this seems quite unlikely. This concern is further mitigated by the LOOCV, which shows the stability of our statistical methods. After validation, the area under the ROC curve remained high at 0.84. Our results will still need to be replicated in larger studies testing the prediction rule prospectively.
In conclusion, we show for the first time that the microvascular blood content in the histologically normal rectal mucosa is elevated in patients with multiple and advanced lesions. Our study is a proof-of-concept that the quantification of rectal blood content may offer a new method for colon cancer screening. For clinical application, we have developed a fiber-optic polarization-gated probe that allows in vivo interrogation of the rectal mucosa. If validated in future larger clinical studies, this thin and flexible probe could be potentially used by primary care physicians during the annual rectal exam to determine the need for colonoscopy.
Acknowledgments
Grant support: NIH grants R01CA128641, R01CA109861, R01CA118794, U01 CA111257, R42CA130508, and R01CA112315; a grant from the Coulter Foundation; and NSF grant CBET-0733868.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors have an ownership interest in ABO, LLC, which holds license on an EIBS patent (H. K. Roy, M. J. Goldberg, and V. Backman).
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. American Cancer Society Colorectal Cancer Advisory Group, US Multi-Society Task Force, American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Cooper GS, Doug Kou T. Underuse of colorectal cancer screening in a cohort of Medicare beneficiaries. Cancer. 2008;112:293–8. doi: 10.1002/cncr.23176. [DOI] [PubMed] [Google Scholar]
- 4.Roy HK, Backman V, Goldberg MJ. Colon cancer screening: the good, the bad, and the ugly. Arch Intern Med. 2006;166:2177–9. doi: 10.1001/archinte.166.20.2177. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JD, Ng K, Hung KE, et al. Detection of proximal adenomatous polyps with screening sigmoidoscopy: a systematic review and meta-analysis of screening colonoscopy. Arch Intern Med. 2003;163:413–20. doi: 10.1001/archinte.163.4.413. [DOI] [PubMed] [Google Scholar]
- 6.Schoenfeld P, Shad J, Ormseth E, et al. Military Colo-rectal Cancer Screening Trials Group. Predictive value of diminutive colonic adenoma trial: the PREDICT trial. Clin Gastroenterol Hepatol. 2003;1:195–201. doi: 10.1053/cgh.2003.50029. [DOI] [PubMed] [Google Scholar]
- 7.Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl JMed. 1998;339:1277–84. doi: 10.1056/NEJM199810293391803. [DOI] [PubMed] [Google Scholar]
- 8.Hao CY, Moore DH, Chiu YS, et al. Altered gene expression in normal colonic mucosa of individuals with polyps of the colon. Dis Colon Rectum. 2005;48:2329–35. doi: 10.1007/s10350-005-0153-2. [DOI] [PubMed] [Google Scholar]
- 9.Polley AC, Mulholland F, Pin C, et al. Proteomic analysis reveals field-wide changes in protein expression in the morphologically normal mucosa of patients with colorectal neoplasia. Cancer Res. 2006;66:6553–62. doi: 10.1158/0008-5472.CAN-06-0534. [DOI] [PubMed] [Google Scholar]
- 10.Aotake T, Lu CD, Chiba Y, Muraoka R, Tanigawa N. Changes of angiogenesis and tumor cell apoptosis during colorectal carcinogenesis. Clin Cancer Res. 1999;5:135–42. [PubMed] [Google Scholar]
- 11.Shpitz B, Gochberg S, Neufeld D, et al. Angiogenic switch in earliest stages of human colonic tumorigenesis. Anticancer Res. 2003;23:5153–7. [PubMed] [Google Scholar]
- 12.Kim YL, Liu Y, Wali RK, et al. Simultaneous measurement of angular and spectral properties of light scattering for characterization of tissue microarchitecture and its alteration in early precancer. IEEE J Sel Top Quantum Electron. 2003;9:243–56. [Google Scholar]
- 13.Roy HK, Liu Y, Wali RK, et al. Four-dimensional elastic light-scattering fingerprints as preneoplastic markers in the rat model of colon carcinogenesis. Gastroenterology. 2004;126:1071–81. doi: 10.1053/j.gastro.2004.01.009. discussion 948. [DOI] [PubMed] [Google Scholar]
- 14.Wali RK, Roy HK, Kim YL, et al. Increased microvascular blood content is an early event in colon carcinogenesis. Gut. 2005;54:654–60. doi: 10.1136/gut.2004.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy HK, Gomes A, Turzhitsky V, et al. Spectroscopic microvascular blood detection from the endoscopically normal colonic mucosa: biomarker for neoplasia risk. Gastroenterology. 2008;135:1069–78. doi: 10.1053/j.gastro.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turzhitzsky V, Gomes A, Kim YL, et al. Measuring mucosal blood supply in vivo with a polarization gating probe. Appl Opt. 2008;47:6046–57. doi: 10.1364/ao.47.006046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Kim YL, Li X, Backman V. Investigation of depth selectivity of polarization gating for tissue characterization. Opt Express. 2005;13:601–11. doi: 10.1364/opex.13.000601. [DOI] [PubMed] [Google Scholar]
- 18.Backman V, Gurjar R, Badizadegan K, et al. Polarized light scattering spectroscopy for quantitative measurement of epithelial cellular structures in situ. IEEE J Sel Top Quantum Electron. 1999;5:1019–26. [Google Scholar]
- 19.Myakov A, Nieman L, Wicky L, Utzinger U, Richards-Kortum R, Sokolov K. Fiber optic probe for polarized reflectance spectroscopy in vivo: design and performance. J Biomed Opt. 2002;7:388–97. doi: 10.1117/1.1483314. [DOI] [PubMed] [Google Scholar]
- 20.Siegel M, Kim YL, Roy HK, et al. Assessment of blood supply in superficial tissue by polarization-gated elastic light scattering spectroscopy. Appl Opt. 2006;45:335–42. doi: 10.1364/ao.45.000335. [DOI] [PubMed] [Google Scholar]
- 21.Prahl S. Tabulated molar extinction coefficient for hemoglobin in water. Oregon Medical Laser Center. 1998 Available at http://omlc.ogi.edu/spectra/hemoglobin/summary.html.
- 22.Finlay JC, Foster TH. Effect of pigment packaging on diffuse reflectance spectroscopy of samples containing red blood cells. Opt Lett. 2004;29:965–7. doi: 10.1364/ol.29.000965. [DOI] [PubMed] [Google Scholar]
- 23.Reif R, Amorosino MS, Calabro KW, A’Amar O, Singh SK, Bigio IJ. Analysis of changes in reflectance measurements on biological tissues subjected to different probe pressures. J Biomed Opt. 2008;13:010502. doi: 10.1117/1.2870115. [DOI] [PubMed] [Google Scholar]
- 24.van Veen RL, Verkruysse W, Sterenborg HJ. Diffuse-reflectance spectroscopy from 500 to 1060 nm by correction for inhomogeneously distributed absorbers. Opt Lett. 2002;27:246–8. doi: 10.1364/ol.27.000246. [DOI] [PubMed] [Google Scholar]
- 25.Hunter M, Backman V, Popescu G, et al. Tissue self-affinity and polarized light scattering in the born approximation: a new model for precancer detection. Phys Rev Lett. 2006;97:138102. doi: 10.1103/PhysRevLett.97.138102. [DOI] [PubMed] [Google Scholar]
- 26.Zwas FR, Cirillo NW, el-Serag HB, Eisen RN. Colonic mucosal abnormalities associated with oral sodium phosphate solution. Gastrointest Endosc. 1996;43:463–6. doi: 10.1016/s0016-5107(96)70286-9. [DOI] [PubMed] [Google Scholar]
- 27.Sandler RS, Baron JA, Tosteson TD, Mandel JS, Haile RW. Rectal mucosal proliferation and risk of colorectal adenomas: results from a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2000;9:653–6. [PubMed] [Google Scholar]
- 28.Bostick RM, Fosdick L, Lillemoe TJ, et al. Methodological findings and considerations in measuring colorectal epithelial cell proliferation in humans. Cancer Epidemiol Biomarkers Prev. 1997;6:931–42. [PubMed] [Google Scholar]
- 29.Anti M, Marra G, Armelao F, et al. Rectal epithelial cellproliferationpatterns aspredictors ofadenomatous colorectalpolyp recurrence. Gut. 1993;34:525–30. doi: 10.1136/gut.34.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen LC, Hao CY, Chiu YS, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004;64:3694–700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 31.Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl JMed. 2004;351:2704–14. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 32.Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129:422–8. doi: 10.1016/j.gastro.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 33.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl JMed. 2008;359:1207–17. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am. 2002;12:1–9. v. doi: 10.1016/s1052-5157(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–85. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–85. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 37.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 38.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl JMed. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 39.Gopalswamy N, Shenoy VN, Choudhry U, et al. Is in vivo measurement of size of polyps during colonoscopy accurate? Gastrointest Endosc. 1997;46:497–502. doi: 10.1016/s0016-5107(97)70003-8. [DOI] [PubMed] [Google Scholar]