Abstract
We examined the effects of voluntary (16 weeks of wheel running) and forced (16 weeks of treadmill running) exercise on memory-related behavior, hippocampal volume, thioflavine-stained plaque number, and soluble Aβ levels in brain tissue in the Tg2576 mouse model of Alzheimer’s disease (AD). Voluntary running animals spent more time investigating a novel object in a recognition memory paradigm than all other groups. Also, voluntary running animals showed fewer thioflavine S stained plaques than all other groups, whereas forced running animals showed an intermediate number of plaques between voluntary running and sedentary animals. Both voluntary and forced running animals had larger hippocampal volumes than sedentary animals. However, levels of soluble Aβ-40 or Aβ-42 did not significantly differ among groups. The results indicate that voluntary exercise may be superior to forced exercise for reducing certain aspects of AD-like deficits – i.e., plaque deposition and memory impairment, in a mouse model of AD.
Keywords: Alzheimer’s Disease, Voluntary Exercise, Forced Exercise, Tg2576, Object Recognition, Hippocampal Volume
INTRODUCTION
Regular physical activity has clear health benefits (Fox et al., 1971; Stummer et al., 1994; Blumenthal et al., 1999; Colcombe and Kramer, 2003; Dishman et al., 2006). However, the results of epidemiological studies of physical activity in patients with dementing disorders, such as Alzheimer’s disease (AD), have been inconsistent, some reporting an inverse association between physical activity and cognitive decline (Laurin et al., 2001; Rovio et al., 2005), while others report no relationship (Broe et al., 1998; Verghese et a., 2003). More recently, exercise has been reported to attenuate behavioral deficits and CNS plaque deposition in a transgenic mouse model of AD (Adlard et al., 2005), although not in others (Wolf et al., 2006). Thus, while physical exercise has long been seen to contribute to physical and psychological health, there is now interest in whether it can delay progression of neurodegenerative diseases, such as AD.
The beneficial effects of exercise on stress management in people are well documented (Dishman et al., 1997; Starkweather 2007). In turn, stress is now being identified as a risk factor for the development of dementing disorders, such as AD (Black et al., 1991; Grootendorst et al., 2001; Wilson et al., 2005). Plasma cortisol levels, a widely used physiological marker of the stress state, have been correlated with the progression of AD (Csernansky et al., 2006). Further, chronic isolation stress has been reported to accelerate plaque development and cognitive decline (Dong et al., 2004; 2008) in the Tg2576 mouse model of AD. Moreover, acute restraint stress increases interstitial Aβ levels, possibly through a mechanism that involves corticotropin-releasing factor (CRF) and increases in neuronal firing (Kang et al., 2007).
The circumstances in which exercise occurs may determine its effects on the brain. For example, some human exercise regimens may be considered forced (O’Callaghan et al., 2007), and therefore could have deleterious effects on health by increasing stress levels. In most studies using animal models of neurodegenerative disorders, exercise is administered in a “voluntary” fashion; yet, forced exercise has also been shown to improve cognitive function in such models (Albeck et al., 2006; Ang et al., 2006). This would seem contrary to the conceptualization of forced exercise as “stressful” (Narath et al., 2001). In support of forced exercise as a stressful experience, forced exercise has been reported to increase hypothalamic CRF activity more than voluntary exercise in rodents (Yanagita et al., 2007).
The purpose of our study was to differentiate between the effects of voluntary and forced exercise on specific features of Alzheimer’s disease, the dependent variable being the exercise regimen, therefore we focused on Tg2576 transgenic mice. This model has been previously characterized to show reliable deficits in cognitive ability and hippocampal volume at 9 months of age, coinciding with plaque deposition (Hsiao et al., 1996; Dong et al, 2005; 2008). We hypothesized that both voluntary and forced exercise would be beneficial in reducing the rate of cognitive decline, plaque development, and hippocampal atrophy in Tg2576 mice and voluntary exercise would provide greater benefits than forced exercise.
MATERIALS AND METHODS
Animals
The Tg2576 mouse strain, created by Hsiao, et al (1996), over-expresses human APP 695. It contains a double mutation (Lys670-Asn, Met671-Leu [K670N, M671L]) driven by a hamster prion protein promoter. Tg2576 males (Taconic Farms Inc. Germantown, NY) were bred with C57B6/SJL (The Jackson Laboratory, Bar Harbor, Maine) females. Genotyping for transgenic screening in the offspring was performed using DNA obtained from post-weaning tail biopsies (Hanley and Merlie, 1991). PCR products were sequenced to confirm the presence of human APP DNA in offspring (Tg+). A total of 38 Tg+ animals were randomly assigned to one of four groups (described below): voluntary exercise (VOL) = 10, forced exercise (FOR) = 10, forced exercise control (FCON) = 9, sedentary (SED) = 9. Males and females were equally distributed among groups.
Experimental Design
Animals were individually housed and randomly assigned to one of four groups beginning at 5 months of age. Voluntary runners (VOL) were placed in a cage with a running wheel for one hour each day, five days per week, for 16 weeks. The number of revolutions was monitored by a counter and running distance was determined by multiplying revolution number by wheel circumference. Finally, average daily distance for the group was converted to a velocity (m/min). This velocity was then used to determine the running conditions for a forced exercise group (FOR). The FOR group began ran on a motor driven treadmill at the average velocity recording for the VOL group ran on a corresponding day. The FOR group was motivated by an electric grid at the back of the treadmill that provided a mild foot shock upon contact. To account for the potential stress effect of the electric grid, the number of shocks each animal received during the daily hour run was counted and a paired animal was placed on the grid, but without access to the treadmill belt, and subjected to the same number of shocks over a one hour period. These animals constituted the forced control group (FCON). Finally, a sedentary group (SED) remained in their home cages throughout the course of the experiment. Daily running took place during the dark phase of the light/dark cycle to maximize activity levels and to avoid stresses of sleep disruption. A timeline for experimental manipulations is presented in Figure 1.
Figure 1. Experimental Timeline.
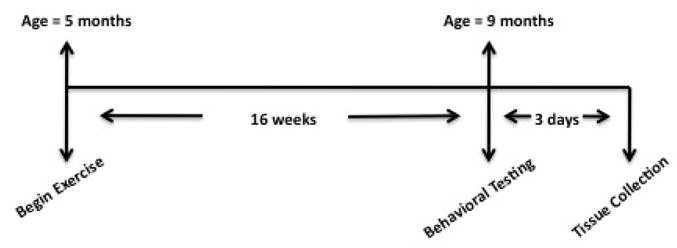
Time line of experimental manipulations. Mice in exercise groups began daily 1 hr sessions beginning at 5 months of age and continuing until 9 months of age. Behavioral testing was conducted on 3 consecutive days and tissue was collected immediately following behavioral testing on the last day.
Behavioral Analysis
Behavioral testing began at 9 months of age, four months after the initiation of the exercise paradigm. Notably, Tg2576 begin to show reliable signs of plaque deposition and behavioral impairment at 9 months of age (Hsiao et al., 1996). General activity, spontaneous alternation and object recognition memory was measured in each animal. Tests were conducted in the order presented and only one test was performed each day over a period of 3 consecutive days.
Open Field
A table (20×15 in) with a grid of 2×2 in squares on the surface was placed in the corner of a room such that two sides of the table were against a wall and two sides were open. Mice were placed on top of the table and allowed to explore freely for 20 minutes. Sessions were video recorded for later analysis. The numbers of lines crossed during the 20 min session were counted as a measure of general activity.
Spontaneous Alternation
Each animal was placed in the center of a Y-maze and allowed to explore for 8 minutes. Sessions were video recorded for later analysis. The number of arm choices and pattern of choices were recorded for each animal. When all four paws had entered the arm, the animal was considered to have made an arm entry. Alternations were defined as 3 different consecutive arm entries. Alternation percentage was calculated by summing the total number of alternations that occurred and dividing by the number of alternations possible (total number of arm choices minus 2). Chance alternation in this task is 33%. Number of arm choices and pattern of arm choices (alternating arms) were analyzed as measures of general activity and spatial working memory.
Object Recognition
Pilot studies were conducted using Tg2576 mice to evaluate the level of interest of specific objects and two different objects showing equal interest levels were chosen for this study. Mice were habituated to the test arena (8.5×17×8 in) for 20 min per day, 2 days before testing. Each mouse received a sample trial and a test trial. During the sample trial mice were placed in a familiar arena with 2 copies of the same object and allowed to explore for 10 min then returned to their home cage. Following a 50 min delay mice were placed back in the arena where a novel object was presented with one of the familiar objects explored during the sample trial and allowed to explore for 10 min. Objects and test arena were cleaned with 70% ethanol after each trial. Sessions were video recorded for later analysis. The amount of time each animal spent actively investigating the objects was manually scored using Stopwatch+ software (CBN, Emory University, Atlanta GA). Active investigation was defined as facing the object within close proximity (2 cm) and/or touching the object with forepaws. The amount of time spent investigating the novel object as compared to the familiar object was analyzed as a measure of recognition memory as previously described (Dere et al., 2005) (Figure 2). Novelty preference scores were derived by calculating the percentage of the total investigation time spent investigating the novel object for each animal.
Figure 2. Object Recognition Procedure.
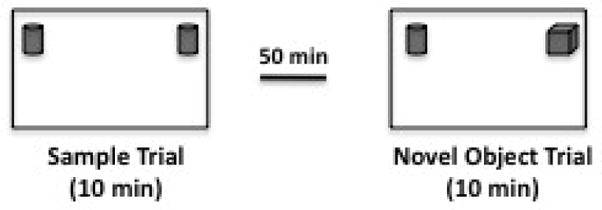
Schematic drawing of novelty-preference paradigm. Mice should spend a roughly equal percentage of their total investigation time investigating both objects during the sample trial and a higher percentage of their total investigation time on the novel object during the test trial. Pilot tests were conducted to identify objects of equal interest to this strain of mice.
Tissue preparation
Following behavioral testing, the animals were sacrificed by rapid decapitation, the brains were removed and the hemispheres were separated on ice. The hippocampus and cortex from one hemisphere, selected at random, were snap-frozen on dry ice, then stored at −80° C for tissue Aβ analysis. The other hemisphere was placed in fixative containing 4% paraformaldehyde for 1 week. The fixed hemispheres were subsequently immersed in same fixative with 30% sucrose for 48hr prior to embedding in Tissue-Tek embedding medium (Electron Microscopy Sciences, Hatfield, PA, USA) and cut into 35 μm thick serial sections in the coronal plane using a cryostat (Lecia CM 1850 UV, Nussloch, Germany). Every 8th section was saved in a series. One series was used for Thioflavin S staining and one used for Nissl staining.
Soluble Aβ analysis
Tissue levels of Aβ-40 and Aβ-42 from selected brain areas were determined by Enzyme-Linked ImmunoSorbent Assay (ELISA). The hippocampus and cortex from one hemisphere were homogenized in PBS to measure soluble Aβ, and centrifuged at 10,000 rpm for 5 min. Supernatants were collected and protein quantification was performed using the bicinchoninic acid (BCA) assay (Bio-Rad, Hercules, CA). Samples were analyzed for soluble Aβ using a sandwich ELISA specific for human Abx-40 or ABx-42. Briefly, Aβx-40 and Aβx-42 were captured using monoclonal antibodies targeted against amino acids 35–40 (mHJ2) or 33–42 (m21F12) of Aβ, respectively. Samples were loaded in duplicate onto plates and stored at 4°C overnight. For both assays, a biotinylated central domain monoclonal antibody (mHJ5.1) followed by streptavidin-poly-HRP 40 (Fitzgerald, Concord, MA) was used to detect. Assays were developed using Super Slow ELISA TMB (Sigma, St. Louis, MO) and read on a Bio-Tek FL-600 plate reader (Winooski, Vermont) at 650 nm. Resultant values were normalized to total protein concentration.
Histology
Thioflavin S (Sigma, St. Louis, MO) was used to stain sections for beta-pleated sheet structures. Tissue sections were incubated in 0.5% thioflavin S in 50% ethanol for 3–5 min, mounted on slides and coverslipped. Thioflavin S positive plaques in the hippocampus and cortex were counted by an observer blind to treatment conditions. Another set of serial sections was mounted on gelatin-coated slides and allowed to air-dry. The slides were then rehydrated in double distilled water, submerged in a standard thionin solution for 15 to 45 s until the desired depth of staining was achieved, and then gradually dehydrated for 5 min in successive baths of ethanol (i.e. 50%, 75%, 90%, 95% and 100%). Each slide was then given two 15 min baths in 100% xylenes and coverslipped for hippocampal volume measurement under light microscope.
Hippocampal Volume
Nissl-stained coronal sections were used to estimate the total volume of the hippocampal formation of one hemisphere. All sections containing the hippocampus were photographed. The final resolution of the images were one pixel2 = 0.00056787 mm2. Using Analyze 8.0 stereology module, the area of hippocampus on each photograph was measured in each coronal section using Cavalieri’s principle. The contours of the hippocampus were identified using landmarks derived from a mouse brain atlas (Paxinos and Franklin, 2001). A grid was randomly placed over each photograph. The points on the grid were spaced eight pixels apart on the X and Y axes. Thus, the total number of grid points over the target area was multiplied by 64 to obtain an estimate of the total pixels encompassing the hippocampal formation in each brain. The total pixels were then multiplied by 0.00056787 mm2 and 0.28 mm (the distance between sections) to obtain hippocampal volume (mm3).
Statistical Analysis
Repeated measures ANOVA was used to determine where there were significant effects of group status between objects in object recognition tests. When ANOVA suggested significant effects of group, Fisher’s LSD was used post-hoc to evaluate between-group differences. One-way ANOVAs were performed to evaluate group effects on novelty preference scores, open field activity, spontaneous alternation, total arm choice, plaque load, hippocampal volume, and soluble Aβ levels. Pearson’s r was used for correlations between shock and other dependent measures. All analyses were conducted using Statistica software (Statsoft, Tulsa, OK) with statistical significance a priori set at p < 0.05.
RESULTS
Running distance and shock exposure
The average running velocity for the VOL mice over the 4 month training period was 10.9±1.6 m/min with a range from 4.4±2.3 m/min (Week 1) to 15.5±3.5 m/min (Week 4). The overall daily mean number of shocks received by the FOR animals was 32.8±38.6 shocks/day. Shock number in these animals ranged from 1.9±2.9 to 129.5±119.0 shocks/day. As stated in the Methods the FCON received an equal number of shocks to the FOR group in a pair matched manner.
Open Field Activity
The analysis of general activity level of animals completing the session indicated no significant differences among groups [F(3,22) = 0.922, p = 0.446] (data not shown). Open field activity was conducted on a tabletop such that two of the edges were open and several of the mice jumped off the table and did not complete the 20-min session. Interestingly, more animals in the SED (n = 3), FOR (n = 3), and FCON (n = 4) groups jumped off the table compared to the VOL (n = 1) group. Total number of animals completing the session in each group were as follows: VOL = 9, SED = 5, FOR = 7 and FCON = 5.
Spontaneous Alternation
Alternation rate did not significantly differ among groups [F(3,30) = 0.44, p = 0.72] and all groups alternated above chance levels in this test (data not shown). Mean alternation rates and standard errors for each group were as follows: VOL = 43.62 ±4.1%; SED = 43.74 ±5.8%; FOR = 45.70 ±3.1%; FCON = 38.51 ±6.1%. Total arm choice also did not significantly differ among groups [F(3,30) = 1.43, p = 0.25] suggesting similar levels of activity in this measure.
Object Recognition
Repeated measures ANOVA of object investigation in the sample trial indicated no significant effects of group [F(3,32) = 0.59, p = 0.63], Object [F(1,32) = 0.04, p = 0.84] or interaction between Group and Object [F(3,32) = 0.58, p = 0.62] suggesting that animals in all groups investigated each object equally (Figure 3B). Analysis of the total amount of time spent in active investigation during the sample trial was also insignificant [F(3,32) = 0.72, p = 0.543] indicating that the groups did not respond differently to novelty (Figure 3A).
Figure 3. Object Recognition Sample Trial.
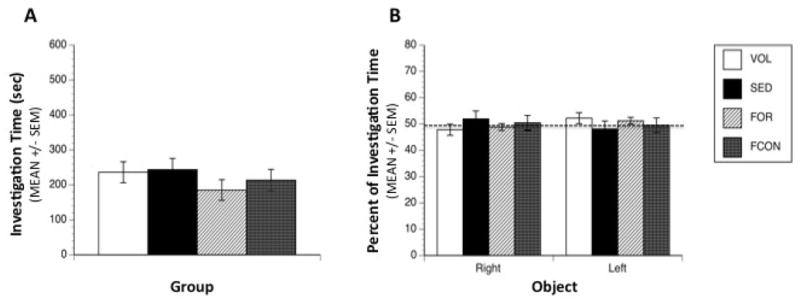
Object investigation during the sample trial. (A) No significant differences were observed between groups in total time spent investigating both objects, and all groups spent an average of 220 sec in active investigation of the objects during the 600 sec trial. (B) All groups spent an equal percentage of their investigation time on both copies of the object during the sample trial. Dashed line represents chance level of investigation.
In the novel object test we found a significant interaction between Group and Object [F(3,33) = 3.20, p = 0.03] in investigation. Post hoc analysis indicates that the VOL group spent significantly more time investigating the novel object in relation to the familiar object (p = 0.0009) while the other groups did not show significant differences between objects suggesting improved recognition memory in the VOL group. The differences among all other groups were not significant. One-way ANOVA of the novelty preference scores revealed a significant main effect of Group [F(3,33) = 3.78, p = 0.019] with post-hoc tests indicating that the VOL group spent more time investigating the novel object compared to all other groups (SED, p = 0.0069; FCON, p = 0.0077; FOR, p = 0.036) (Figure 4B). Total investigation times were not significantly different among groups [F(3,33) = 0.105, p = 0.956] (Figure 4A).
Figure 4. Object Recognition Test Trial.
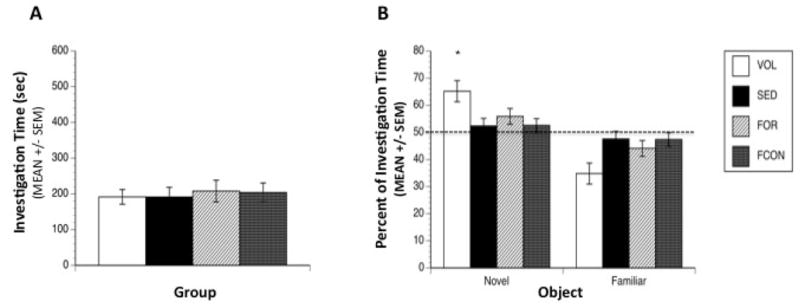
Object investigation during the test trial. (A) No significant differences were observed among groups in total time spent in active investigation of the objects during the test trial. Average investigation time was 199 sec during the 600 sec trial. (B) Animals in the VOL group showed a significant preference for the novel object compared to all other groups. (* denotes p < 0.05). Mice exposed to voluntary running were the only group to show significant differences in investigation time between the novel object and the familiar object. Dashed line represents chance level of investigation.
Soluble Aβ-40 and Aβ-42
The analysis of tissue Aβx-40 and Aβx-42 levels in the cortex and hippocampus revealed no significant differences among groups [F(3,33) = 0.314, p = 0.82 and F(3,33) = 0.885, p = 0.459] (data not shown). Means and standard deviations for Aβx-40 were VOL = 106.44 +/− 2.83, SED = 88.06 +/− 2.53, FOR = 99.03 +/− 1.54 and FCON = 96.02 +/− 3.19 pg/mg total protein. Means and standard deviations for Aβx-42 were VOL = 100.32 +/− 2.75, SED = 94.87 +/− 3.73, FOR = 101.19 +/− 2.54 and FCON = 90.11 +/− 1.95 fg/mg total protein.
Plaque Counts
Small amyloid plaques (<50 μm in diameter) were observed in the hippocampus and overlying cortex of Tg2576 mice, in keeping with previous findings (Dong et al., 2004; 2008). One-way ANOVA on total number of Thioflavin S stained plaques in the brain shows a significant effect of Group [F(3,31) = 10.56, p = 0.00001] with post-hoc tests indicating that the VOL group had fewer plaques than SED, FOR and FCON (all p’s < 0.003) (Figure 5) and that the FOR group had fewer plaques than the SED (p = 0.018).
Figure 5. Thioflavine-stained Plaque Count.
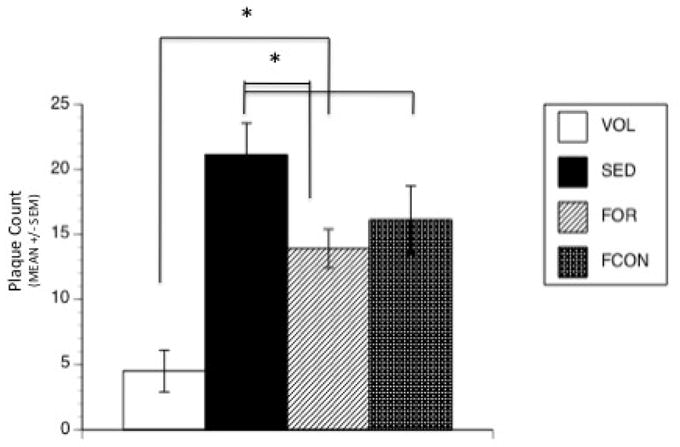
Quantative analysis of Thioflavine S stained plaques in the brains of Tg2576 mice at 9 months of age. Plaque number was significantly decreased in VOL group compared to all other groups. The FOR group had fewer plaques than the SED group. (* denotes p < 0.05).
Hippocampal Volume
The analysis of hippocampal volumes revealed a significant effect of group [F(3,31) = 4.577, p = 0.009] with post-hoc tests showing that the VOL and FOR groups were significantly larger than the SED group (p = 0.005 and p = 0.002, respectively), but not the FCON group (p = 0.26) (Figure 6A).
Figure 6. Hippocampal Volume.
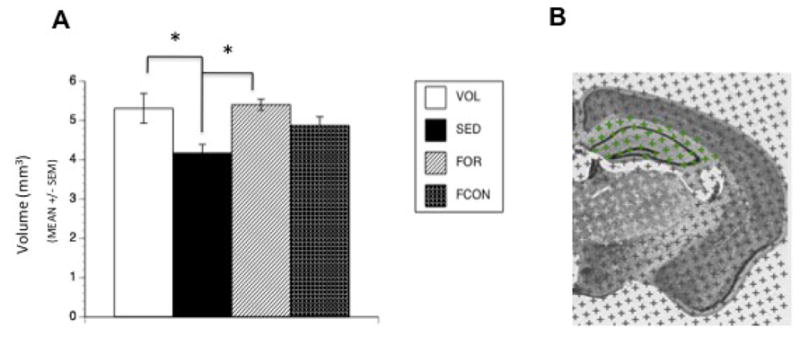
Hippocampal volume measurements. (A) Both VOL and FOR groups show increased hippocampal volume after 16 weeks of exercise compared to SED controls. (* denotes p < 0.05). (B) Photograph of coronal hemisection containing Analyze 8.0 stereology module grid. Points on the grid are spaced 8 pixels apart on the X and Y axes, and total number of pixels encompassing the hippocampal formation in each brain were calculated to obtain hippocampal volume using Cavalieri’s principle.
Correlations between Shock exposure and other variables
Due to the large variation in shock exposure within the forced exercise and forced control groups we conducted a series of correlations between average shock exposure and the other dependent measures to determine whether there may be indications of stress that are insignificant in overall ANOVA analysis. There was a trend towards a correlation between soluble Aβ-40 and shock exposure, however all other analyses were not significant (Table 1).
Table 1. Correlations between shock exposure and other variables.
Shock exposure was not significantly correlated with measures of hippocampal volume, recognition memory, or Aβ-related variables.
| Variable | r | N | p |
|---|---|---|---|
| Recognition Memory | −0.2251 | 19 | 0.3541 |
| Thioflavine-stained plaque | 0.2576 | 19 | 0.2873 |
| Soluble Aβx-40 | 0.4514 | 19 | 0.0522 |
| Soluble Aβx-42 | 0.0994 | 19 | 0.6864 |
| Hippocampal volume | −0.1171 | 19 | 0.6433 |
DISCUSSION
Psychosocial stress (Wilson et al., 2003; 2006), level of education (Letenneur et al., 1999; Snowdon et al., 1996), socioeconomic status (Karp et al., 2004; Fotenos et al., 2008), head trauma (Plassman et al, 2000), and physical activity (Podewils et al., 2004; Larson et al., 2006) are being increasingly recognized as environmental factors that can affect the development and progression of late-onset AD. In rodents, studies investigating the effects of environmental enrichment on the cognitive and pathological features of Alzheimer’s disease have revealed equivocal results. Lazarov et al (2005) reported dramatically reduced Aβ levels in APPSW/PS1 mice while Jankowsky et al (2005) demonstrated increased plaque deposition in an environmental enrichment paradigm. Wolf et al (2006) and Arendash et al (2004) both report improved cognition but stable plaque deposition. Taken together, these studies highlight the importance of environmental factors in modifying aspects of the disease and indicate that there are probably many factors involved in environmental enrichment. Comparison of these studies can be difficult because of the multiple components contributing to the various enriched environments utilized (i.e., assorted toys, colorful funnels, running wheels, large cages housing multiple animals). The specific influence of any one factor remains unclear, however, it has been suggested that physical and cognitive activity differentially affect progression of pathology in enriched environments (Lazarov et al, 2005; Wolf et al, 2006). For this reason, our study attempts to isolate the effect of exercise.
Our findings of reduced decline in recognition memory and decreased plaque load in the voluntary exercise group relative to the sedentary controls are consistent with the results of exercise in previous studies of mouse models of AD (Adlard et al., 2005, Nichol et al., 2007, Nichol et al., 2008, Parachikova et al., 2008). A finding unique to this study is that when Tg2576 mice were forced to exercise they did not realize the same benefits as the animals in the voluntary exercise group. These differences were not likely due to differences in exercise intensity, which has been proposed as a complicating factor when comparing forced and voluntary exercise (Dishman et al. 1997), because the velocity of the forced running group was matched to the average velocity of the voluntary running group, but may be related to qualitative differences in the exercise regimens.
A large body of work (for reviews see: Sapolsky 1990; McEwen and Sapolsky 1995; McEwen 2000) has demonstrated that various types of behavioral stressors can impact cognition and alter the pathology underlying cognitive decline in rodents. In previous work in our laboratory (Dong et al. 2004; 2008), we demonstrated that isolation stress accelerated the rate of amyloid plaque deposition and cognitive decline in Tg2576 mice. The results of this study suggest that stress associated with forced running may block, at least in part, the beneficial effects of exercise in the Tg2576 mouse model of AD.
Stress in our forced running program could be conceptualized in one of two ways: 1) the physical discomfort of foot shock resulting from the animal contacting the grid at the back of the treadmill belt, or 2) the mental distress associated with having to run at a constant predetermined speed. In an attempt to differentiate between these two possible stressors, we included a group that received foot shock, but did not exercise. This group did not differ significantly from sedentary controls in recognition memory or level of plaque deposition. Moreover, there was a wide range in foot shock number within this group, but there was no correlation between foot shock number and recognition memory, plaque count, hippocampal volume, soluble Aβ-40 or Aβ-42. These data suggest that foot shock associated with the forced running program was not a significant stressor for these animals. By exclusion, it appears more likely that mental distress associated with forced running, per se, mitigated the beneficial effects of voluntary exercise in the forced exercise group. This interpretation of our results is consistent with the isolation stress model previously studied by our group (Dong et al. 2004; 2008) in that being housed in isolation is a mental stressor unrelated to physical discomfort.
The type of exercise regimen did not have significant effects on measures of general activity or spontaneous alternation in this study. Spontaneous alternation was above chance levels for all groups, but similar to reported levels for Tg2576 (King and Arendash, 2002) suggesting that exercise did not improve alternation in these mice. Our previous work has shown consistent deficits in contextual fear conditioning at 7 months of age after exposure of Tg2576 mice to isolation stress (Dong et al., 2004; 2008). In the current study, we began behavioral testing at 9 months of age, which is an age at which cognitive decline is usually detectable in Tg2576 animals even under non-stressed conditions (Dong et al., 2005; Kawarabayashi et al., 2001; Westerman et al., 2002). However, testing animals at a later age may lead to larger differences between groups, and allow for a powerful evaluation of the influence of forced versus voluntary exercise on behavioral measures in this model of AD.
Voluntary exercise was associated with significantly lower brainplaque number when compared to the other groups in this study. However, there were no differences in soluble Aβ levels among groups. These data suggest that voluntary exercise may decrease the rate at which soluble Aβ aggregates into insoluble species. The plaque load of the forced exercise group was intermediate between the voluntary and sedentary groups indicating that forced running offsets the benefit of the physical activity. Increased ISF Aβ levels resulting from neuronal activity have been reported with acute restraint stress in Tg2576 mice (Kang et al., 2007). The intermediate plaque counts in the forced exercise group could have resulted from stress-related increases in Aβ production (Jankowsky et al., 2005; Kang et al., 2007), perhaps related toincreases in clathrin-mediated endocytosis that were caused in turn by increases in neuronal activity (Cirrito et al., 2008).
Exercise, both forced and voluntary, appeared to diminish hippocampal atrophy in these animals. This is consistent with findings of increased neurogenesis related to exercise in animals (van Praag et al., 1999b, a; 1999b), and more recently a report by Burns et al. (2008) in which physical fitness levels were correlated with hippocampal volume in AD patients. However, while forced exercise was partially beneficial in reducing plaque load and diminished hippocampal atrophty, it was not similarly successful in preventing memory deficits. Arida et al. (2004) demonstrated that both voluntary and forced exercise increased parvalbumin-positive neurons in the hippocampal formation of rats compared to controls, while voluntary exercise resulted in a stronger fiber staining in the dentate gyrus hilus. These results, combined with our own, suggest that voluntary exercise may promote neuronal plasticity needed for establishing and maintaining hippocampal function, whereas, forced exercise is not as successful in this regard.
Stress has been associated with reductions in hippocampal volume in both humans and animals (Sapolsky 1985; Bremner 1999; McEwen 2006), and previously we observed decreased hippocampal volumes in association with isolation stress in Tg2576 mice (Dong et al., 2008). However, similar to the pattern of results observed with plaque deposition, in this study, hippocampal volume of the shock-control group was not significantly different from sedentary controls. This result was unexpected and further supports the interpretation that the daily exposure to the shock grid was not a significant stressor for Tg2576 animals in the context of this study, and may serve as some form of environmental enrichment.
The cellular mechanisms by which exercise might mediate enhanced cognitive effects in animal models of AD remains unclear, although it has been suggested that exercise may preserve neuronal plasticity, increase the release of neurotrophic factors (Neeper et al., 1996), decrease oxidative stress (Pratico et al., 2001; Yao et al., 2004), or exert indirect effects on CNS metabolism by improving cardiovascular function (Hicks and Birren, 1970; Colcombe et al., 2004). Growth factors, such as BDNF, may also mediate behavioral improvements associated with exercise, and are known to be involved in the proliferation and survival of new neurons in the hippocampus (Cotman and Berchtold, 2002; Vaynman et al., 2004). Further studies will be needed to determine the relative influence of growth factors in forced exercise compared to voluntary exercise.
A recent review by Dishman et al. (2006) emphasized that more information is needed to understand the neurobiology of exercise, and specifically the impact of exercise on various disease states. In summary, the results of this study suggest that voluntary exercise is more beneficial for mitigating the behavioral and neuropathological components of the AD process than forced exercise. While forced exercise was better than no exercise for mitigating certain pathological changes, it was not sufficient to produce beneficial behavioral changes.
Acknowledgments
The authors would like to thank Vince Butano, Shannon Kensinger and Keith Miller for their help in exercise testing, and Eli Lilly for the generous donation of the 21F12 antibody. This work was supported in part by PHS grants MH060883 and AG025824 (J.G.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeck DS, Sano K, Prewitt GE, Dalton L. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behav Brain Res. 2006;168:345–348. doi: 10.1016/j.bbr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Ang ET, Dawe GS, Wong PT, Moochhala S, Ng YK. Alterations in spatial learning and memory after forced exercise. Brain Res. 2006;1113:186–193. doi: 10.1016/j.brainres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Garcia MF, Costa DA, Cracchiolo JR, Wefes IM, Potter H. Environmental enrichment improves cognition in aged Alzheimer’s transgenic mice despite stable beta-amyloid deposition. Neuroreport. 2004;15:1751–54. doi: 10.1097/01.wnr.0000137183.68847.4e. [DOI] [PubMed] [Google Scholar]
- Arida RM, Scorza CA, da Silva AV, Scorza FA, Cavalheiro EA. Differential effects of spontaneous versus forced exercise in rats on the staining of parvalbumin-positive neurons in the hippocampal formation. Neurosci Lett. 2004;364:135–138. doi: 10.1016/j.neulet.2004.03.086. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Greenough WT. Usual vs. successful aging: some notes on experiential factors. Neurobiol Aging. 1991;12:325–328. doi: 10.1016/0197-4580(91)90009-9. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan KR. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Does stress damage the brain? Biol Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Broe GA, Creasey H, Jorm AF, Bennett HP, Casey B, Waite LM, Grayson DA, Cullen J. Health habits and risk of cognitive impairment and dementia in old age: a prospective study on the effects of exercise, smoking and alcohol consumption. Aust N Z J Public Health. 1998;22:621–623. doi: 10.1111/j.1467-842x.1998.tb01449.x. [DOI] [PubMed] [Google Scholar]
- Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psych Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness: recent findings and future directions. J Mol Neurosci. 2004;24:9–14. doi: 10.1385/JMN:24:1:009. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. Episodic-like memory in mice: simultaneous assessment of object, place and temporal order memory. Brain Res Brain Res Protoc. 2005;16:10–19. doi: 10.1016/j.brainresprot.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton R, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of Exercise. Obesity. 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neurosci. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Dong H, Csernansky CA, Martin MV, Bertchume A, Vallera D, Csernansky JG. Acetylcholinesterase inhibitors ameliorate behavioral deficits in the Tg2576 mouse model of Alzheimer’s disease. Psychopharmacology. 2005;181(1):145–52. doi: 10.1007/s00213-005-2230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Yuede CM, Yoo HS, Martin MV, Deal C, Mace AG, Csernansky JG. Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice. Neurosci. 2008;155:154–163. doi: 10.1016/j.neuroscience.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol. 2008;65:113–120. doi: 10.1001/archneurol.2007.27. [DOI] [PubMed] [Google Scholar]
- Fox SM, 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404–432. [PubMed] [Google Scholar]
- Grootendorst J, de Kloet ER, Vossen C, Dalm S, Oitzl MS. Repeated exposure to rats has persistent genotype-dependent effects on learning and locomotor activity of apolipoprotein E knockout and C57Bl/6 mice. Behav Brain Res. 2001;125:249–259. doi: 10.1016/s0166-4328(01)00294-7. [DOI] [PubMed] [Google Scholar]
- Hanley T, Merlie JP. Transgene detection in unpurified mouse tail DNA by polymerase chain reaction. Biotechniques. 1991;10(1):56. [PubMed] [Google Scholar]
- Hicks LH, Birren JE. Aging, brain damage, and psychomotor slowing. Psychol Bull. 1970;74:377–396. doi: 10.1037/h0033064. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Melnikova T, Fadale DJ, Xu GM, Slunt HH, Gonzales V, Younkin LH, Younkin SG, Borchelt DR, Savonenko AV. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s disease. J Neurosci. 2005;25(21):5217–24. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci USA. 2007;104:10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp A, Kåreholt I, Qiu C, Bellander T, Winblad B, Fratiglioni L. Relation of education and occupation-based socioeconomic status to incident Alzheimer’s disease. Am J Epidemiol. 2004;159:175–183. doi: 10.1093/aje/kwh018. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DL, Arendash GW. Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiol Behav. 2002 Apr 15;75(5):627–42. doi: 10.1016/s0031-9384(02)00639-x. [DOI] [PubMed] [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo JM, Dartiques JF. Are sex and educational level independent predictors of dementia and Alzheimer’s disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry. 1999;66:177–183. doi: 10.1136/jnnp.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narath E, Skalicky M, Viidik A. Voluntary and forced exercise influence the survival and body composition of ageing male rats differently Exp. Gerontol. 2001;36:1699–1711. doi: 10.1016/s0531-5565(01)00145-0. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nichol KE, Parachikova AI, Cotman CW. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behav Brain Res. 2007;184:124–132. doi: 10.1016/j.bbr.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13–27. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30:121–129. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates: Second Edition (Deluxe) Academic Press; New York: 2001. [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JC. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: finds from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2004;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanowski, Lee VM-Y. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovio S, Kåreholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoid toxicity in the hippocampus: temporal aspects of neuronal vulnerability. Brain Res. 1985;359:300–305. doi: 10.1016/0006-8993(85)91440-4. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, hippocampal damage and the glutamatergic synapse. Prog Brain Res. 1990;86:13–23. doi: 10.1016/s0079-6123(08)63163-5. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Marksbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun study. JAMA. 1996;275:528–532. [PubMed] [Google Scholar]
- Starkweather AR. The effects of exercise on perceived stress and IL-6 levels among older adults. Biol Res Nurs. 2007;8:186–194. doi: 10.1177/1099800406295990. [DOI] [PubMed] [Google Scholar]
- Stummer W, Weber K, Tranmer B, Baethmann A, Kempski O. Reduced mortality and brain damage after locomotor activity in gerbil forebrain ischemia. Stroke. 1994;25:1862–1869. doi: 10.1161/01.str.25.9.1862. [DOI] [PubMed] [Google Scholar]
- van Pragg H, Chistie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pragg H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, Ambrose AF, Sliwinski M, Buschke H. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between A β and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, Kempermann G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer’s Disease. Biol Psychiatry. 2006;60(12):1314–23. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Yanagita S, Amemiya S, Suzuki S, Kita I. Effects of spontaneous and forced running on activation of hypothalamic corticotropin-releasing hormone neurons in rats. Life Sci. 2007;80:356–363. doi: 10.1016/j.lfs.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Yao Y, Chinnici C, Tang H, Trojanowski JQ, Lee VM-Y, Pratico D. Brain inflammation and oxidative stress in a transgenic mouse model of Alzheimer-like brain amyloidosis. J Neuroinflammation. 2004;1:21–29. doi: 10.1186/1742-2094-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]


