Abstract
Objective
The purpose of this paper was to determine whether antiretroviral medications, especially the nucleoside analogue reverse transcriptase inhibitors, lead to altered brain activation due to their potential neurotoxic effects in patients with human immunodeficiency virus (HIV) infection.
Methods
Forty-two right-handed men were enrolled in three groups: seronegative controls (SN, n=18), HIV subjects treated with antiretroviral medications (HIV+ ARV, n=12), or not treated with antiretroviral medications (HIV+NARV, n=12). Each subject performed a set of visual attention tasks with increasing difficulty or load (tracking two, three or four balls) during functional magnetic resonance imaging.
Results
HIV subjects, both groups combined, showed greater load-dependent increases in brain activation in the right frontal regions compared to SN (p-corrected=0.006). HIV+ARV additionally showed greater load-dependent increases in activation compared to SN in bilateral superior frontal regions (p-corrected=0.032) and a lower percent accuracy on the performance of the most difficult task (tracking four balls). Region of interest analyses further demonstrated that SN showed load-dependent decreases (with repeated trials despite increasing difficulty), while HIV subjects showed load-dependent increases in activation with the more difficult tasks, especially those on ARVs.
Interpretation
These findings suggest that chronic ARV treatments may lead to greater requirement of the attentional network reserve and hence less efficient usage of the network and less practice effects in these HIV patients. As the brain has a limited reserve capacity, exhausting the reserve capacity in HIV+ARV would lead to declined performance with more difficult tasks that require more attention.
Keywords: HIV, antiretroviral, NRTI, fMRI, brain reserve, attention
Prior functional magnetic resonance imaging (fMRI) studies demonstrated increased usage of reserve brain regions during working memory and attention tasks in HIV patients with mild dementia (Chang et al. 2001, 2004) as well as in those who were neuroasymptomatic (Ernst et al. 2002). With effective antiretroviral treatments (ARVs), HIV-infected individuals are living longer, and the full clinical manifestation of HIV-dementia is less common (Dore et al. 2003; Ghafouri et al. 2006). However, the prevalence of a milder form of cognitive impairment appears to be rising among HIV-infected individuals, possibly due to the longer duration of illness and the additional effect of aging in these patients. Although treatment with ARVs typically leads to improved cognitive performance in HIV patients with dementia or cognitive deficits (Larussa et al. 2006; Sacktor et al. 2006; Sacktor et al. 2000), some studies suggest that some ARV treatments may not benefit the cognitive performance of those with lower nadir CD4 counts (Cysique et al. 2006). In addition, patients treated with nucleoside analogue reverse transcriptase inhibitors (NRTIs) develop a varying degree of myopathy or neuropathy after long-term therapy (Dalakas 2001); however, the effects of these drugs on brain function are not well understood. As NRTIs inhibit gamma-DNA polymerase, which is responsible for replication of mitochondrial DNA (mtDNA), its long-term use may cause mtDNA dysfunction (Dalakas 2001). Recent studies further indicate that some antiretrovirals, particularly azidothymi-dine (AZT) and NRTIs, may induce direct endothelial dysfunction (Jiang et al. 2006), which may ultimately lead to compromised blood-brain barrier (BBB) and further brain injury.
However, the few brain-imaging studies that evaluated the effects of ARVs showed varied results, from greater metabolite abnormalities in those treated with NRTIs (Schweinsburg et al. 2005) to persistent brain abnormalities during early periods of treatment (Chang et al. 2003) and improved or better brain function (e.g., metabolite levels or BBB integrity) after longer periods of treatment (Avison et al. 2004; Chang et al. 1999; Tarasow et al. 2004). Therefore, whether ARVs exacerbate HIV-associated brain injury and contribute to the increased brain activation observed in prior fMRI studies remains controversial. We aimed to determine whether regional blood-oxygen-level-dependent (BOLD) activation on fMRI during a set of visual attention tasks is different between HIV patients on stable regimens of ARVs that included NRTIs compared to HIV patients not treated with ARVs. Based on our prior studies, we expected the HIV patients to show greater usage of brain reserve capacity, as assessed by greater load-dependent increases in the BOLD response with the more difficult tasks; however, we did not expect the two HIV subject groups to show significant differences in brain activation.
Methods
Participants
Forty-two male volunteers were enrolled into three subject groups: 12 HIV-positive men who were either naïve to ARVs or had not received ARVs for the past 6 months (HIV+NARV), 12 HIV-positive men stable on ARV regimens (viral load <50 copies per ml; HIV+ARV), and 18 HIV-seronegative healthy men (SN). Each subject provided a written consent that was approved by our Institutional Review Board. All subjects were carefully screened with neuropsychiatric evaluations and laboratory screening tests to ensure that they fulfilled all study criteria. The inclusion criteria for all subjects were as follows: (1) male, ages ≥18 years; (2) right-handed; (3) healthy and on no medications that might affect brain function (except HIV medications, e.g., efavirenz); and (4) willing and able to provide consent for participation in the study. The HIV+ subjects had the following additional inclusion criteria: (1) seropositive for HIV-1; (2) nadir CD4 count <500/mm3; (3) the NARV subjects were either naïve for ARV or had not taken any ARVs in the past 6 months, while the ARV subjects were required to have been on stable ARV regimens for the past 6 months. The seronegative comparison subjects were required to be seronegative for HIV-1. Subjects who fulfilled any of the following criteria were excluded: (1) history of co-morbid psychiatric illness that may confound the analysis of the study (e.g., schizophrenia, bipolar disorder, severe major depression requiring acute treatment); (2) confounding neurological disorder (e.g., multiple sclerosis, degenerative brain diseases, any brain infections or neoplasms, cortical infarcts, cerebral palsy); (3) abnormal screening laboratory tests or abnormal electrocardiogram that might affect brain-imaging measures (e.g., hematocrit <37 or >55% or significant hepatic or renal dysfunction); (4) current or history of drug dependence according to the Diagnostic Statistical Manual (DSM)-IV criteria (including methamphetamine, ecstasy, cocaine, alcohol, opiates, and barbiturates but not nicotine); (5) positive urine toxicology screen for drugs of abuse (cocaine, amphetamines, benzodiazepines, and opiates); (6) history of head trauma with loss of consciousness for >30 min; (7) contraindication for magnetic resonance (MR) studies, such as presence of ferromagnetic substances or electronic implants in the body (e.g., pacemaker, surgical clips, metallic pumps, etc.) or significant claustrophobia; and (8) less than eighth grade level of English reading skills to ensure that the subjects could understand the written consent. Urine toxicology tests were performed on the day of the fMRI scan using the Multi Drug 6-Panel Drug Test from Medimpex United (Bensalem, PA). The cognitive status of each subject was evaluated by the Mini-Mental Status examination (Folstein et al. 1975), the HIV-dementia scale (Power and Johnson 1995), and a well-validated battery of neuropsychological tests known to be sensitive for detecting cognitive deficits in HIV patients (Chang et al. 2004). As deficits in attention might affect all cognitive tasks, a global cognitive deficit score was also calculated for each subject using neuropsychological tests from established clinical domain ratings and an algorithm reported previously (Woods et al. 2004). A similar global deficit score has been shown to be accurate in classifying HIV subjects with neuropsychological impairments (Carey et al. 2004).
Activation paradigm
Each subject performed a set of fMRI studies comprising three nonverbal visual-attention tasks with different levels of difficulty (Chang et al. 2004; Culham et al. 1998). The tasks required mental tracking of multiple (two, three, or four) briefly highlighted target balls amongst ten balls moving randomly (in a simulated Brownian motion) and colliding with each other. The target tracking period (60 s) alternated with a fixation period (also 60 s) during which the balls moved in the same random motion without the targets being highlighted (block design: 120 s/period×3 periods). The movies showing the stimuli were created in MATLAB and presented to the subjects on a magnetic resonance imaging (MRI)-compatible liquid crystal display (LCD) projector screen connected to a computer, which was also used to record events from a push button. The response-button events were used to determine task performance (percent accuracy and reaction times). An MRI trigger pulse was used to synchronize the stimulus display software with the MR acquisition.
MRI acquisition
Before undergoing the MR studies, each subject was trained outside of the scanner to ensure that he or she was able to perform the tasks correctly. The fMRI studies were performed on a 3 Tesla whole-body Siemens Trio MRI scanner, equipped with a head-specific eight-channel receiver coil. T1- and T2-weighted images were first acquired to evaluate for possible pathology, using the MPRAGE [echo time (TE)/repetition time (TR)=4.91/2,200 ms, 1×1×1 mm3 resolution, 256×256 matrix, 160 sagittal slices, 7 min) and FLAIR (TE/TR=85/9,100 ms, 256×256 matrix, 44 axial slices, 0.9×0.9 mm in-plane resolution, 3 mm thickness, 4 min) sequences. BOLD signal changes were measured using a T2*-weighted single-shot gradient-echo echo-planar imaging sequence (TE/TR=30/3,000 ms, 3 mm slice thickness, 42 axial slices, 64×64 matrix, 3 mm in-plane resolution, 90°-flip angle, 126 time points) covering the whole brain. Padding was used to minimize motion. Task performance and subject motion were determined immediately after each fMRI trial, using motion parameters extracted from a text file automatically saved by the scanning software, to assure performance accuracy better than 80%, and head motion <1-mm translation and <1° rotation in any direction.
fMRI data pre-processing
Siemens proprietary software was used for image reconstruction, and fMRI time series were further analyzed with SPM02. First, activation maps for each subject and condition were calculated using a fixed-effects model, using a box-car design convolved with a canonical hemodynamic response function (HRF), and low- and high-pass filters (1/246 Hz cut-off frequency). The individual BOLD activation maps were then used to calculate activation patterns for each task and group, using a random effects model (Friston et al. 1999; Woods 1996). Additionally, a repeated measures analysis of variance (ANOVA) model was created to evaluate the effects of HIV status and of the number of balls tracked on the BOLD signal. Both a pure attention effect (average activation across the three tasks, independent of load) and a pure load effect (increment in activation across the three tasks, independent of attention) were evaluated. A mask of voxels with a significant attention effect (p<0.05) in any group was used to assess contrasts involving attention effect differences across groups and load effects within a group. Similarly, a mask of voxels with a significant load effect (p<0.05) in any group was used to evaluate interactions between HIV status and attentional load. Cluster significance was defined as p<0.05 (corrected for multiple comparisons) and size >100 voxels (Friston et al. 1994).
To further validate voxel-by-voxel comparisons in statistical parametric mapping (SPM), regions-of-interest (ROI) comparisons were specifically tabulated for brain regions that showed group differences. Each of the cubic ROIs had a volume of 0.729 cm3 (27 SPM voxels) and was centered at the maximum of representative clusters with group differences. The ROI values were analyzed with Statistical Analysis Software (SAS Institute, Cary, NC) using a repeated measures ANOVA model; p=0.05 was considered significant.
Results
Participants' clinical data
The three subject groups were well-matched by age, education, and estimated premorbid verbal intelligence quotient (Table 1). All three groups also had similar hematocrit, which is important, as BOLD signals may vary with percent hematocrit (Levin et al. 2001). The two HIV subgroups were well matched by CD4 counts. As the HIV+ NARV group were not taking antiretrovirals and despite the trend for higher nadir CD4 count (HIV+NARV, 289±34; HIV+ARV, 148±31; p=0.06), they had higher viral burden than the HIV+ARV group (undetectable viral load in all 12 HIV+ARV subjects vs Log viral load of 4.17±0.16 copies/ml in the HIV+NARV group; p<0.0001). The two HIV groups were not different on estimated duration of HIV infection, the Karnofsky scores and cognitive performance as measured by the HIV dementia scale (14.5±0.4 vs 15.0± 0.4), Mini-Mental State examination (28.8±0.4 vs 29.2± 0.2), and a global measure of cognitive deficits based on neuropsychological tests (6.2±0.2 vs 6.0±0.9). However, compared to the SN group (2.7±0.5), the global cognitive deficit scores were higher in both HIV groups (p<0.004 for both groups).
Table 1.
Characteristics of seronegative controls (SN) and HIV subjects (mean±S.E) and group comparisons
| SN(n=18) | HIV+ARV (n=12) | HIV+NARV (n=12) |
p values* |
|||
|---|---|---|---|---|---|---|
| SN vs HIV+ARV | SN vs HIV+NARV | HIV+ARV vs HIV+NARV | ||||
| Age (years) | 39.8±2.9 | 41.5±1.2 | 38.8±3.1 | NS | NS | NS |
| Education (years) | 13.9±0.5 | 13.6±0.7 | 13.7±0.8 | NS | NS | NS |
| Hematocrit (%) | 45.1±0.6 | 44.5±0.9 | 43.4±1.5 | NS | NS | NS |
| CD4 (number/mm3) | 511±66 | 424±42 | NS | |||
| Nadir CD4 (number/mm3) | 148±31 | 289±34 | 0.063 | |||
| Viral load (copies/ml) | All <50 | 28,964±12,009 | <0.0001a | |||
| Log viral load (copies/ml) | All <1.69 | 4.17±0.16 | <0.0001a | |||
| Duration HIV infection (months) | 142.8±19.9 | 117±22 | NS | |||
| Duration ART (months) | 134.8±21.7 | 0 | N/A | |||
| HIV Dementia scale (0-16) | 14.5±0.4 | 15.0±0.4 | NS | |||
| Estimated VIQb | 111.0±1.9 | 105.8±2.8 | 106.2±3.1 | NS | NS | NS |
| MMSE (0-30) | 29.2±0.2 | 28.8±0.4 | 29.2±0.2 | NS | NS | NS |
| Global cognitive deficit | 2.7±0.5 | 6.2±0.2 | 6.0±0.9 | 0.0036 | 0.0039 | NS |
| score (0-9) | ||||||
| Tracking 2 balls | ||||||
| Accuracy (%) | 95.3±2.0 | 94.0±2.8 | 94.5±3.8 | NS | NS | NS |
| Reaction times(ms) | 686±74 | 794±97 | 852±106 | NS | NS | NS |
| Tracking 3 balls | ||||||
| Accuracy (%) | 94.9±1.9 | 94.8±1.9 | 96.9±2.2 | NS | NS | NS |
| Reaction times (ms) | 867±65 | 690±83 | 767±88 | NS | NS | NS |
| Tracking 4-balls | ||||||
| Accuracy (%) | 97.6±1.3 | 90.6±2.7 | 93.2±2.1 | 0.016 | NS | NS |
| Reaction times (ms) | 714±51 | 871±90 | 609±96 | NS | NS | NS |
NS Not significant; N/A not applicable
p values are from post-hoc analyses (nonparametric or parametric as indicated) between any two groups as indicated.
"Undetectable viral load" was <50 copies/ml; therefore, comparisons between the two HIV groups for viral load and Log viral load were performed with 49 for all subjects in the HIV+ARV group.
Estimated premorbid verbal IQ is derived from the National Adult Reading Test
All 12 HIV+ARV subjects were on at least three ARVs. Eleven of 12 were on NRTI (eight tenofovir, five lamivudine, three zidovudine, two abacavir, one didanosine, one stavudine, and two emtricitabine) in combination with either one or more non-NRTIs (four efavirenz and three nevirapine) and/or protease inhibitors (three lopinavir/ritonavir, two ritonavir, one saquinavir mesylate, one atazanavir, and one fosamprenavir). One subject was on a NNRTI and PI combination (nevirapine and lopinavir/ritonavir). Eleven of 12 of these subjects were on at least one cerebrospinal fluid (CSF)-penetrating ARV, as defined previously (i.e., those with CSF concentrations that exceeded the level needed to inhibit replication of HIV; Letendre et al. 2004), which included stavudine, zidovudine, abacavir, efavirenz, or nevirapine. Although all of the HIV+ARV subjects were taking medications that would likely lead to increases in triglycerides (i.e., NNRTI and PI), only one subject was taking lipid-lowering medication, and as expected, triglyceride levels were higher in the HIV+ARV subjects compared to the HIV+NARV subjects (p=0.04, one tailed).
Task performance and BOLD activation during visual attention tasks
All three groups showed >90% accuracy in task performance, but only the HIV+ARV subject group showed poorer performance on the most difficult task (four-ball tracking) compared to SN controls (Table 1). However, no group difference was observed on the subjects' reaction times fMRI (Table 1).
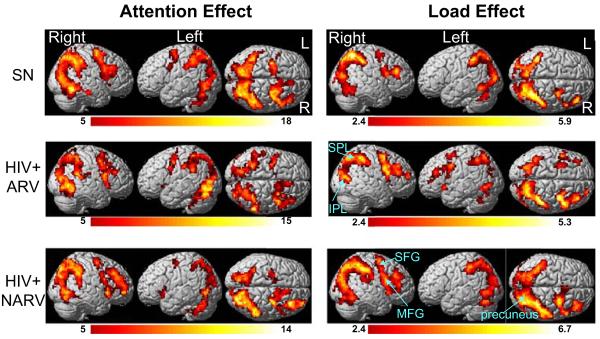
Fig. 1 (left) shows BOLD fMRI activation patterns in each subject group (average activation across all three tasks). Activated brain regions include bilateral prefrontal and dorsolateral prefrontal regions (right greater than left), as well as bilateral parietal (right greater than left) and medial cerebellar regions. This activation pattern is consistent with prior observations during visual attention tasks performed in different subject populations and different MR scanner field strengths (1.5 or 4 Tesla; Chang et al. 2004; Jovicich et al. 2001). Both HIV+ARV and HIV+NARV groups showed less maximum BOLD signals than the SN subjects (Fig. 1, left column), although these differences did not reach statistical significance.
Fig. 1.
Left panels: Parametric maps showing the significance of the attention effect across all three tasks, independent of load, in the two HIV groups and SN controls (cluster size> 100 voxels or 2.7 ml; voxel t threshold>5 or p<0.00001). Right panels: Parametric maps showing brain regions that show load-dependent increase in activation (attentional load effect), independent of mean activation, in each subject group (cluster size> 100 voxels 2.7 ml; voxel t threshold> 2.38 or p<0.05). See also Table 2 for the corresponding cluster volumes and significance of these brain regions that show load effects. SPL Superior parietal lobe; IPL inferior parietal lobule; SFG superior frontal gyrus; MFG middle frontal gyrus. R right; L left
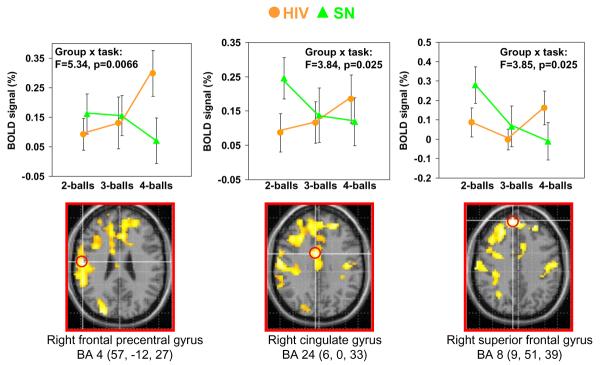
With increasing task difficulty (from tracking two balls to three to four balls), the subjects typically showed load-dependent increases in brain activation (Fig. 1, load effect, right column). Compared to the SN, the load-dependent increase in activation was more extensive in the frontal regions in both HIV+ groups (Fig. 1, right column). Direct group comparisons showed that HIV subjects had greater load-dependent activation than SN in the right frontal and cingulate regions (cluster size, 2,315 voxels, corrected p value=0.006). Table 2 also shows the three local maxima in this cluster. Fig. 2 shows the ROI analyses of the BOLD signals centered at these three local maxima within the significant cluster: right frontal precentral gyrus or Broadmann area 4 (BA4: 57, -12, 27), right cingulate gyrus or BA 24 (6, 0, 33) and right superior frontal gyrus or BA 8 (9, 51, 39). These ROI analyses confirmed the group-by-task interactions in these regions, such that while SN subjects (green) showed load-dependent decreases in BOLD signals in the frontal regions, the HIV+ subjects (orange) showed load-dependent increase in these regions (Fig. 2, graphs in top row).
Table 2.
Brain regions showing attentional load effects in each subject group and group differences across all three visual attention tasks (cluster size>100 voxels; t>2.38)
| Brain region(s) in local maximum | Z-max Talairach coordinate* x,y,z (mm) | Voxel Z-max score | Cluster size and corrected p value |
|---|---|---|---|
| Load effects in SN controls (n=18), Fig. 1 | |||
| R superior parietal lobule BA 7 | 33, -60, 575.37 | 5.37 | 327 voxels (8.83 ml)p<0.001 |
| R superior parietal lobule BA 7 | 18, -75, 54 | 4.80 | |
| R inferior parietal lobule | 45, -42, 51 | 4.52 | |
| Load effects in HIV+ARV (n=12), Fig. 1 | |||
| R inferior parietal lobule BA 40 | 51, -39, 57 | 4.91 | 287 voxels (7.75 ml)p<0.001 |
| R superior parietal lobule | 33, -57, 51 | 4.82 | |
| R inferior parietal lobule BA 40 | 39, -45, 48 | 4.55 | |
| Load effects in HIV+NARV (n=12), Fig. 1 | |||
| R superior parietal lobule | 30, -63, 48 | 5.90 | 1,035 voxels (27.9 ml) p< 0.001 |
| R inferior parietal lobule | 36, -45, 48 | 5.87 | |
| R precuneus | 30, -78, 36 | 5.61 | |
| L superior parietal lobule BA 7 | -24, -63, 51 | 5.54 | 335 voxels (6.7 ml) p<0.001 |
| L inferior parietal lobule | -42, -39, 51 | 4.97 | |
| L inferior parietal lobule BA 40 | -33, -51, 54 | 4.79 | |
| R middle frontal gyrus BA 6 | 27, -6, 51 | 5.27 | 113 voxels (3.05 ml) p<0.001 |
| R superior frontal gyrus BA 6 | 30, 0, 63 | 4.34 | |
| Significant differences in attentional load: HIV subjects > SN controls (Fig. 2) | |||
| Right frontal precentral gyrus or BA 4 | 57, -12, 27 | 3.11 | 2,315 voxels (62.5 ml) p=0.006 |
| Right cingulate gyrus BA 24 | 6, 0, 333 | 3.10 | |
| Right superior frontal gyrus BA 8 | 9, 51, 39 | 2.85 | |
| Significant differences in attentional load: HIV+ARV subjects > SN controls (Fig. 3) | |||
| Right superior frontal gyrus BA 8 | 15, 45, 36 | 3.57 | 1,730 voxels (46.7 ml) p=0.032 |
| Left superior frontal gyrus BA 9 | -18, 42, 333 | 3.29 | |
| Left superior frontal gyrus BA 8 | -39, 18, 48 | 3.12 |
Fig. 2.
Top row: Region of interest (ROI) analyses from local maxima of significant clusters (see red circles below) showing interaction effects between attentional load and subject groups. Load-dependent increases in BOLD signals were observed in the HIV+ subjects (orange dots) while BOLD signals decreased with greater attentional load (repeated trials) in the SN subjects (green triangles). Bottom row: Parametric maps showing brain regions with significantly greater attentional load effect in HIV+ subjects than SN subjects (cluster size> 100 voxels; t scores >2.38). The local maxima where the ROIs are extracted are indicated with red circles. See also Table 2
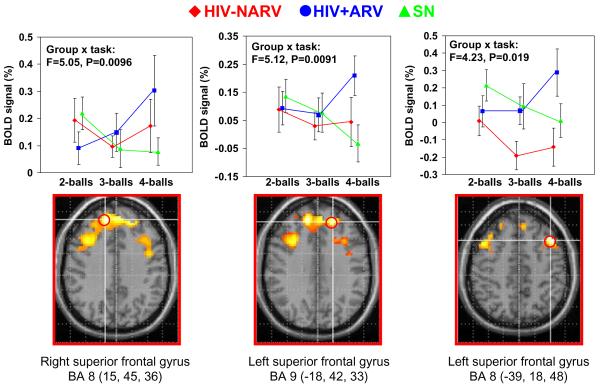
Similarly, further voxel-by-voxel comparisons of the two HIV subgroups showed that the HIV+ARV group had greater load-dependent increases in activation than SN subjects in the frontal regions (cluster size, 1,730 voxels, p=0.032), with local maxima at the right superior frontal gyrus (BA 8: 15, 45, 36) and the left superior frontal gyrus (BA 9: -18, 42, 33; BA 8: -39, 18, 48), Table 2. ROI analyses further demonstrate that while SN subjects showed load-dependent decreases in BOLD signals in these frontal regions, the HIV+ARV subjects showed load dependent increases in these regions (Fig. 3, scatterplots on top row). Therefore, interaction effects were observed in these brain regions between these two subject groups (15, 45, 36: F=5.05, p=0.0096; -18, 42, 33: F=5.12, p=0.0091; -39, 18, 48: F=4.23, p=0.019; Fig. 3). In contrast, the HIV+NARV subjects showed load-dependent decreases in activation between tracking two balls and three balls but increases in activation between three balls and four balls (Fig. 3, top row).
Fig. 3.
Top row: Region of interest (ROI) analyses from local maxima of significant clusters (see red circles below) showing interaction effects between attentional load and subject groups. Load-dependent increase in BOLD signals were observed in the HIV+ARV subjects (blue squares), while BOLD signals decreased with increased attentional load in the SN subjects (green triangles), and the BOLD signals for HIV+NARV subjects decreased from tracking two balls to three but increased only with tracking four balls (red dots). Bottom row: Parametric maps showing brain regions with significantly greater attentional load effect in HIV+ARV subjects than SN subjects (cluster size>100 voxels; t scores >2.38). The location of each of the ROIs at local cluster maxima are indicated with red circles. See also Table 2
BOLD activation in relation to clinical variables
No significant correlations were observed between activation in brain regions that showed greater load effects with Logarithmic viral load, CD4 cell count, nadir CD4 cell count, or HIV dementia scale in the HIV subjects, both with or without ARVs. Furthermore, neither attention nor load activation correlated with the global cognitive deficit score or task performance (accuracy or reaction time) in all subjects. As the triglyceride levels were higher in the HIV+ARV subjects, we also evaluated for possible relationship between the BOLD signals and triglyceride levels in both groups of HIV subjects and found none.
Discussion
We observed greater load-dependent increased brain activation in the right frontal lobes of both HIV subject groups compared to SN controls, which suggests greater usage of their brain reserve capacity as the visual attention tasks became more difficult. However, the slopes were similar in both HIV subject groups in most brain regions except for the right and left superior frontal lobes, where only the HIV+ARV group showed greater load-dependent increase in activation than SN subjects. As the brain may have a limited capacity to further increase the BOLD response with the more difficult tasks, task performance in the HIV+ARV subjects may decline with more difficult tasks (e.g., tracking four balls), as is observed in this study.
The two HIV subject groups were well matched on many variables, including hematocrit, immune status (CD4 cell count), estimated duration of HIV infection, age, education, premorbid intelligence, and cognitive performance on three clinical measures [HIV dementia scale, Mini-Mental Status Examination (MMSE), and a global cognitive score based on neuropsychological tests]. Therefore, our findings suggest that ARV may lead to greater usage of brain reserve in HIV subjects as shown by the greater load-dependent increase in brain activation.
The majority of HIV patients were maintained on ARV regimens that included at least one NRTI. NRTIs include zidovudine (AZT), zalcitabine (ddC), didanosine (ddl), lamuvidine (3TC), stavudine (d4T), Tenofovir (TDF), Emtricitabine (FTC) and Abacavir (ABC), many of which are known to cause mitochondrial toxicity that may lead to distal sensory neuropathy or myopathy (Dalakas 2001). However, whether chronic treatment with ARVs or NRTI in particular might cause brain injury in the long term has been less clear. N-acetylaspartate (NAA), a neuronal marker that can be measured with proton magnetic resonance spectroscopy (1H-MRS), was lower in the frontal white matter of HIV patients treated with didanosine and/or stavudine but not in those who were treated with zidovudine or were ARV-naïve, compared to HIV-seronegative controls (Schweinsburg et al. 2005). The decreased NAA suggests that NRTI treatment in HIV might lead to mitochondrial toxicity also in the brain (Schweinsburg et al. 2005). However, other 1H-MRS studies found either no change or even improvement in NAA or NAA/creatine in patients treated with ARVs (Chang et al. 1999, 2003; Tarasow et al. 2004; Vion-Dury et al. 1995), although most studies did not distinguish those treated with NRTIs from those on non-NRTIs. A preclinical study also found that ddC, but not 3TC, at CSF-achievable concentrations, induced oxidative stress and increased proteins associated with induction of apoptosis (Opii et al. 2006). Therefore, whether NRTIs may contribute to brain injury that could exacerbate HIV dementia or cognitive impairment remains controversial.
Our findings demonstrate that HIV infection is associated with greater activation of the attention network with more difficult tasks, and HIV subjects treated with ARVs may have even more attentional requirements in the superior frontal regions. It is interesting to note that SN control subjects showed load-dependent decrease in activation in these frontal regions, where increases were observed in the HIV subjects. Based on prior reports, frontal brain regions may show decreased BOLD signals with repeated trials, due to practice effects (Tomasi et al. 2004). Therefore, it appears that SN subjects were able to become more efficient at performing the tasks with repeated trials, even as the tasks became more difficult, and required less activation in these frontal regions. Similarly, the HIV+NARV subjects appear to show some practice effect (less activation) in the superior frontal regions with the repeat trials (tracking three balls after the first trial of tracking two balls) but then required greater activation when the tasks became more difficult (tracking four balls). The HIV subjects, especially those on ARVs, required greater usage of this attention network to maintain performance. When the reserve attentional network reached capacity limit, as might be the case for our HIV+ARV subjects, performance may decline. Our HIV+ARV subjects indeed performed more poorly on the four-ball track task than the SN (90 vs 97%). Therefore, our findings suggest that the brains of HIV-infected individuals typically required increased usage of the reserve capacity to perform more difficult tasks and did not demonstrate strong practice effects, and those treated with ARVs may require even greater usage of the brain reserve and reach the network capacity limit sooner.
Surprisingly, untreated HIV subjects, despite their higher viral burden, did not have higher BOLD signals during the attention tasks. As HIV viral proteins (e.g., tat, gp120) and the associated inflammatory responses (e.g., macrophage and microglial activation and associated cytokine and chemokine release) are known to cause additional brain injury, the higher concentrations of viruses in the untreated patients might lead to additional brain injury and hence higher BOLD signals. However, we observed higher BOLD signals in those treated with ARVs. It is possible that the NRTIs with low lipophilicity (e.g., d4T) do not readily enter the brain tissue despite levels found in the CSF due to the higher paracellular permeability characteristics of the choroid plexus (blood-CSF barrier) compared to the BBB (Thomas and Segal 1998). Hence, HIV+ARV subjects may have had higher viral burden in the brain parenchyma, despite undetectable plasma viral load, and hence greater inflammatory responses in the brain and greater BOLD signals. Previous study has shown that higher levels of glial markers, myoinositol, and to a lesser degree, total creatine and choline compounds, predicted higher BOLD signals in the brain (Ernst et al. 2003). Whether the ARVs cause direct neurotoxicity or increase inflammatory response in the brain remains unclear, but most of the NRTIs in our HIV patients' regimen were of the less neurotoxic type; only one patient was on ddI and one on d4T.
As long-term HIV infection as well as ARVs are independent risk factors for atherosclerosis (Lorenz et al. 2007), and as ARVs may lead to endothelial dysfunction (Jiang et al. 2006), our HIV+ARV subjects might also have reduced vascular reactivity, which in turn could lead to abnormal BOLD responses on fMRI. While our HIV+ARV subjects did have slightly longer duration of HIV infection, it was not significantly different from the untreated HIV group. Another mechanism for possible altered neuro-vascular response or atherosclerosis might be due to elevated triglycerides that have been associated with the majority of the NNRTIs and protease inhibitors. However, we did not observe a correlation between serum triglyceride levels and the BOLD signals from brain regions that showed greater load effects in the HIV-subjects. This lack of correlation argues against such a mechanism; however, it also may be due to the relatively small sample size in our study. To assess whether the microvasculature might be affected, future studies with simultaneous BOLD and perfusion MRI and studies that could assess the cerebro-vascular reserve may help to determine whether the microvasculature associated with the brain responses might be affected significantly by the ARVs.
Furthermore, recent studies demonstrated that lower nadir CD4 count was associated with decline on cognitive performance over time (Cysique et al. 2006). Our HIV+ ARV subjects had nearly significantly lower nadir CD4 than the HIV+NARV subjects, but the two groups' cognitive performance were similar on several gross measures of cognitive function (MMSE, HIV dementia scale and the global cognitive deficits scores). On fMRI, however, only the HIV+ARV subjects but not the untreated HIV subjects showed greater BOLD signals and poorer performance on the most difficult task (tracking four balls) compared to the SN subjects. Therefore, using fMRI tasks with increasing cognitive load may provide a sensitive measure to assess brain injury and can serve as a parametric “stress test” for assessing brain function.
There are some limitations to the current study. One potential confound of the study is that differences in brain activation may be related to baseline group differences in brain reserve rather than to the treatment status of the subjects. To minimize the baseline group differences, we carefully matched the subject groups by age, gender, education, handedness, estimated premorbid intelligence, and hematocrit. The two HIV groups were also well-matched by CD4, by duration of HIV infection, and by their cognitive performance. Nevertheless, longitudinal fMRI studies of the same HIV subjects before and after ARV treatment would help to validate our initial observation. Another potential confound is that the sample sizes of the HIV subgroups were relatively small, which could increase the chance for type II errors. Lastly, the HIV+ARV subjects were prescribed various antiretroviral regimens, with different combinations of NRTI, NNRTI and PIs. Future studies with larger sample size of HIV subjects may allow us to further delineate the separate effects of these different classes of drugs.
In summary, the findings from this study suggest that stable ARV regimens are associated with abnormal BOLD response and may exacerbate HIV-associated brain injury in the frontal brain regions. As the brain has a limited reserve capacity, the greater requirement for the usage of the attention network in the ARV-treated patients may exhaust the reserve capacity sooner, leading to decline in performance with attention-related tasks.
Acknowledgements
We thank our research participants and support by the NIH (2R01MH61427; K24-DA16170; K02-DA16991; 5P20-RR11091; G12-RR003061) and the Office of National Drug Control Policy (ONDCP).
References
- Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Greenberg RN, Berger JR. Neuroimaging correlates of HIV-associated BBB compromise. J Neuroimmunol. 2004;157:140–6. doi: 10.1016/j.jneuroim.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Leonido-Yee M, Witt M, Speck O, Walot I, et al. Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology. 1999;53:782–789. doi: 10.1212/wnl.53.4.782. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt M, Ames N, Walot I, Jovicich J, et al. Persistent brain abnormalities in antiretroviral-naïve HIV patients three-months after HAART. Antivir Ther. 2003;8:17–26. [PubMed] [Google Scholar]
- Chang L, Speck O, Miller E, Braun A, Jovicich J, Koch C, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, et al. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol. 2004;56:259–272. doi: 10.1002/ana.20190. [DOI] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RBH. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Variable benefit in neuropsychological function in HIV-infected HAART-treated patients. Neurology. 2006;66:1447–1450. doi: 10.1212/01.wnl.0000210477.63851.d3. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J Peripher Nerv Syst. 2001;6:14–20. doi: 10.1046/j.1529-8027.2001.006001014.x. [DOI] [PubMed] [Google Scholar]
- Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 2003;17:1539–1545. doi: 10.1097/00002030-200307040-00015. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Arnold S. Increased glial markers predict increased working memory network activation in HIV patients. NeuroImage. 2003;19:1686–1693. doi: 10.1016/s1053-8119(03)00232-5. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. NeuroImage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Hebert VY, Zavecz JH, Dugas TR. Antiretrovirals induce direct endothelial dysfunction in vivo. J Acquir Immune Defic Syndr. 2006;42:391–395. doi: 10.1097/01.qai.0000228790.40235.0c. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Peters RJ, Koch C, Braun J, Chang L, Ernst T. Brain areas specific for attentional load in a motion tracking task. J Cogn Neurosci. 2001;13:1048–1058. doi: 10.1162/089892901753294347. [DOI] [PubMed] [Google Scholar]
- Larussa D, Lorenzini P, Cingolani A, Bossolasco S, Grisetti S, Bongiovanni M, et al. Highly active antiretroviral therapy reduces the age-associated risk of dementia in a cohort of older HIV-1-infected patients. AIDS Res Hum Retrovir. 2006;22:386–392. doi: 10.1089/aid.2006.22.386. [DOI] [PubMed] [Google Scholar]
- Letendre SL, McCutchan JA, Childers ME, Woods SP, Lazzaretto D, Heaton RK, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004;56:416–423. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- Levin JM, Frederick BB, Ross MH, Fox JF, vonRosenberg HL, Kaufman MJ, et al. Influence of baseline hematocrit and hemodilution on BOLD fMRI activation. Magn Reson Imaging. 2001;19:1055–1062. doi: 10.1016/s0730-725x(01)00460-x. [DOI] [PubMed] [Google Scholar]
- Lorenz MW, Stephan C, Harmjanz A, Staszewski S, Buehler A, Bickel M, et al. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2006.12.022. in press. [DOI] [PubMed] [Google Scholar]
- Opii WO, Sultana R, Abdul HM, Ansari MA, Nath A, Butterfield DA. Oxidative stress and toxicity induced by the nucleoside reverse transcriptase inhibitor (NRTI)-2′,3′-dideoxycytidine (ddC): Relevance to HIV-dementia. Exp Neurol. 2006;204:29–38. doi: 10.1016/j.expneurol.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Johnson RT. HIV-1 associated dementia: clinical features and pathogenesis. Can J Neurol Sci. 1995;22:92–100. doi: 10.1017/s0317167100040154. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Nakasujja N, Skolasky R, Robertson K, Wong M, Musisi S, et al. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology. 2006;67:311–314. doi: 10.1212/01.wnl.0000225183.74521.72. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky R, Lyles R, Esposito D, Selnes O, McArthur J. Improvement in HIV-associated motor slowing after antiretroviral therapy including protease inhibitors. J Neurovirol. 2000;6:84–88. doi: 10.3109/13550280009006385. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Gonzalez R, Brown GG, Ellis RJ, et al. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol. 2005;11:356–364. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]
- Tarasow E, Wiercinska-Drapalo A, Jaroszewicz J, Orzechowska-Bobkiewicz A, Dzienis W, Prokopowicz D, et al. Antiretroviral therapy and its influence on the stage of brain damage in patients with HIV-1H MRS evaluation. Med Sci Monit. 2004;10(Suppl 3):101–106. [PubMed] [Google Scholar]
- Thomas SA, Segal MB. The transport of the anti-HIV drug, 2′,3′-didehydro-3′-deoxythymidine (D4T), across the blood-brain and blood-cerebrospinal fluid barriers. Br J Pharmacol. 1998;125:49–54. doi: 10.1038/sj.bjp.0702044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. NeuroImage. 2004;23:1414–1421. doi: 10.1016/j.neuroimage.2004.07.065. [DOI] [PubMed] [Google Scholar]
- Vion-Dury J, Nicoli F, Salvan A, Confort-Gouny S, Dhiver C, Cozzone P. Reversal of brain metabolic alterations with zidovudine detected by proton localised magnetic resonance spectroscopy. Lancet. 1995;345:60–61. doi: 10.1016/s0140-6736(95)91184-7. [DOI] [PubMed] [Google Scholar]
- Woods RP. Modeling for intergroup comparisons of imaging data. NeuroImage. 1996;4:S84–S94. doi: 10.1006/nimg.1996.0058. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]