(A) The Strange Case Of Extracellular Heat Shock Proteins
Stonewalls do not a prison make nor iron bars a cage.
Richard Lovelace, (1618–1657)
Of course, heat shock proteins (Hsp) are essential for intracellular molecular chaperone function. Without the Hsp, protein integrity in the crowded intracellular spaces would be uncertain and the sensitive protein complexes that make cells responsive to their environments, would become prone to aggregation and pose a perilous problem for cells [1, 2]. Hsps are therefore established as key intracellular proteins. However, it is now also difficult to argue against extracellular roles for the Hsps in immune and inflammatory responses [3]. This was not always so, and until recently the existence of extracellular Hsp was the subject of skepticism or ascribed to episodes of mass cell carnage. The first indications that Hsp may transit through the extracellular spaces to carry out their functions were uncovered by the Tytell and Hightower labs [4, 5]. Further evidence was that the Hsp 60 and 70 appear in the serum of human patients in substantial quantities-indirect evidence for escape from the confines of the cell [6]. Again, such extracellular Hsps may have originated from dying tissues and indeed, elevated serum Hsp60 and 70 levels have been associated with some diseases or pathological episodes. However the most compelling studies, those that riveted attention on extracellular Hsp, were from tumor immunology and showed that tumor Hsp carry out the function of sampling intracellular antigenic peptides and transporting such peptides to antigen presenting cells for further display to cytotoxic T lymphocytes [3]. But how do such Hsp abandon their intracellular tasks, exit the cell and journey to distant cellular destinations? There are still many questions to answer. Understanding the mechanisms of Hsp release from cells poses a number of theoretical and practical problems, including: How does a leaderless protein such as Hsp70 cross the plasma membrane? What is the molecular signal for hsp70 release? And, how is the fraction of intracellular Hsp70 fated for secretion “sorted” from Hsp destined for cytoplasmic or nuclear roles?
Model Systems
Until recently the physiological stimuli that lead to Hsp70 secretion were unknown. We initially used heat shock as a model stimulus for Hsp70 release. This was used as other groups had previously shown that heat shock stimulates the secretion of a number of cytokines such as interleukin-1α and fibroblast growth factor-1 indicating that this was a useful model system [7]. In addition there was the notion from immunotherapy studies that heat shock could stimulate a cascade that led from Hsp70 transcription to release of Hsp70-peptide complexes [8]. The rationale behind heat-induced release of leaderless proteins is not known. Heat shock does not appear to have a major effect on intracellular monovalent ion content. It does however lead to the activation of membrane phospholipases A and C (whose stimulation is required for IL-1β secretion) and gives rise to intracellular Ca++ transients, another factor in secretion [9, 10]. Recent studies now indicate that a number of bacterial products including the chaperone-60 protein GroEL from E. coli can also stimulate Hsp70 release [11]. We have found that exposure to live E coli gives rise to abundant Hsp70 secretion and have adopted this as our current model system (S.S. Mambula & S. K. Calderwood, in preparation). Interestingly E.coli cell wall lipopolysaccharides (LPS), which are the dominant factor in secretion of cytokines including Il-1β do not seem to play a major role in mediating Hsp70 secretion from macrophages (S.S. Mambula & S. K. Calderwood, in preparation). Thus secretion of Hsp70 and Il-1β, although utilizing a similar pathway for secretion, appear to respond to different proximal signals in E. coli.
(B) No Way Out For Hsp70?
We have now reached a quite advanced understanding of the processes involved in secretion of most proteins. Lipid membrane-bounded structures such as cells or intracellular organelles are privileged environments that exclude charged and polar compounds. Charged and bulky molecules such as proteins are incapable of penetrating such membranes under normal circumstances. However, cells have solved these problems in a number of ways. Most secreted proteins utilize a two-fold strategy to solve protein secretion or insertion into membranes. They encode an N-terminal, hydrophobic “leader” sequence; as such proteins are translated the leader sequence becomes inserted into ER membranes and they enter the secretion pathway in a co-translational manner; the leader is synthesized first, penetrates the ER membrane and draws in the remainder of the protein until leader cleavage and protein folding within the lumen of the ER [12]. Such proteins are then passed along within the confines of lipid-bounded organelles and vesicles, until these structures fuse with the plasma membrane and their contents are released into the extracellular spaces [12]. Thus once inserted into the ER, secreted proteins are not again required traverse a lipid membrane before release. However there are other strategies which cytoplasmic proteins use to penetrate membrane-bounded spaces. For instance, many integral mitochondrial matrix proteins are fully synthesized in the cytoplasm prior to translocation and their translocation does not involve co-translation on the mitochondrial membrane surface [13]. Traverse of the mitochondrial membrane involves an N-terminal matrix targeting sequence [13]. The problem of folding is solved by the binding of these proteins to Hsp70 homolog Hsc70, an association which prevents premature folding and permits their entry into the mitochondrial matrix while still unfolded [13].
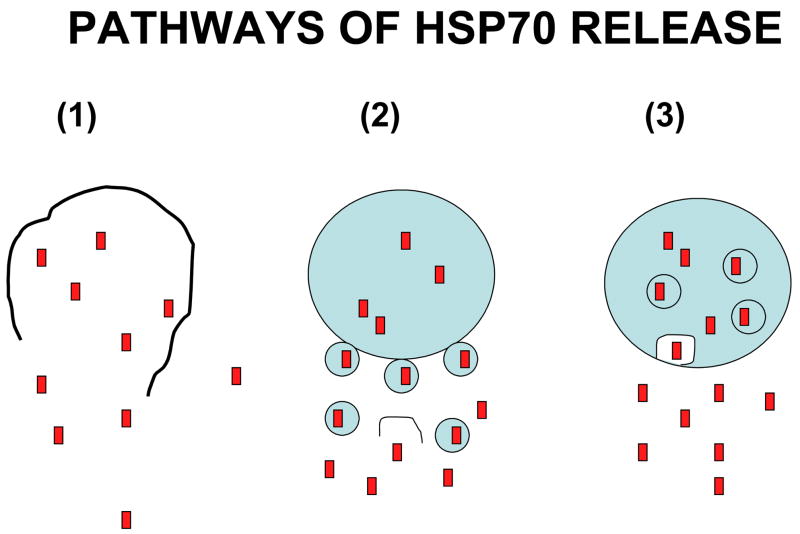
The genes encoding intracellular hsp70, of course encode no ER signal sequence. The canonical secretion pathway is thus closed to them. However, there are known non-canonical pathways for protein release used by a minority of cell proteins, most notably interleukin-1β (IL-1β), IL-1α, IL-18, IL-33 and IL-1 receptor antagonist IL1-Ra [14, 15]. Such proteins encode no leader sequence but nonetheless possess the primary function of secreted proteins which interact with receptors on adjacent or distant target cells [14]. Studies of IL-1β secretion indicate three potential scenarios for secretion [16], including: (a) lysis of IL-1β containing secretary cells and release of contents, (b) cell surface blebbing and release of IL-1β in microvesicles that lyse in the extracellular fluid and (c) entry of IL-1β into intracellular structures known as endolysosomes that transport the cytokine to the cell surface, fuse with the plasma membrane and release the contents upon fusion (Fig. 1). Each of these mechanisms is supported by some evidence and may be utilized for Hsp70 release [17–19].
Figure 1. Pathways of Hsp70 Release.
We depict the three main hypotheses for the release of proteins by non-canonical secretion. These include: (a) lysis of the cell of origin, (b) release in vesicles by a “blebbing” mechanism followed by subsequent lysis of vesicles and (c) secretion through an endolysosomes and relese when the endolysosome fuses with the cell surface.
(C) Dead Cells Tell No Stories: Hsp70 Leaks From Necrotic And Apoptotic Cells
Approaches to the use of Hsp70 in cancer immunotherapy have stressed the approach of inducing high Hsp expression in tumors by exposure to stress or by injecting expression vectors intratumorally, and then induction of necrotic death [20, 21]. Necrotic death rather than apoptotic death is emphasized, as apoptotic bodies have been shown to inhibit immunogenicity in contrast to lysed, necrotic cells which are immunogenic [8, 20]. Heat shocked cell lysates have been shown to have enhanced vaccine activity compared with non-heat shocked controls [3]. Indeed, combination of forced Hsp70 overexpression in target tissues with their killing by a necrotic mechanism has been shown to induce a powerful T cell mediated anti-tumor response and is a highly promising approach to immunotherapy [20, 22]. Nonetheless, recent studies indicate that Hsp70 can be released from intact cells by active mechanisms [18]. In a carefully controlled study of heat shocked prostate carcinoma cells it was shown that mild levels of heat shock, within the fever range lead to Hsp70 release while more severe conditions inhibit release consistent with the inactivation of a protein based secretion mechanism [19]. Severe heat shock (45°–55°C) cause delayed necrosis and a gradual release of Hsp70 as necrosis developed [19]. It is thus apparent that there are at least two pathways for Hsp70 release, including passive leakage from necrotic cells and active secretion.
(D) Leaving By The Back Door; Active Hsp70 trafficking Within Lysosomal Endosomes
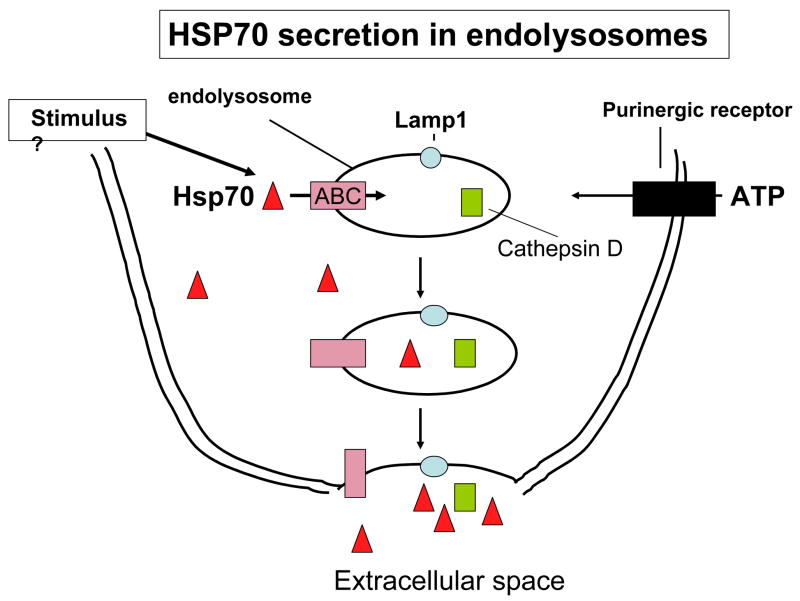
The mechanisms for secretion of leaderless proteins such as Hsp70 are complex and incompletely understood. While a number of plausible mechanisms, supported by considerable amounts of data have been deduced, there is currently little consensus as to their relative merits [16, 23]. One such mechanism (i), utilized by proteins such as fibroblast growth factor 1 (FGF-1) and IL-1α, involves translocation across the plasma membrane after partial denaturation of these cytokines to a “molten globule” form [7]. It has been conjectured that heat shock stimulates this pathway by causing partial unfolding of FGF-1 and IL-1α to conformations permissive for membrane translocation [24], [25]. However, this does not indicate how secretion be induced by a non-physical stimulus such as exposure to danger signal. In a related mechanism (ii) suggested by the studies of Suprenant et al, after stimulation of cells by the combined secretion stimulus of bacterial lipopolysaccharide (LPS) and extracellular ATP, IL-1β is packaged into plasma membrane blebs along with caspase 1 and the blebs are then released from cells as microvesicles [23]. Another potential pathway (iii) involves the entry of the secreted protein (IL-1β) into intracellular secretary endolysosomes along with caspase 1, organelle transport to the cell surface and protein release from the cell after phospholipase-mediated fusion of the endosomes to the plasma membrane [26]. Our studies have largely concentrated on investigating a role for mechanism (iii) in Hsp70 secretion, and we have examined whether secretory lysosomes are involved Hsp70 release. We initially investigated mechanisms of Hsp70 release in prostate carcinoma cell lines chosen for their spectrum of Hsp70 expression [18]. One such line, PC-3, expresses high intracellular levels of Hsp70 while Hsp70 levels in another line (LnCap) are undetectable by immunoblot. However, release rates by fever range heat shock were similar in each cell line and our studies indicate that Hsp70 release is independent of its basal intracellular levels or de novo expression; heat-induced Hsp70 release appears to constitute an independent “heat shock response” to the transcriptional stress response that is normally envisaged [18]. We therefore next investigated whether inhibition of lysosomal function affects Hsp70 secretion. Indeed, lysosomotropic agents methylamine and ammonium chloride markedly decrease the rate of Hsp70 release in PC-3 and LNCap cells after heat shock [18]. Hsp70 release can also be stimulated by uptake of Gram-negative bacteria such as E.coli or exposure to E coli proteins and such Hsp70 release is also inhibited by targeting lysosomal intraluminal pH (Mambula, S.S. & Calderwood, S. K., submitted). Thus lysosomal function is required for in Hsp70 release. We further confirmed a role for these cellular organelles in Hsp70 release by isolating the lysosomal fraction from LnCap and PC-3 cells and analyzing Hsp70 levels in this compartment after stimulation [18]. Hsp70 levels were minimal in the lysosomal fraction under normal conditions but increased after heat shock conditions and were co-expressed in the lysosome along with marker protein cathepsin D (Fig 2). These findings are consistent with heat causing Hsp70 entry into lysosomal endosomes. We further investigated route for Hsp70 secretion by examining the transport of secretory lysosomes to the cell surface, using the lysosomal membrane protein LAMP1 as a marker [18]. LAMP1 was detected by indirect immunofluorescence, in small patches on the cell surface of both LnCap and PC-3 at 37°C. However, conditions that lead to Hsp70 secretion including heat shock at 30 min at 43°C or for 6 hr at 40°C lead to a major increase in cell surface LAMP1, which becomes concentrated in large caps on the surface of PC-3 and LNCap cells [18]. These data are consistent with a pathway of transport of secretory lysosomes, containing Hsp70 to the cell surface, fusion with the plasma membrane and insertion of LAMP1 into the extracellular face of the plasma membrane accompanied by Hsp70 secretion accompanied by other lysosomal proteins (Fig 2). Indeed, IL-1β secretion after monocyte exposure to ATP involves the coordinate release of lysosome resident proteins cathepsin D and caspase 1 into extracellular compartment [26].
Figure 2. Secretion of Hsp70 through the endolysosome pathway.
Our experiments suggest that hsp70 secretion involves the entry of Hsp70 into endolysosomes through ABC family transporters, where they co-localize with intravesicular cathepsin D. These organelles then are transported to the cell surface. Subsequent fusion of Hsp70 containing endolysosomes with the cell surface results in the localization of LAMP1 in the plasma membrane and release of Hsp70 along with other proteins such as cathepsin D.
Several other schemes for Hsp70 release have been proposed and indeed may be operable under some circumstances. It has been proposed that Hsp70 may be released from some cells on the surface of lipid-bounded “endosomes” [27]. Such a scheme is not supported by our study of prostate carcinoma cells in which we only detected Hsp70 in the extracellular medium in free solution. However, it is possible that fraction of Hsp70 is released from other cell types by such a mechanism [28] [17]. Secretion mechanisms may vary between cell types and, for instance, IL-1β secretion may involve quite distinct mechanisms in different cell types [29, 30]. In either case, Hsp70 transits though a lipid-bounded vesicular structure along the pathways to secretion- which in the case of the present study is a secretary lysosomal compartment, and finally passes the plasma membrane to be secreted [31].
Detection of extracellular HSP70 by ELISA
Sandwich enzyme-linked immunosorbant assay ELISA is used for detection of Hsp70 in supernatants [32]. We have used ultra high binding 96-well microtiter ELISA plates (ThermoLabsystems)coated overnight with a concentration 2ug/ml Hsp70 monoclonal antibody (Stressgen # SPA-810) in carbonate buffer pH9.5 (Sigma # C3041) followed by 3 washes in Dulbecco’s phosphate buffered saline (PBS; Sigma # P4417) containing 0.1% v/v Tween 20 Sigma # P1379). The plates were blocked with 1% bovine serum albumin (BSA; Sigma # A2153) in PBST followed by 3 washes in PBST. Samples and recombinant human HSP70 protein (Stressgen# NSP-555) standard were added to the wells and incubated for 1 hour followed by 3 washes in PBST. Rabbit polyclonal anti-HSP70 antibody (Stressgen #SPA-812) at a dilution of 1/1000 in PBST containing 2% v/v normal mouse serum (Sigma # M5905) was added to each well and incubated for 1 h at room temperature followed by 3 washes with PBST. Anti- rabbit IgG alkaline phosphatase conjugated monoclonal antibody (Sigma # A-2556) was added to the 96-well plates followed by 3 washes with PBST. Finally, the substrate p-nitro phenyl phosphate substrate (Sigma # N2770) was added and absorbance was read by spectrophotometer (Bio-Rad) at absorbances of 405 nm and 650 nm (reference wavelength).
Isolation of Cell Lysosomal Fraction
PC3 and LNCap cells were grown to a packed cell volume of 3ml, which was resuspended in an equal volume of packed cells with hypotonic buffer (10mM HEPES, 1.5mM MgCl2, 10mM KCl, 1mM DTT) and allowed to swell for 15 min on ice. Cells were lysed by rapid passage cells through 1.0 ml syringe with 25 gauge needle 5 times while keeping cells/lysates on ice. Crude nuclear pellet was prepared by centrifugation for 30 sec at 12,000 × g. Supernatants were then stored and the lysosomal fraction isolated as described by Andrei et al (1999). Briefly, supernatants were treated with proteinase K for 30 min on ice and then inhibited with complete protease inhibitors (Roche # 1697498). The supernatant was then diluted 10-fold in homogenizing buffer and centrifuged at 50,000 × g for 20 min. The pellet was isolated, washed once in homogenizing buffer and then solubilized in reducing sample buffer. The presence of Hsp70 and lysosomal resident proteins cathepsin D and LAMP1 was detected by western analysis after 10 % SDS-PAGE.
The hsp70 containing lysosomal fraction can be further analyzed by Percoll density gradient centrifugation that separates high-density lysosomes rich in hydrolytic enzymes from the lighter endolysosomal structures. Distribution of hsp70 will be assayed in the Percoll gradient fractions by immunoblot with anti-hsp70 antibodies and compared with that of CD that distributes to denser lysosomal vesicles and Rab7, which is found in endolysosomes.
However, an alternative approach that can be used to analyze the vesicles is transmission electron microscopy after probing fixed ultracentrifuge fixed fractions with immunogold labeled hsp70 antibodies. Distribution of Hsp70 in vesicles is compared with CD and Lamp1.
Use of Lysosomotropic Agents to examine lysosomal/endolysosomal function
Lysosomal function is dependent on maintaining a low pH relative to the bulk cytoplasm. As most lysosomal enzymes function optimally at pH values below 7.0, lysosomal function can be blocked by weak bases which readily cross cell membranes and accumulate preferentially in zones of low pH. Weakly basic lysomotropic agents that lead to increased endoluminal pH can be used to study vesicular accumulation and secretion of Hsp70 after heat shock or pathogenic danger signals. Cells are pretreated for 2 h with 30 uM methylamine or 50 uM ammonium chloride (Aldrich-Sigma, St Louis) compared to control untreated cells. The agents are minimally toxic at these concentrations.
Intracellular Localization of LAMP-1 and Hsp70 by Immunofluorescence microscopy
Cells were plated onto fibronectin-coated 8-well chamber glass slides with cover (Nalge Nunc) at 105 cells per well. 24 h later, the culture medium was removed and replaced with fresh medium before the cells were hyperthermia treated as described above. The cells were immediately fixed with 3.7% paraformaldehyde. After fixation for 15 min, the cells were washed once with PBS and blocked with blocking buffer (PBS containing 3% BSA) for 30 min. For intracellular staining the cells were permeabilized with 0.1% triton X-100 (Sigma) for 15 min at room temperature after the fixing step. The cells were next washed once with PBS, incubated for 1 h anti-HSP70 (Stressgen) or LAMP1 (CD107a) (BD Pharmingen) monoclonal antibodies followed by 5 washes in wash buffer (PBS containing 0.05% Tween). The cells were incubated with flourescein-conjugated anti-mouse/rabbit IgG antibody (Alexa 594 and Alexa-488) (Molecular Probes) respectively. Slides were then washed and mounted in aqueous mounting solution using cover glass (Fisher Scientific) prior to fluorescent microscopy. Images were acquired using a Nikon Eclipse E600 microscope fitted with a RT SPOT digital camera and processed using SPOT software (Diagnostic Instruments, Inc.
(E) Crossing The Frontier: Folding, Flipping And Pumping Across Lipid Membranes?
One of the major unresolved problems for non-canonical protein secretion is- how do leaderless proteins cross an impermeable lipid bilayer in order to either access the intraluminal space of an organelle such as a secretary lysosome or cross the plasma membrane? A number of scenarios are envisaged for such transport. Mechanisms include pumping of proteins across membranes by members of the highly conserved ABC cassette transport proteins which can pump large and small molecules across membranes, “flipping” of proteins across membranes associated with phospatidylserine or binding to molecular chaperones to prevent folding [7, 13, 33].
As previous studies have implicated a role for ABC family transporters in the secretion of IL-1β, we further pursued this paradigm and asked whether this mechanisms might also mediate Hsp70 secretion [29]. Our preliminary studies using ABC inhibitors suggest such a scenario for transport in cells exposed to fever range heat or responding to PAMPS exposure. Cells treated with the ABC family inhibitor glibenclamide showed significant inhibition of Hsp70 release [18]. In addition, previous studies implicate one member of the large the ABC gene family member ABCA-1 in leaderless secretion [34]. The role for ABCA-1 can be examined using another chemical, inhibitor 4,4-diisothiocyanato-stilbene-2, 2-disulfonic acid (DIDS). DIDS inhibits ABCA-1 specifically by blocking the chloride current necessary for its function [30]. DIDS proved to significantly inhibit Hsp70 secretion triggered by PAMPS exposure or temperature shock [18]. These experiments therefore suggest that Hsp70 secretion requires the ABCA-1 transmembrane transporters. However the exact role of ABCA-1 in Hsp70 secretion is not yet clear and further studies will be required to confirm its role and to determine its place in the secretion pathway. One open question is whether the Hsp70 is transported in a folded form or unfolds for translocation across the lysosomal membrane. Indeed Hsp70 proteins carry out the ATP-dependent function of maintaining mitochondrial matrix proteins in unfolded form prior to crossing the mitochondrial outer membrane and arrival at their destination [13]. It is not known whether Hsp70 molecules can catalyze their own unfolding under specialized circumstances. Other secreted proteins that are largely of cytoplasmic/nuclear distribution in addition to HSP70, such as high mobility protein b1 (HMGB1) and engrailed-2 appear to be secreted along a similar pathway under the specialized conditions that promote secretion [35], [36]. Curiously heat shock has been shown to cause the secretion of FGF1 and IL-1α though an alternative pathway (i), involving heat induced membrane translocation [7]. Hsp70 secretion by heat shock could therefore use a hybrid pathway involving mechanisms found in pathways (i) and (ii). For instance binding of FGF1 and IL-1α to phosphatidylserine on the inner leaflet of the plasma membrane prior to translocation may be involved in a “flipping “ mechanism in which phosphatidylserine is externalized to the outer leaflet of the plasma membrane accompanied by associated proteins [37]. Intriguingly, Hsp70 has been shown to bind phosphatidylserine, although the significance of this interaction for Hsp70 release has not been determined [38]. There is previous evidence that heat shock stimulates lysosomal exocytosis in vivo in liver cells and peritoneal macrophages [39] [40].
To study the role of ABC transporters in Hsp7-0 secretion, cells were pretreated for 2 h with either 1 mM glibenclamide or 250 uM 4,4-diisothiocyanato-stilbene-2, 2-disulfonic acid (DIDS) (Sigma #D3514) and compared to controls treated without drug.
(F) Passport Control: Sorting signals for secreted hsp70?
The studies carried out to date do not indicate the sorting mechanism required for a fraction of the intracellular Hsp70 to translocate to lysosomal endosomes during heat shock or infection and enter the lumen of these organelles. However as the majority of intracellular Hsp70 is either retained in the cytoplasm or translocates to the nucleus after stress, it seems likely that a signal such as posttranslational modification is required for its sorting to the secretion pathway [18, 41]. Hsp70 can be phosphorylated under some circumstances and may be ubiquitinylated on association with the ubiquitin E3 ligase CHIP during recovery from stress [42, 43]. Secretion signaling has been carefully studied in other systems. Translocation of a fraction of the nuclear proteins HMGB1 and engrailed-2 to the secretory pathway involves, respectively, hyperacetylation and dephosphorylation [44, 45]. Hsp70 family protein Hsc70 frequently traffics to lysosomal compartments and indeed mediates lysosomal proteolysis of many intracellular proteins as well as autophagy [41]. The sorting mechanism for Hsp70 mediated secretion remains however, mysterious.
So far no progress has been made in this area. An important initial approach might be to analyze secreted Hsp70 by mass spectrometric approaches. This might permit us to analyze potential modifications such as phosphorylation, ubiquination, acetylation or sumoylation. One problem to be overcome in this respect would be the presence of serum albumin as an overwhelming component; Hsp70 release by heat shock requires full serum containing medium [18].
(F) A Call To Arms: Signals Of Death And Danger Bring Forth Hsp70
It is becoming apparent that Hsp70 and other heat shock proteins are mediators of innate immunity [11, 46, 47]. As abundant endogenous proteins, their release may signal danger to the host in a similar way to endogenous danger signals such as sodium urate or HMGB1 [48, 49]. We have therefore examined potential trigger mechanisms for release following the paradigm of cytokine triggering by exogenous danger signals [50]. For molecules such as LPS, which have been classified as pathogen-associated molecular patterns or PAMPS, a double signal is often required for optimal secretion. This includes binding of PAMPS to primary pathogen recognition receptors (PRR) such as toll-like receptor 4 which leads to activation of a signaling network that involves iκB kinases and MAP kinases and transcription of cytokines such as IL-1β [51]. However, release of newly synthesized cytokines also involves an increase in extracellular ATP levels released from adjacent stressed cells or from platelets [15]. The effects of ATP on secretion of cytokines can be mediated through the purinergic receptor P2X7 [15]. Engagement of P2X7, which contains a small cationic channel leads to K+ efflux from the target cell [52, 53]. K+ efflux is a key trigger in IL-1β release and appears to exert effects on processing of pro- IL-1β as well as secretion [15, 54]. It should be noted that prolonged stimulation of P2X7 can lead to cell lysis through the opening of a larger channel that permits passage of larger particles and cell lysis [15]. There have been great advances in this area recently and the massive K+ efflux in response to P2X7 activation acts as a trigger for assembling the “inflammasome” a multiprotein complex that mediates secretion of a number of cytokines in response to stress [55]. The inflammasome particle is involved in activation of caspase 1, an essential proteolytic enzyme involved in the cleavage of pro-IL-1β to the mature form (IL-1β ready for secretion. The complex contains NALP3, the caspase 1 activator and adaptor proteins ASC and CARDINAL which each bind directly to caspase 1 through their CARD domains [56]. The inflammasome may, in addition to mediating processing of pro-IL-1β by caspase 1, orchestrate the events of non-canonical secretion and could potentially be involved in Hsp secretion [55]. Caspase 1 is loaded into secretary endolysosomes in response to LPS and extracellular ATP exposure [55]. The proximal signal which leads to Hsp70 release is however not clear. For instance, abundant Hsp70 release is strongly induced by live E. coli or E. coli intracellular protein GroEL but not by LPS ([11] S.S. Mambula & S.K. Calderwood, in preparation). This would appear to rule out TLR4 as the PRR responsible for Hsp70 secretion [57]. Other possibilities include other TLR members, c-type lectins or intracellular PRR such as Nod-like proteins (NLR) [56–58]. We have begun to examine trigger mechanisms for Hsp70 release first examining fever stress. We first examined the potential role of extracellular ATP and P2X7. P2X7 is sensitive to extracellular ion concentrations and its cationic channel is effectively blocked by extracellular Mg 2+ [29]. Its role in secretion can be probed by inhibiting K+ efflux or alterating extracellular Mg2+ [29]. When we incubated carcinoma cells or macrophages in growth medium supplemented with elevated Mg2+ prior to stimulus, Hsp70 release was strongly inhibited [19]. By contrast, Mg++ chelation by EDTA significantly enhanced HSP70 release after heat shock (Fig. 4C and 4D) [30]. This is therefore indirect evidence for a role for P2X receptors. However addition of ATP alone did not induce secretion of Hsp70 in the absence of stimulus, suggesting that ATP may be necessary but is not sufficient for HSP70 release, as is the case with some other molecules [30]. ABC1 transporter activity may also be modulated by the activation of ATP receptors and cation efflux, as ABC1 is a K+ antiporter [33]. However, potassium efflux also heralds the activation of phospholipases A and C, enzymes whose activity brings about membrane remodeling events and may participate in the mechanisms required for secretary lysosomes to fuse with the plasma membrane and be released [26]. These workers suggest that phosphatidylcholine-phospholipase C activated by K+ depletion leads to an elevation in intracellular Ca 2+ that next activates phospholipase A2 and permits the later stages of exocytosis [26]. Somewhat perplexingly, we did not observe a major effect of depletion of intracellular or extracellular Ca 2+ on Hsp70 release by heat shock [19]. However, heat shock can directly activate phospholipases an effect that requires intracellular GTP and Mg 2+ but occurs without added Ca 2+ [10]. It should also be noted that signals other than extracellular ATP may be involved in non-canonical protein secretion and for instance lysophosphatidylcholine, a product of phospholipase A1 activity induces IL-1β release in an ATP independent manner [59].
Up- And Down Regulation Of ATP receptor function
P2X7 receptor function in cytokine secretion has been probed using commercially available blocking antibodies to restrict nucleotide access and by gene targeting [52, 60]. P2X7 receptors are also strongly inhibited by extracellular divalent cations whose relative effectiveness ranges in order from Cu 2+ > Cd 2+ >Zn 2+ > Ni 2+ > Mg 2+ > Mn 2+ > Ca 2+>Ba 2+, and addition of such ions can be used as a rapid method to test for P2X7 requirement [61]. We used a protocol of cell pretreatment for 2 h with 20 mM MgCl2 to antagonize P2X7 or 5 mM ethylenediaminetetraacetic acid (EDTA) to chelate extracellular Mg++[19]. Such treatments led to, respectively, down- and up-regulation of Hsp70 secretion [19]. Receptor function can also be inhibited by monovalent anions such as Cl−; for example receptor activity of recombinant P2X7 is increased 19-fold when NaCl is replaced by sucrose [53]. An alternative cell physiological approach to inhibiting P2X7 is to block K+ efflux using agents such as nigericin [62, 63]. Finally, selective chemical P2X7 antagonists are now available to study the role of this receptor [64].
(G) Brave New World: Role of Extracellular Hsp In The Inflammatory Response-Feeding Back Or Forward?
Hsp70 along with other molecular chaperones can be a powerful mediator of inflammation and immunity [46]. It can under certain circumstances mediate the release of cytokines from macrophages and dendritic cells, induce co-stimulatory molecules in APC and cross prime T lymphocytes to attack tumor cells [3, 46, 65]. Under other conditions, such as in chronic inflammatory diseases, mammalian Hsp70 is immunomodulatory and antagonizes the pro-inflammatory effects of for instance bacterial Hsp70 from invading pathogens [66, 67]. We have discovered a pathway for Hsp70 release that resembles those in pro-inflammatory molecules such as the interleukins 1, 16 and 33, and HMGB1 [19]. Hsp70 is released in macrophages and tumor cells exposed to E coli, bacterial proteins and febrile temperatures. Further studies are required to determine its place in the inflammatory response.
Acknowledgments
We acknowledge the support of the Department of Radiation Oncology at Beth Israel Deaconess Medical Center, Boston. We thank out colleagues Jimmy Theriault and Abdul Khaleque for sharing their thoughts and Rong Zhong for managing the laboratory. These studies were supported by grants 5RO1CA047407 and 3RO1CA094397.
Abbreviations
- Hsp70
heat shock protein 70
- IL-1β
interleukin I beta
- ATP
adenosine triphosphate, ABC transporter
- LAMP1
lysosomal-associated membrane protein 1
- FGF-1
fibroblast growth factor-1
- IL-18
interleukin 18
- IL-1α
interleukin-1alpha
- DIDS
4,4-diisothiocyanato-stilbene-2, 2-disulfonic acid
- BAPTA-AM
1,2-bis (2-aminophenoxy)ethane-N,N,N′,N′-tetra acetic acid
- EGTA
ethylene glycol bis(2-aminoethyl ether)-N,N,N′N′-tetra acetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bukau B, Weissman J, Horwich A. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Mayer MP, Bukau B. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderwood SK, Theriault JR, Gong J. Eur J Immunol. 2005;35:2518–2527. doi: 10.1002/eji.200535002. [DOI] [PubMed] [Google Scholar]
- 4.Hightower LE, Guidon PT., Jr J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- 5.Tytell M, Greenberg SG, Lasek RJ. Brain Res. 1986;363:161–164. doi: 10.1016/0006-8993(86)90671-2. [DOI] [PubMed] [Google Scholar]
- 6.Pockley AG, Shepherd J, Corton JM. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- 7.Prudovsky I, Mandinova A, Soldi R, Bagala C, Graziani I, Landriscina M, Tarantini F, Duarte M, Bellum S, Doherty H, Maciag T. J Cell Sci. 2003;116:4871–4881. doi: 10.1242/jcs.00872. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava P. Cancer Immun. 2003;3:4. [PubMed] [Google Scholar]
- 9.Calderwood SK, Stevenson MA. J Cellul Physiol. 1993;155:248–256. doi: 10.1002/jcp.1041550205. [DOI] [PubMed] [Google Scholar]
- 10.Calderwood SK, Stevenson MA, Price BD. J Cell Physiol. 1993;156:153–159. doi: 10.1002/jcp.1041560121. [DOI] [PubMed] [Google Scholar]
- 11.Davies EL, Bacelar MM, Marshall MJ, Johnson E, Wardle TD, Andrew SM, Williams JH. Clin Exp Immunol. 2006;145:183–189. doi: 10.1111/j.1365-2249.2006.03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapoport TA, Matlack KE, Plath K, Misselwitz B, Staeck O. Biol Chem. 1999;380:1143–1150. doi: 10.1515/BC.1999.145. [DOI] [PubMed] [Google Scholar]
- 13.Neupert W, Brunner M. Nat Rev Mol Cell Biol. 2002;3:555–565. doi: 10.1038/nrm878. [DOI] [PubMed] [Google Scholar]
- 14.Chimini G, Rubartelli A. Novel Pathways of Protein Secretion. Cambridge Univ. Press; Cambridge: 2005. [Google Scholar]
- 15.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 16.Wewers MD. Proc Natl Acad Sci U S A. 2004;101:10241–10242. doi: 10.1073/pnas.0403971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Cancer Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mambula SS, Calderwood SK. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- 19.Mambula SS, Calderwood SK. Int J Hyperthermia. 2006;22:575–585. doi: 10.1080/02656730600976042. [DOI] [PubMed] [Google Scholar]
- 20.Daniels GA, Sanchez-Perez L, Diaz RM, Kottke T, Thompson J, Lai M, Gough M, Karim M, Bushell A, Chong H, Melcher A, Harrington K, Vile RG. Nat Biotechnol. 2004;22:1125–1132. doi: 10.1038/nbt1007. [DOI] [PubMed] [Google Scholar]
- 21.Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, Stoppacciaro A, Vile RG. J Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- 22.Calderwood SK. Trends Biotechnol. 2005;23:57–59. doi: 10.1016/j.tibtech.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 23.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 24.Arai M, Kuwajima K. Adv Protein Chem. 2000;53:209–282. doi: 10.1016/s0065-3233(00)53005-8. [DOI] [PubMed] [Google Scholar]
- 25.Ptitsyn OB. Adv Protein Chem. 1995;47:83–229. doi: 10.1016/s0065-3233(08)60546-x. [DOI] [PubMed] [Google Scholar]
- 26.Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Proc Natl Acad Sci U S A. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tytell M. Int J Hyperthermia. 2005;21:445–455. doi: 10.1080/02656730500041921. [DOI] [PubMed] [Google Scholar]
- 28.Lancaster GI, Febbraio MA. J Biol Chem. 2005 doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 29.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marty V, Medina C, Combe C, Parnet P, Amedee T. Glia. 2005;49:511–519. doi: 10.1002/glia.20138. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez A, Webster P, Ortego J, Andrews NW. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mambula SS, Sau K, Henneke P, Golenbock DT, Levitz SM. J Biol Chem. 2002;277:39320–39326. doi: 10.1074/jbc.M201683200. [DOI] [PubMed] [Google Scholar]
- 33.Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, Chimini G. Nat Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- 34.Hamon Y, Luciani MF, Becq F, Verrier B, Rubartelli A, Chimini G. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- 35.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maizel A, Bensaude O, Prochiantz A, Joliot A. Development. 1999;126:3183–3190. doi: 10.1242/dev.126.14.3183. [DOI] [PubMed] [Google Scholar]
- 37.Peterson EA, Sutherland MR, Nesheim ME, Pryzdial EL. J Cell Sci. 2003;116:2399–2408. doi: 10.1242/jcs.00434. [DOI] [PubMed] [Google Scholar]
- 38.Arispe N, Doh M, De Maio A. Cell Stress Chaperones. 2002;7:330–338. doi: 10.1379/1466-1268(2002)007<0330:lidtca>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barni S, Bertone V, Silvotti MG, Freitas I, Mathe G, Pontiggia P. Biomed Pharmacother. 1996;50:79–84. doi: 10.1016/0753-3322(96)84717-2. [DOI] [PubMed] [Google Scholar]
- 40.Pontiggia P, Barni S, Mathe G, Bertone V, Pontiggia E. Biomed Pharmacother. 1995;49:429–430. doi: 10.1016/0753-3322(96)82680-1. [DOI] [PubMed] [Google Scholar]
- 41.Cuervo AM, Dice JF. J Mol Med. 1998;76:6–12. doi: 10.1007/s001090050185. [DOI] [PubMed] [Google Scholar]
- 42.Evdonin AL, Guzhova IV, Margulis BA, Medvedeva ND. Cancer Cell Int. 2004;4:2. doi: 10.1186/1475-2867-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim YP, Wong CY, Ooi LL, Druker BJ, Epstein RJ. Clin Cancer Res. 2004;10:3980–3987. doi: 10.1158/1078-0432.CCR-03-0663. [DOI] [PubMed] [Google Scholar]
- 44.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Embo J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wisniewski JR, Szewczuk Z, Petry I, Schwanbeck R, Renner U. J Biol Chem. 1999;274:20116–20122. [PubMed] [Google Scholar]
- 46.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 47.Vabulas RM, Wagner H. Toll-like receptor-dependent activation of antigen presenting cells by hsp60, Gp96 and hsp70. Cambridge University Press; Cambridge: 2005. [Google Scholar]
- 48.Shi Y, Evans JE, Rock KL. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 49.Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. Trends Immunol. 2005;26:381–387. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Matzinger P. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 51.Takeda K, Kaisho T, Akira S. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 52.Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WS, Dixon SJ, Sims SM, Thompson DD. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 53.Michel AD, Chessell IP, Humphrey PP. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:102–109. doi: 10.1007/pl00005328. [DOI] [PubMed] [Google Scholar]
- 54.Baraldi PG, Di Virgilio F, Romagnoli R. Curr Top Med Chem. 2004;4:1707–1717. doi: 10.2174/1568026043387223. [DOI] [PubMed] [Google Scholar]
- 55.Ogura Y, Sutterwala FS, Flavell RA. Cell. 2006;126:659–662. doi: 10.1016/j.cell.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 57.Sansonetti PJ. Nat Immunol. 2006;7:1237–1242. doi: 10.1038/ni1420. [DOI] [PubMed] [Google Scholar]
- 58.Robinson MJ, Sancho D, Slack EC, Leibundgut-Landmann S, Sousa CR. Nat Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 59.Stock C, Schilling T, Schwab A, Eder C. J Immunol. 2006;177:8560–8568. doi: 10.4049/jimmunol.177.12.8560. [DOI] [PubMed] [Google Scholar]
- 60.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 61.Virginio C, Church D, North RA, Surprenant A. Neuropharmacology. 1997;36:1285–1294. doi: 10.1016/s0028-3908(97)00141-x. [DOI] [PubMed] [Google Scholar]
- 62.Kahlenberg JM, Dubyak GR. Am J Physiol Cell Physiol. 2004;286:C1100–1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 63.Perregaux D, Gabel CA. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 64.Stokes L, Jiang LH, Alcaraz L, Bent J, Bowers K, Fagura M, Furber M, Mortimore M, Lawson M, Theaker J, Laurent C, Braddock M, Surprenant A. Br J Pharmacol. 2006;149:880–887. doi: 10.1038/sj.bjp.0706933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 66.Quintana FJ, Carmi P, Mor F, Cohen IR. Arthritis Rheum. 2004;50:3712–3720. doi: 10.1002/art.20635. [DOI] [PubMed] [Google Scholar]
- 67.Quintana FJ, Cohen IR. Heat shock proteins regulate inflammation by both molecular and network cross-reactivity. Cambridge University Press; Cambridge: 2005. [Google Scholar]