Abstract
Our recent observations of reduced total nitric oxide synthesis in renal failure patients on peritoneal dialysis and haemodialysis suggest that hypertension in end-stage renal disease involves lack of vasodilatory endothelial NO. To directly test this, uraemic plasma was obtained from dialysis patients and incubated with cultured vascular endothelial cells, to determine the effect on nitric oxide synthase (NOS) activity in comparison with plasma from subjects with normal renal function. After incubation for 6 h with 20% uraemic plasma from peritoneal dialysis and immediately prehaemodialysis patients, NOS activity was reduced in human dermal microvascular endothelial cells. Haemodialysis did not remove the NOS-inhibitory activity of uraemic plasma nor did it activate inducible NOS, as NOS activity was always similar in control and dexamethasone pretreated cells. Nitric oxide production (accumulation of nitrite and nitrate) was lower in cells incubated with uraemic vs. normal plasma and excess arginine increased nitric oxide production by cells previously exposed to uraemic medium. This inhibitory effect was not associated with co-factor deficiency but did correlate with plasma concentrations of endogenous NOS inhibitors. These in vitro findings suggest that low endothelial NOS activity may contribute to hypertension in end stage renal disease patients.
Keywords: asymmetric dimethylarginine (ADMA), bovine aortic endothelial cells, dermal microvas cular endothelial cells, endogenous nitric oxide synthase inhibitors, glomerular capillary endothelial cell, kidney failure, nitrate and nitrite, NOx, plasma
The synthesis of nitric oxide (NO) by endothelial cells is continual and plays an important role in minute-to-minute control of vascular tone, blood pressure (BP) and blood flow (Moncada et al. 1995). Hypertension occurs in mice with knockout of the endothelial nitric oxide synthase (eNOS) gene (Huang et al. 1995) and in man with certain eNOS gene polymorphisms (Soma et al. 1999). There is evidence that regional vascular endothelial NO production is defective in some patients with primary and secondary hypertension (Baylis & Vallance 1996). Therefore, insufficient NO production from eNOS may play a role in some forms of hypertension in man.
Hypertension is a major complication of end stage renal disease (ESRD) (Rostand et al. 1991) and although in part, caused by volume overload, may also involve NO deficiency. Indeed, we have reported reductions in total NO synthesis (from 24 h NO2 + NO3 = NOx production) in both peritoneal dialysis (PD) and haemodialysis (HD) patients (Schmidt et al. 1999a, b). Patients with ESRD accumulate endogenous circulating compounds which may competitively inhibit the l-arginine : NO pathway (Vallance et al. 1992).
The purpose of this study was to assess the effects of uraemic plasma on NOS activity in cultured vascular endothelial cells. The majority of studies were on human dermal microvascular endothelium although some experiments were done on human glomerular endothelial cells and bovine thoracic aortic endothelium.
METHODS
Human dermal microvascular endothelial cells (HDMEC) and endothelium growth medium (EGM-MV) were obtained from Clonetics Corporation (San Diego, CA). Human glomerular endothelial cells (HGEC) and CS-C growth medium were from Cell System Corporation (Kirkland, WA). The bovine thoracic aortic endothelial cells (BAEC) were established by us in primary culture. Human plasma was from PD patients, pre- and immediately posthaemodialysis (pre-HD and post-HD) and normal controls. These studies were performed with the consent of each subject and permission of the West Virginia University Institutional Review Board. Clinical characteristics of the study populations are shown in Table 1. Each type of plasma was pooled from two to three patients, stored frozen at –80 °C, and thawed immediately prior to use. All HD patients were dialysed with polysulfone membranes on F-80 dialysers (Fresenius USA, Lexington, MA).
Table 1.
The clinical characteristics of the patients with end stage renal disease and normal control
| Blood pressure (mmHg) |
||||||
|---|---|---|---|---|---|---|
| Systolic | Diastolic | BUN (mg dL–1) | Pcr(mg dL–1) | PADMA (μm) | Medications† | |
| Control (n = 5) | 117 ± 6 | 72 ± 4 | 10 ± 3 | 0.8 ± 0.2 | 0.45 ± 0.10 | None |
| PD (n = 6) | 127 ± 4 | 74 ± 5 | 42 ± 6* | 10.1 ± 3.2* | 2.16 ± 0.27* | BB, CEI, D |
| HD (n = 5) Pre/post | 136 ± 8* | 69 ± 3 | 74 ± 8* | 10.2 ± 1.4* | 4.13 ± 0.78* | BB,CEI, D |
| 32 ± 4*# | 3.2 ± 0.4*# | 1.46 ± 0.44*# | ||||
Antihypertensive treatment: BB, Beta-blocker; CEI, angiotensin converting enzyme inhibitor; D, diuretic drugs.
P < 0.05 vs. control.
After haemodialysis and P < 0.05 vs. control.
Cell culture
HDMEC (passage 4–7) were maintained in EGM-V media containing 10 pg mL–1 human recombinant epidermal growth factor, 1 μg mL–1 hydrocortisone, 50 μg mL–1 gentamicin, 50 ng mL–1 amphotericin-B, 12 μg mL–1 bovine brain extract and 5% fetal bovine serum. HGECs (passage 4–7) were maintained in CS-C medium and BAECs (passage 2–4) in DMEM + 10% FBS. All cells were incubated at 37 °C in a humidified atmosphere of 5% CO2 and 95% air and had tested positive for Factor VIII and acetylated low density lipoprotein (LDL) uptake and negative for smooth muscle alpha actin, confirming that they are pure endothelial cells. Cells were subcultured onto 12-well plates and when confluent, culture medium was replaced with MEM containing 20% human plasma (uraemic or control). Initial time course experiments showed that cells remained viable when incubated for 6–12 h but that cell detachment occurred at 24 h and longer, when any of the human plasmas (including control) were used. In all experiments cells were incubated for 6 h, then studied for NOS activity over the following 60 min.
Determination of nitric oxide synthase activity in living endothelial cells
NOS activity was determined in all cells by measuring l-[3H]arginine conversion to l-[3H]citrulline according to the method of Davda et al. (1993), using Dowex 50WX8-400 resin (Na+ form) to remove unconverted l-[3H]arginine.
Determination of nitric oxide synthase activity in fractionated endothelial cells
Confluent endothelial cells grown in T-75 flasks were disrupted by freeze-thawing and the NOS activity in the cell lysate was determined by conversion rate of 3H-l-arginine to 3H-l-citrulline (Bredt & Synder 1994).
Measurement of NO production from nitrate + nitrite = NOx level
To obtain a sufficient amount of NOx for analysis, cells were grown to confluence in T25 flasks, then incubated for 6 h with 20% normal or uraemic plasma, with and without 100 μm l-arginine or 100 μm d-arginine. Following this, cells were incubated with 1 mL MEM (a NOx-free medium) for 2 h (rocked to ensure that medium always covered the cells). The medium was harvested and NOx determined by the Greiss reaction as reported by us (Suto et al. 1995) with modifications to increase the sensitivity of the assay (Funai et al. 1997, Verdon et al. 1995).
Measurement of cell protein
The total cellular protein was determined by the Bio-Rad detergent method which uses a modification of the 2Lowry assay (Peterson 1979) with bovine serum albumin as a standard.
Determination of plasma concentration of asymmetric dimethyl arginine
Asymmetric dimethyl arginine (ADMA) was measured by reverse phase HPLC with AccQ Tag as described in detail recently (Anderstam et al. 1997, Schmidt et al. 1999b).
Each measurement was in triplicate and experiments were repeated at least three times (n given in table or figure legend). Results are expressed as mean ± SEM. Statistical analysis was performed with the use of Student's unpaired t-test and one way anova. Values of P < 0.05 are considered to be significantly different.
RESULTS
As shown in Table 1, the HD patients whose plasma was used in this study, had systolic hypertension, despite the fact that they, and the PD patients, were on one or more antihypertensive drugs. Both BUN and plasma creatinine were elevated in the ESRD population and HD reduced these blood levels but did not normalize them (Table 1).
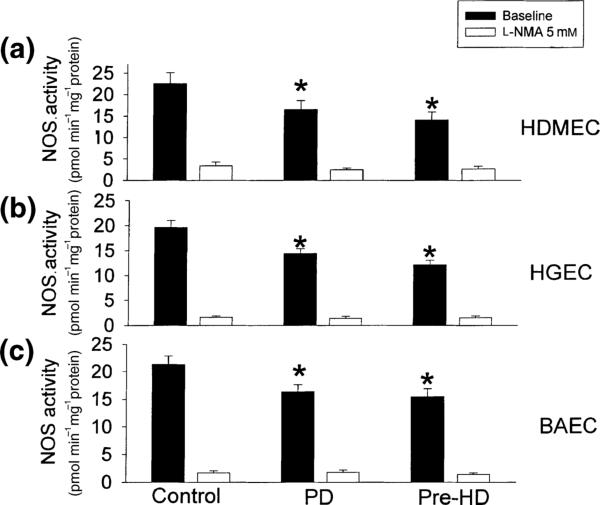
After 6 h incubation with 20% uraemic plasma from PD and pre-HD patients, NOS activity was significantly, and similarly inhibited compared with control (P < 0.05) by ≈25–30% in all three cell types studied (Fig. 1). As most of the l-arginine to l-citrulline conversion can be inhibited with l-NMA (Fig. 1, open columns), we are confident that we are measuring NOS activity. In separate studies we showed that there was no difference between the NOS inhibitory effect of pre- vs. post-HD plasma in HDMEC (14 ± 1 vs. 14 ± 2, conversion of 3H-l-arginine to 3H-l-citrulline in pmol min–1 mg–1 protein) and both were low (P < 0.05) vs. control (22 ± 3 pmol min–1 mg–1 protein). Also, the NOS activity in dexamethasone-pretreated cells was similar to the baseline level in control (22 ± 2 pmol min–1 mg–1 protein), pre- (14 ± 1 pmol min–1 mg–1 protein) and post-HD (11 ± 3 pmol min–1 mg–1 protein), suggesting that no activation of inducible NOS (iNOS) occurs in these studies.
Figure 1.
Effects of 20% human plasma (from controls, PD and pre-HD patients) on the NOS activity in human dermal microvascular endothelial cells (HDMEC, a), human glomerular capillary endothelial cells (HGEC, b) and bovine thoracic aortic endothelial cells (BAEC, c). After 6 h incubation, NOS activity was determined by measuring the conversion rate of l-arginine to l-citrulline. The NOS inhibitor l-NMA (5 mm) was added to some wells to confirm that the l-arginine to l-citrulline conversion reflected specific NOS activity. Results are mean ± SEM of three separate experiments, each performed in triplicate. * P < 0.05 compared with control. PD: peritoneal dialysis, pre-HD: prehaemodialysis.
To determine if the inhibition of eNOS activity is related to insufficient co-factors rather than an intrinsic reduction in eNOS activity, we measured NOS activity in fractionated HDMEC in the presence of excess co-factors. The inhibition of NOS activity persisted in the fractionated endothelial cells incubated with PD (8.2 ± 0.5 pmol min–1 mg–1 protein; P < 0.05), pre-HD (7.3 ± 0.4 pmol min–1 mg–1 protein; P < 0.05) and post-HD plasma (7.5 ± 0.6 pmol min–1 mg–1 protein; P < 0.05) vs. controls (10.6 ± 0.6 pmol min–1 mg–1 protein).
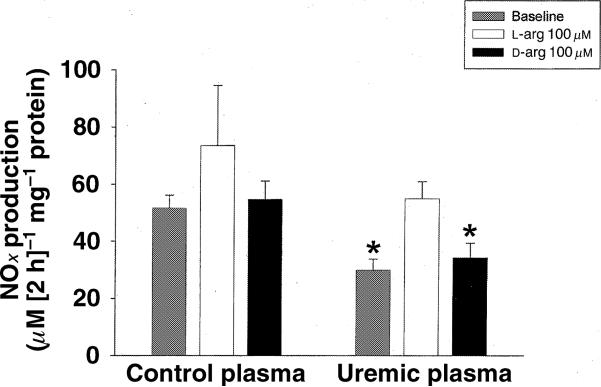
We also investigated whether excess l-arginine or d-arginine in the incubation medium could prevent the ‘NOS inhibitory’ activity of ESRD plasma on HDMEC. As shown in Fig. 2, NOx production from cells incubated in uraemic plasma was lower than with control plasma. Addition of excess l-arginine, but not d-arginine, increased NOx production from the cells incubated with uraemic plasma so that it was no longer different to the control value.
Figure 2.
Effects of plasma from normal control and uraemic plasma on NOx (nitrate and nitrite) production on human dermal microvascular endothelial cells (HDMEC). After 6 h incubation with normal control plasma or uraemic plasma with or without 100 μm l-arginine or d-arginine, the cells were incubated with MEM medium for 2 h. The NOx concentration was then measured in the cell medium. * P < 0.05 compared with control baseline.
The plasma concentration of the endogenous NOS inhibitor ADMA (methylated l-arginine analogue) was increased to ≈5× control in PD patients and ≈10× in HD patients (Table 1).
DISCUSSION
We have recently conducted carefully controlled clinical studies in ESRD patients (both PD and HD) and have reported a reduction in total NO production compared with subjects with normal renal function (Schmidt et al. 1999a, b). We speculated that diminished total NO production reflected, at least in part, a reduction in ‘haemodynamically active NO’, produced by vascular endothelial cells. The present study was designed to directly test this possibility and we have provided evidence which implicates uraemia as a cause of eNOS inhibition. Plasma from both PD and HD patients reduced NOS activity in cultured vascular endothelial cells by ≈30% compared with control. As these cultured cells are only exposed to 20% plasma for 6 h, it is possible that in vivo, the eNOS-inhibitory actions of uraemic plasma are more pronounced. Our observations are at variance with a report that uraemic plasma has no effect on human umbilical vein endothelium and can increase, have no effect or inhibit NOS activity in transformed murine endothelial cells or stimulated macrophages (Arese et al. 1995). However, as we observed a NOS inhibitory action of uraemic plasma in two normal human, and one normal bovine endothelial cell types, the NOS inhibitory effect of uraemic plasma appears to be widespread throughout the circulation. This observation implicates eNOS inhibition as a cause of the increased peripheral resistance and elevated blood pressure in ESRD.
There are endogenous arginine analogues that can inhibit NOS and which accumulate in ESRD. For example, plasma concentrations of ADMA increase in renal failure patients, possibly to concentrations that inhibit NO synthesis (Vallance et al. 1992), although this is controversial (Marescau et al. 1997, Anderstam et al. 1997). In the present studies we have found increases in plasma ADMA in both PD and HD patient plasma, to concentrations where NOS activity is affected (Xiao et al. 1999), in the range reported earlier by Vallance et al. (1992). It is likely that the NOS inhibitory activity of uraemic plasma is due to arginine analogues, as we were able to reverse the inhibition of NO production (measured by NOx levels) with excess unlabelled arginine in the cell incubation medium. This observation provides a mechanism for the observed protective effect of chronic arginine supplementation in some forms of renal disease (Reyes et al. 1993).
It has been reported that some types of human endothelial cells express both constitutive eNOS and iNOS when appropriately stimulated (Liang et al. 1994). Haemodialysis can provoke the release of cytokines which are iNOS stimulators (Amore et al. 1997) and we were concerned that some of the remaining NOS activity seen in endothelial cells incubated with HD patient plasma, might reflect iNOS activity. However, pretreatment of vascular endothelial cells with glucocorticoids such as dexamethasone prevents cytokine-stimulated increases in iNOS activity (Simmons et al. 1996). As dexamethasone pretreatment had no effect on NOS activity in cells incubated with control, pre- or post-HD patient plasma in our study, we conclude that only eNOS is active.
We also observed that post-HD plasma contains similar eNOS inhibitory activity to pre-HD plasma, suggesting that NOS inhibitory activity in ESRD plasma is not adequately removed by HD. Indeed, although plasma ADMA levels fall with HD, they remain substantially higher than in controls. Of note, plasma l-arginine concentration is also reduced during haemodialysis such that the ratio of ADMA to l-arginine remains very high in ESRD plasma, even immediately post-HD (Schmidt et al. 1999b). The ability of HD to acutely remove methylated arginines seems variable. Both Vallance et al. (1992) and Kielstein et al. (1999) report similar pre-HD values of ADMA to those seen by us, and although Vallance reports ≈40% reduction in plasma ADMA, Kielstein reports no change immediately post-HD, with a decline delayed by several hours (Vallance et al. 1992, Kielstein et al. 1999). It has recently been reported that HD corrects the impaired venodilation in response to acetylcholine in ESRD, suggesting that dialysis removes eNOS inhibitors (Hand et al. 1998). We are uncertain about the reason for the difference in these studies but differences in duration and materials used in HD, as well as the time after HD when blood is sampled, are likely explanations. The existence of higher MW NOS inhibitors (not cleared by HD) is also a possibility, although there is no evidence for this at present.
ESRD patients are on many different medications which could impact on eNOS activity, especially various antihypertensive agents. Studies by others indicate that the drugs used by ESRD patients in our study are either not related to NO production (β-receptor blocker) (Saijonmaa et al. 1997) or can actually increase NO production (diuretics and angiotensin II converting enzyme inhibitors) (Wiemer et al. 1994, Zhang et al. 1999) in vascular endothelial cells. Therefore, the inhibition of eNOS activity by ESRD plasma is presumably directly related to the renal disease.
In conclusion, intracellular NOS activity and NOx production were reduced in HDMECs incubated with uraemic plasma. These cell culture studies complement clinical studies that implicate a role for a low NO state in hypertensive ESRD patients.
Acknowledgments
These studies were supported by a Beatrice A. Madera grant from the School of Medicine, WVU, Office of the Dean and NIH grant # R01 DK 45517. We are grateful to Kevin Engels, Glenn Kuenzig, Jennifer Domico, Marilyn Howton and Lennie Samsell for technical assistance.
REFERENCES
- Amore A, Cirina P, Mitola S, et al. Acetate intolerance is mediated by enhance synthesis of nitric oxide by endothelial cells. J Am Soc Nephrol. 1997;8:1431–1436. doi: 10.1681/ASN.V891431. [DOI] [PubMed] [Google Scholar]
- Anderstam B, Katzarski K, Bergstrom J. Serum levels of NG, NG-dimethyl-l arginine, a potential endogenous nitric oxide inhibitor in dialysis patients. J Am Soc Nephrol. 1997;8:1437–1442. doi: 10.1681/ASN.V891437. [DOI] [PubMed] [Google Scholar]
- Arese M, Strasly M, Ruva C, et al. Regulation of nitric oxide synthesis in uremia. Nephrol Dial Transplant. 1995;10:1386–1397. [PubMed] [Google Scholar]
- Baylis C, Vallance P. Nitric oxide and blood pressure: effects of NO deficiency. Curr Op Nephrol Hypert. 1996;5:80–88. doi: 10.1097/00041552-199601000-00014. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Synder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–196. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Davda RK, Chandler LJ, Crews FT, Guzman NJ. Ethanol enhances endothelial nitric oxide synthase response to agonists. Hypertension. 1993;21:939–943. doi: 10.1161/01.hyp.21.6.939. [DOI] [PubMed] [Google Scholar]
- Funai EF, Davidson A, Seligman SP, Finlay TH. S-Nitrosohemoglobin in the fetal circulation may represent a cycle for blood pressure regulation. Biochem Biophys Res Commun. 1997;293:875–877. doi: 10.1006/bbrc.1997.7565. [DOI] [PubMed] [Google Scholar]
- Hand MF, Haynes WG, Webb DJ. Hemodialysis and l-arginine, but not d-arginine, corrects renal failure-associated endothelial dysfunction. Kidney Int. 1998;53:1068–1077. doi: 10.1111/j.1523-1755.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Boger RH, Bode-Boger SM, et al. Asymmetric dimethylarginine plasma concentrations differ in patients with ESRD: relationship to treatment method and atherosclerotic disease. J Am Soc Nephrol. 1999;10:594–600. doi: 10.1681/ASN.V103594. [DOI] [PubMed] [Google Scholar]
- Liang Y, Vandivier RW, Suffredini AF, Danner RL. Human polymorphonuclear leukocytes lack detectable nitric oxide synthase activity. J Immunol. 1994;153:1825–1834. [PubMed] [Google Scholar]
- Marescau BG, Nagels G, Possemiers I, et al. Guanidino compounds in serum and urine of nondialysed patents with chronic renal insufficiency. Metabolism. 1997;46:1024–1031. doi: 10.1016/s0026-0495(97)90273-0. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1995;43:109–142. [PubMed] [Google Scholar]
- Peterson GL. Review of the folin phenol protein quantitation method of Lowry, Rosebrough, Farr, and Randall. Anal Biochem. 1979;100:201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Reyes AA, Karl IE, Kissane J, Klahr S. l-arginine administration prevents glomerular hyperfiltration and decreases proteinuria in diabetic rats. J Am Soc Nephrol. 1993;4:1039–1045. doi: 10.1681/ASN.V441039. [DOI] [PubMed] [Google Scholar]
- Rostand SG, Brunzell JD, Cannon RO, Victor RG. Cardiovascular complications in renal failure. J Am Soc Nephrol. 1991;2:1053–1062. doi: 10.1681/ASN.V261053. [DOI] [PubMed] [Google Scholar]
- Saijonmaa O, Metsarinne K, Fyhrquist F. Carvedilol and its metabolites suppress endothelin-1 production in human endothelial cell culture. Blood Press. 1997;6:24–28. doi: 10.3109/08037059709086442. [DOI] [PubMed] [Google Scholar]
- Schmidt RJ, Yokota S, Tracy TS, Sorkin MI, Baylis C. Nitric oxide production is low in end stage renal disease patients on peritoneal dialysis. Am J Physiol. 1999a;276:F794–F797. doi: 10.1152/ajprenal.1999.276.5.F794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Domico J, Samsel L, et al. Indices of activity of the nitric oxide system in patients on hemodialysis. Am J Kidney Dis. 1999b;34:228–234. doi: 10.1053/AJKD03400228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WW, Ungureanu-Longrosis D, Smith GK, Smith TW, Kelly RA. Glucocorticoids regulate inducible nitric oxide synthase by inhibiting tetrahydrobiopterin synthesis and l-arginine transport. J Biol Chem. 1996;271:23928–23937. doi: 10.1074/jbc.271.39.23928. [DOI] [PubMed] [Google Scholar]
- Soma M, Nakayama T, Kanmatsuse K. NOS gene polymorphism and its influence on cardiovascular disease. Curr Op Nephrol Hypert. 1999;8:83–87. doi: 10.1097/00041552-199901000-00013. [DOI] [PubMed] [Google Scholar]
- Suto T, Losonczy G, Qiu C, et al. Acute changes in urinary excretion of nitrite + nitrate do not necessarily predict renal vascular NO production. Kidney Int. 1995;48:1272–1277. doi: 10.1038/ki.1995.411. [DOI] [PubMed] [Google Scholar]
- Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- Verdon CP, Burton BA, Prior RL. Sample pretreatment with nitrate reductase and Glucose-6-phosphate dehydrogenase quantitatively reduces nitrate while avoiding interference by NADP+ when the Griess reaction is used to assay for nitrite. Anal Biochem. 1995;224:502–508. doi: 10.1006/abio.1995.1079. [DOI] [PubMed] [Google Scholar]
- Wiemer G, Fink E, Linz W, Hropot M, Scholkens BE, Wohlfart P. Furosemide enhances the release of endothelial kinins, nitric oxide and prostacylin. J Pharmacol Exp Ther. 1994;271:161–165. [PubMed] [Google Scholar]
- Xiao S, Schmidt R, Howton M, Engels K, Baylis C. Chronic renal disease plasma on nitric oxide synthase activity in cultured endothelial cells (Abstract). FASEB. 1999;13:125.37. [Google Scholar]
- Zhang X, Recchia FA, Bernstein R, Xu X, Nasjletti A, Hintze TH. Kinin-mediated coronary nitric oxide production contributes to the therapeutic action of angiotensin-converting enzyme and neutral endopeptidase inhibitors and amlodipine in the treatment in heart failure. J Pharmacol Exp Ther. 1999;288(2):742–751. [PubMed] [Google Scholar]