Abstract
Background:
Previous studies indicate that nitric oxide (NO) is involved in the regulation of blood pressure (BP) and natriuresis in response to high sodium intake. We investigated the role of inducible NO synthase (iNOS) in response to an increased salt intake.
Methods:
Conscious, chronically catheterized rats were exposed to a high-salt (6%) diet for 14 days while receiving vehicle or aminoguanidine ([AG]; 250 mg/kg/24 h), which selectively inhibits iNOS. A group of rats on normal salt intake + AG were also studied.
Results:
Aminoguanidine had no impact on BP (120 ± 2 v 116 ± 1 mm Hg, control v day14) or 24-h urinary nitrite and nitrate excretion (UNOxV), in rats on normal salt but prevented lipopolysaccharide-induced hypotension. High salt alone had no impact on BP (120 ± 1 v 121 ± 1 mm Hg), whereas UNaV (1.3 ± 0.2 v 3.5 ± 0.6 μeq/min, P < .001) and UNOXV increased with high salt intake. The natriuretic response persisted (1.5 ± 0.2 v 4.3 ± 0.8 μeq/min, P < .005), but the increase in UNOXV was prevented with chronic AG although BP fell slightly (121 ± 1 v 115 ± 1 mm Hg, P < .05). There was no change in plasma volume with high salt, and 24-h UNaV increased appropriately in the presence of AG. The in vitro NOS activity was not increased in kidney homogenates by high salt diet, nor was it affected by chronic AG treatment.
Conclusion:
We conclude that NO from an iNOS source is not essential for the regulation of sodium excretion and BP in the presence of a high-salt diet in a normal rat. Am J Hypertens 2002;15:230–235
Keywords: Aminoguanidine, sodium excretion, blood pressure, conscious chronically catheterized rat, urinary nitrite + nitrate, renal hemodynamics
Increased nitric oxide (NO) activity participates in the normal cardiovascular and renal responses to a sodium challenge. For example, total NO production (measured from urinary excretion of NO2+ NO3= NOX, the stable oxidation products of NO) increases in normal rats with elevation of dietary salt intake.1-4 There is also functional evidence of increased peripheral3 and renal4 vascular NO production in rats on high-salt diets. All of the nitric oxide synthase (NOS) proteins are distributed in select locations in the kidney and NO directly inhibits Na+ transport in various tubule segments in vitro and in vivo.5,6
In addition, elegant studies by Chen and Sanders7 and by Chen et al8 have indicated a role for NO deficiency in the genetically salt-sensitive hypertension of the Dahl salt-sensitive (Dahl S) rat, a hypothesis supported by others.9,10 This, together with the observation that acute, experimentally induced NOS inhibition leads to attenuation of the pressure natriuresis,6 suggests that NO deficiency may play a key role in the generation of salt-sensitive hypertension. In confirmation of this possibility, several groups have reported that chronic NOS inhibition, combined with high dietary salt intake, can lead to a volume-dependent hypertension,6,11 and Na+ loading exacerbates the hypertension due to chronic NOS inhibition.8,11-13 All of these studies with experimentally induced NOS inhibition used L-arginine analogs that inhibit all major NOS isoforms; thus it is difficult to isolate the specific NOS responsible for salt-dependent hypertension. However, observations by Chen and Sanders7 and by Chen et al8 in the Dahl S rat suggested that a specific defect may occur in the inducible NOS (iNOS) in this strain. Also, Deng and Rapp14 reported that BP was associated with alleles of the iNOS locus in F2 populations of rats (Dahl S interbred with normotensive rats) that were fed a high-salt diet, although Deng15 subsequently concluded that the iNOS was not a primary regulator of BP in salt sensitivity.
Two distinct iNOS mRNA are expressed constitutively in the normal kidney,5 in afferent arteriole and medullary collecting duct; and upregulation of iNOS protein expression has been reported in medulla in Sprague-Dawley (SD) rats.16 Mattson et al17 have reported that chronic iNOS inhibition in salt-loaded, uninephrectomized SD rats leads to reversible hypertension, and Rudd et al report that iNOS inhibition induces salt sensitivity in the Dahl salt-resistant (Dahl R) rats.18 Taken together, this evidence suggests that the normal physiologic response to salt loading involves an increased NO production by iNOS, which increases sodium excretion and thus preserves volume homeostasis and BP. In the present study, we tested the hypothesis that prevention of iNOS activity would lead to hypertension during 14 days of high dietary salt intake in the normal SD rat. Longitudinal experiments were conducted in conscious, chronically catheterized rats, and renal hemodynamics, electrolyte excretion, BP, plasma volume, and 24-h NOx production were periodically measured. In vitro measurement of NOS activity was performed in kidney and cerebellum in the soluble fraction (the location of the iNOS) from separate groups of rats.
Methods
Studies were conducted in 48 male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) aged 3 to 6 months. All protocols were approved by the Animal Care and Use Committee of West Virginia University.
In 18 rats a preliminary sterile surgery was conducted, with vascular catheters placed in the abdominal aorta and inferior vena cava and a silicone elastomer (Silastic, Dow Corning, Midland, MI) and steel catheter placed in the urinary bladder, as described by us previously.19,20 Experiments were begun 7 to 9 days after surgery; rats were trained to accept handling and activities in the laboratory, and to sit quietly in a restraining cage for several hours at a time. Also during this time, rats were maintained on a normal-salt diet, which contained approximately 0.6g% NaCl. Rats in groups 2 and 3 were also maintained on a low NOX diet (AIN 76C semipurified diet [ICN Pharmaceuticals, Costa Mesa, CA]; NOX content 125 μmol/kg) throughout the study. Longitudinal functional studies were carried out in three groups of conscious rats as follows. Group 1 (n = 6) received chronic aminoguanidine ([AG]; Sigma-Aldrich, St. Louis, MO) by gavage, with normal NaCl intake (approximately 0.6g%) for 14 days. Group 2 (n = 6) was given water by gavage + high salt (approximately 6g%) intake for 14 days. Group 3 (n = 6) rats received chronic AG + high salt intake for 14 days. These rats were also placed in metabolic cages for 24-h urine collections, 2 to 3 days before control measurements and for the 24 h after days 7 and 14, for renal function studies (see later here). The effectiveness of iNOS inhibition by the dose of AG used (250 mg/kg/24 h by gavage once daily) was confirmed at the end of the renal function experiments in group 1. Group 1 rats along with six additional normal rats were anesthetized with inactin (120 mg/kg) intraperitoneally, and BP was monitored. After control BP was measured, rats received lipopolysaccharide ([LPS]; 4 mg/kg) intravenously, and BP was measured for the next 4 h. Because LPS evokes a marked fall in BP by stimulation of iNOS, assessment is possible of the iNOS inhibitory action of the AG.
Studies in the conscious state were as follows. Control renal function measurements were made on day 0 in all rats on normal salt intake and before any drugs were administered. Rats were then assigned to one of the three groups. Renal function (and plasma volume in groups 2 and 3) were measured on days 0, 7, and 14 as described by us previously,19,20 and mean BP was also measured on days 2, 5, 9, 10, and 12. Food intake in groups 2 and 3 was measured daily. After the last metabolic cage collection on day 15, rats were euthanized with Brevital (100 mg/kg; Eli Lilly, Indianapolis, IN) intravenously, and the bladder and kidneys were inspected to ensure that they were free of infection. From the collected samples we measured blood and urine tritiated inulin activity, PAH and sodium concentrations, and hematocrit and total plasma protein concentration in blood samples, as described previously.19,20 From these measures we calculated GFR, renal plasma flow (RPF), renal vascular resistance (RVR), urinary excretion of sodium (UNaV), and fractional excretion of sodium (FENa) using standard calculations. Measurement of plasma volume was performed using Evans blue dye.20 Urinary NO2 and NO3 concentrations were measured using the Griess reaction after reduction of NO3 to NO2, as described by us earlier.19
Four additional groups of rats were fed either a normal-or high-salt diet for 14 days (each n = 10), and five rats on each salt intake were also given daily AG (250 mg/kg/ day). Kidney (separated into cortex and medulla) and cerebellum were then harvested, snap-frozen, and stored at −80°C for later, in vitro measurements NOS activity. The NOS activity was measured by the conversion of 3Harginine to 3H-citrulline in the soluble fractions after removal of endogenous arginine with Dowex resin, as recently described by us in detail.21 For each sample, two sets of triplicates were run, one at baseline and one in the presence of the nonselective NOS inhibitors, N-monomethyl-l-arginine (L-NMA) (10 mmol/L) and Nω-nitro-l-arginine methyl ester (L-NAME) (20 mmol/L) for kidney, and N-nitro-l-arginine (L-NNA) (2 mmol/L) for the cerebellar fractions. Data are expressed as picomoles of [3H]L-citrulline converted per minute per milligram of protein (pmol citrulline/min/mg protein) and corrected for background, determined on heat-inactivated samples. Because of unexpected findings, another 10 rats were fed either a normal- or high-salt diet for 14 days (each n = 5), and the kidneys were harvested as previously described for NOS activity measurement.
Data are expressed as mean ± SE. Statistical analysis was by t test, one- and two-way analysis of variance, with P < .05 taken to indicate statistical significance.
Results
Blood pressure and renal function was similar among the three groups of conscious, chronically catheterized rat at the beginning of the study period (Table 1, Fig. 1). In group 1 rats, 14 days of AG administration during normal salt intake had no impact on body weight, BP, renal hemodynamics, or hematocrit, although the acutely measured UNaV and FENa had decreased by day 14. The 24-h UNOXV was unchanged with time (Fig. 1), which, together with the stable BP and RVR, suggests that this dose of AG was not influencing constitutively generated NO. After completion of renal function studies, rats received an intravenous LPS challenge (4 mg/kg); BP remained relatively constant (96 ± 2 mm Hg in controls v 88 ± 3 mm Hg at 3 h) and, importantly, all rats were alive 4 h after LPS. In contrast, a separate group of normal, iNOS-intact rats showed a precipitous fall in BP from 2 to 3 h after LPS challenge (98 ± 2 mm Hg v 57 ± 12 mm Hg), which led to 100% mortality by 4 h. The results from group 1 rats suggest that chronic AG in the dose used here is a selective iNOS inhibitor.
Table 1.
Chronic aminoguanidine for 14 days during normal salt (NS) or high salt (HS) intake
| Group | BP (mm Hg) |
RVR (mm Hg/ mL/min) |
GFR (mL/min) |
UNaV (μeq/min) |
FENa+ (%) |
Hct (Vol%) |
BW (g) |
Plasma Volume (mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | ||||||||
| 0 | 120 ± 2 | 5.4 ± 0.2 | 3.2 ± 0.1 | 2.0 ± 0.4 | 0.48 ± 0.10 | 43 ± 1 | 381 ± 6 | ND |
| 7 | 116 ± 2 | 5.3 ± 0.2 | 3.2 ± 0.2 | 1.3 ± 0.2 | 0.29 ± 0.05 | 43 ± 1 | 376 ± 9 | ND |
| +AG 14 | 116 ± 1 | 5.0 ± 0.3 | 3.0 ± 0.1 | 0.8 ± 0.1* | 0.21 ± 0.02* | 43 ± 1 | 372 ± 11 | ND |
| 2 | ||||||||
| 0 | 120 ± 1 | 5.4 ± 0.4 | 2.8 ± 0.1 | 1.3 ± 0.2 | 0.34 ± 0.05 | 43 ± 1 | 366 ± 8 | 15.7 ± 0.4 |
| 7 | 120 ± 2 | 6.1 ± 0.5 | 2.8 ± 0.2 | 3.5 ± 0.3* | 0.94 ± 0.12* | 43 ± 1 | 369 ± 8 | 15.7 ± 0.4 |
| 14 | 121 ± 1 | 5.5 ± 0.5 | 2.8 ± 0.2 | 3.5 ± 0.6* | 0.93 ± 0.14* | 44 ± 1 | 371 ± 8 | 15.9 ± 0.4 |
| 3 | ||||||||
| 0 | 121 ± 1 | 5.5 ± 0.7 | 3.1 ± 0.2 | 1.5 ± 0.2 | 0.36 ± 0.06 | 43 ± 1 | 384 ± 16 | 16.5 ± 0.7 |
| 7 | 114 ± 3* | 5.6 ± 0.4 | 2.8 ± 0.3 | 4.8 ± 0.7† | 1.26 ± 0.13* | 41 ± 1 | 382 ± 13 | 16.4 ± 0.6 |
| 14 | 115 ± 3*† | 5.4 ± 0.1 | 3.0 ± 0.3 | 4.3 ± 0.8* | 1.04 ± 0.11* | 42 ± 1 | 392 ± 14 | 16.9 ± 0.6 |
BP = blood pressure; RVR = renal vascular resistance; GFR = glomerular filtration rate; UNaV = excretion of sodium; FENa = fractional excretion of sodium; Hct = hematocrit; BW = body weight; ND = not determined; AG = aminoguanidine.
Group 1, NS + AG; group 2, HS; group 3, HS + AG.
Differences v day 0 within a group.
Difference between HS groups at a given time.
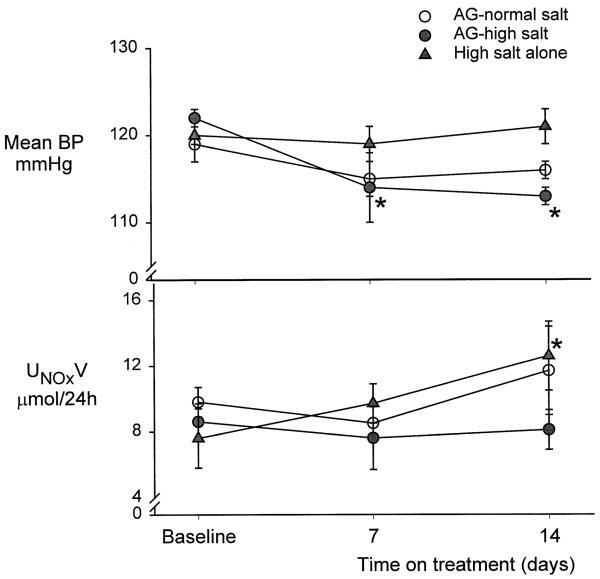
FIG. 1.
Mean arterial blood pressure (BP) and the 24-h urinary excretion of nitrate and nitrite (NOX, UNOXV) in baseline state and at days 7 and 14 of chronic aminoguanidine (AG) while on normal salt intake, high salt intake alone, and on AG with high salt intake in the conscious, chronically catheterized rat.
In group 2 rats with a high-salt intake alone, BP and renal hemodynamics remained unchanged throughout the 14 days on 6% salt (Table 1, Fig. 1), although UNaV and FENa, measured acutely (Table 1), increased significantly. The 24-h UNaV increased appropriately on the high-salt diet, and group 2 rats were in sodium balance on day 14, as UNaV was similar to sodium intake (14.4 ± 1.1 v 14.85 ± 1.1 meq/24 h). Both hematocrit and plasma volume (Table 1) were constant throughout. The 24-h UNOXV was increased at 14 days v day 0 on high salt (Fig. 1). In the rats given high-salt diets and AG (group 3), BP tended to fall and was significantly lower v day 0, on days 7 and 14 (Table 1 and Fig. 1). As with groups 1 and 2, no changes were observed in renal hemodynamics during the 14-day AG treatment, and plasma volume was also unaltered (Table 1). As in group 2 rats, UNaV and FENa rose in group 3 animals, which also maintained an appropriate increase (approximately 10 times) in 24-h UNaV and remained in Na balance (urinary output 16.16 ± 1.3 v intake 17.5 ± 1.0 meq/24 h) for day 14, without a change in plasma volume or hematocrit (Table 1). However, a rise in 24-h UNOXV due to high salt alone was completely prevented by the presence of AG (Fig. 1).
In four separate groups of rats (each n = 5) the NOS activity was measured on the soluble fractions of kidney cortex, medulla, and cerebellum. As shown in Table 2, there was no difference in NOS activity in kidney or cerebellum based on salt intake. Chronic AG for 14 days had no impact on NOS activity in rats on NS or high salt for cerebellum, kidney cortex, or medulla. In kidney cortex, NOS activity tended to be lower in AG-treated rats (P = .06 in high salt alone v high salt +AG), but this was due to the numerically higher baseline NOS activity in high salt alone, which did not persist in group 2 rats on a high-salt diet (see later here). In all cases, cerebellar NOS activity was completely inhibited by the competitive, non-selective NOS inhibitor L-NNA. The NOS activity was inhibited by >90% in renal cortex and >80% in renal medulla by combined L-NMA and L-NAME, indicating that the arginine to citrulline conversion was a NOS-dependent event. Another two groups of rats (each n = 5) were studied on high and normal salt diets; again, sodium intake had no effect on NOS activity in the soluble fraction of either renal cortex or medulla (Table 2).
Table 2.
Nitric oxide synthase (NOS) activity in cerebellum, renal cortex, and medulla
| Group | Cerebellar NOS Activity |
Renal Cortex NOS Activity |
Renal Medulla NOS Activity |
|---|---|---|---|
| Control NS | 29.27 ± 3.56 (100 ± 1) | 0.79 ± 0.07 (96 ± 3) | 0.88 ± 0.12 (84 ± 1) |
| Control HS | 22.50 ± 4.31 (99 ± 2) | 0.91 ± 0.14 (92 ± 2) | 1.13 ± 0.19 (87 ± 2) |
| AG + NS | 27.33 ± 7.90 (101 ± 1) | 0.65 ± 0.11 (95 ± 2) | 1.08 ± 0.07 (87 ± 1) |
| AG ± HS | 28.85 ± 10.74 (102 ± 1) | 0.65 ± 0.09 (94 ± 2) | 1.06 ± 0.06 (89 ± 1) |
| NS-2 | ND | 1.22 ± 0.12 | 1.40 ± 0.16 |
| HS-2 | ND | 0.92 ± 0.12 | 1.46 ± 0.18 |
NS = normal salt intake (~0.6%; NS); HS = high NaCl intake (~6%) for 14 days; AG = rats chronically given AG by gavage (250 mg/kg/day) for 14 days; NS-2 and HS-2 = additional groups of rats fed normal salt (NS-2) and high salt (HS-2) diets, and studied for baseline renal NOS activity only; other abbreviations as in Table 1.
Data given as mean ± SE. Percent inhibition with L-arginine analogs given in parentheses. No values were different from control.
Discussion
These studies were designed to test the hypothesis that iNOS-derived NO is required for natriuretic and hemo-dynamic responses to a chronic increase in dietary salt intake in the normal SD rat. Our major finding is that chronic iNOS inhibition does prevent the high-salt–induced stimulation of total NO production (assessed from 24-h UNOXV) but does not impair the kidneys' ability to increase sodium excretion, to maintain sodium balance, and to regulate BP appropriately. Thus our hypothesis that chronic iNOS inhibition combined with high salt intake would lead to volume-dependent hypertension proved to be wrong.
There is now good evidence to suggest that an increased NOS activity is required for the normal renal/cardiovascular response to high salt intake. In vitro studies show that NO is natriuretic throughout the tubule, that NO is made in numerous locations within the kidney, and that the renal vasculature is under enhanced NO-dependent vasodilatory tone when salt intake is high.4-6,22 Blood flow to the inner medulla is regulated by NO, which probably accounts for the NO dependence of the pressure natriuresis.5,6 Studies in intact animals also indicate that acute NOS inhibition can impair sodium excretion.5,6 The situation with chronic NOS inhibition is more complex in that high level, chronic NOS inhibition leads to intense vasoconstriction and hypertension coexisting with volume depletion.11,20 However, lower levels of NOS inhibition do produce a volum-dependent form of hypertension in the presence of high salt intake, which is associated with blunting of the pressure natriuresis response.6,11
Our findings agree with previous studies in that we confirm that total NO production increases due to high salt intake, as indicated by an increase in 24-h UNOxV.1-4 However, we show here that this increased 24-h UNOXV can be prevented (+AG) without impairing the renal responses to high salt. The interpretation of this result depends on what 24-h UNOXV is actually measuring. We now think that although 24-h UNOXV is a qualitative measure of total NO production, this may not reflect local or regional NO production in the kidney, for example, as renal NO is a small fraction of the total.23 To clarify the impact of high salt (with or without AG) on renal NO production, we conducted additional in vitro studies measuring NOS activity in the soluble fraction from kidney cortex and medulla. Under the conditions of our study (conducted after removal of endogenous L-arginine and in the presence of excess required cofactors), we could not detect a statistically significant increase in NOS activity in either the cortex or medulla that was due to high salt alone. Thus our in vitro studies suggest that moving from a normal- to a high-salt diet does not increase the intrinsic activity of the renal NOS enzymes located in the soluble fraction (ie, iNOS and nNOS) in normal rats. This conflicts with reports that iNOS protein abundance is increased in medulla of SD on high salt16; however, others have reported that high salt downregulates iNOS in cortex and medulla in SD24 and in whole kidney in Dahl R and Dahl S rats.25
Because administering AG inhibits the increase in total NO production caused by high dietary salt, this suggests that the salt-induced increase in NO originates from an iNOS source. Given that AG is inhibiting iNOS everywhere, including the kidney, our results also suggest that NO produced from iNOS in the kidney is not essential for regulation of BP or sodium excretion in normal animals during a salt challenge. Our in vitro data shows that there is no induction of iNOS activity by high salt, inasmuch as combined high salt and AG have no impact on renal cortex or medulla NOS activity. This finding conflicts with a report of decreased medullary NOS activity in SD rats on high salt with chronic AG infusion.17 We also measured NOS activity from the high-output cerebellum and observed no impact of high salt or AG, suggesting that the increased total NO generation seen with high salt does not originate from this source. All of these conclusions rely on the efficiency and selectivity of AG as an iNOS inhibitor. Certainly, AG preferentially inhibits the cytokine-inducible isoform of NOS26 but can also inhibit the constitutive NOS enzymes, depending on dose and route of administration.26,27 We experimented with doses and routes of drug administration before beginning this study and decided on the once-daily gavage (250 mg/kg) regimen, as this had no effect on total NO production (from 24-h UNOXV), BP, or renal hemodynamics in rats on normal salt (group 1) and yet it clearly prevented LPS-induced, iNOS-dependent hypotension. We have also reported that this dose prevents the slow rise in NOX production after nonsterile surgery28; and we therefore conclude that, in the present study, we are blocking the iNOS enzyme, when active, without impact on the constitutive NOS. We recognize, however, that the pharmacologic agents currently available as isoform-selective NOS inhibitors are not absolutely specific.
Our study suggests a lack of relationship between iNOS-generated NO and the generation of salt-sensitive hypertension in normal animals. This is supported by the observations of Waz et al, who found no change in BP or UNaV in rats maintained on a normal salt intake and treated with AG for 2 or 12 months.29 However, our findings conflict with those of two earlier studies. Mattson et al reported that iNOS inhibition with AG leads to mild hypertension in uninephrectomized (but otherwise normal) SD rats fed high salt after 4 days.17 Because excess L-arginine reversed the rise in BP, a role for iNOS inhibition was implicated. Studies by Rudd et al also implicate iNOS derived NO deficiency in salt-dependent hypertension, as Dahl R rats show increased BP with a high-salt diet and when iNOS inhibited.18 We are not sure why our observations conflict. Perhaps the rat strain, extremes of salt intake or uninephrectomy all contribute to this variability. Alternatively, the doses or routes of administration of the “iNOS-selective” drugs may result in inhibition of other NOS isoforms.26,27 Of interest, an intriguing recent report suggests that the impact of dietary salt intake on NO production may reflect salt-dependent regulation of Larginine availability, rather than a direct effect on the NOS enzymes.30
In summary, we confirm an increased 24-h UNOxV during high salt intake, but find this can be ablated without compromising the animals' ability to handle the high salt intake. Because the method of preventing the rise in 24-h UNOXV was by using the iNOS inhibitor AG, we conclude that NO generated from iNOS is not essential for regulation of BP or sodium excretion in normal rats when subjected to a high-salt diet.
Acknowledgments
The technical assistance of Lennie Samsell and Kevin Engels and the secretarial help of Wendy Baker are gratefully acknowledged.
Supported by National Institutes of Health grant R01 DK 45517.
References
- 1.Roczniak A, Zimpelmann J, Burns KD. Effect of dietary salt on neuronal nitric oxide synthase in the inner medullary collecting duct. Am J Physiol. 1998;275:F46–F54. doi: 10.1152/ajprenal.1998.275.1.F46. [DOI] [PubMed] [Google Scholar]
- 2.Shultz PJ, Tolins JP. Adaptation to increased dietary salt intake in the rat. Role of endogenous nitric oxide. J Clin Invest. 1993;91:642–650. doi: 10.1172/JCI116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tolins JP, Shultz PJ. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int. 1994;46:230–236. doi: 10.1038/ki.1994.264. [DOI] [PubMed] [Google Scholar]
- 4.Wilcox CS, Deng X, Welch WJ. NO generation and action during changes in salt intake: roles of nNOS and macula densa. Am J Physiol. 1998;274:R1588–R1593. doi: 10.1152/ajpregu.1998.274.6.R1588. [DOI] [PubMed] [Google Scholar]
- 5.Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol. 1997;272:F561–F578. doi: 10.1152/ajprenal.1997.272.5.F561. [DOI] [PubMed] [Google Scholar]
- 6.Salazar FJ, Llinas MT. Role of nitric oxide in the control of sodium excretion. News in Physiological Science. 1996;11:62–67. [Google Scholar]
- 7.Chen PY, Sanders PW. Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension. 1993;22:812–818. doi: 10.1161/01.hyp.22.6.812. [DOI] [PubMed] [Google Scholar]
- 8.Chen PY, Gladish RD, Sanders PW. Vascular smooth muscle nitric oxide synthase anomalies in Dahl/Rapp salt-sensitive rats. Hypertension. 1998;31:918–924. doi: 10.1161/01.hyp.31.4.918. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda Y, Saito K, Kim JI, Yokoyama M. Nitric oxide synthase isoform activities in kidney of Dahl salt-sensitive rats. Hypertension. 1995;26:1030–1034. doi: 10.1161/01.hyp.26.6.1030. [DOI] [PubMed] [Google Scholar]
- 10.Miyata N, Cowley AW., Jr Renal intramedullary infusion of L-arginine prevents reduction of medullary blood flow and hypertension in Dahl salt-sensitive rats. Hypertension. 1999;33:446–450. doi: 10.1161/01.hyp.33.1.446. [DOI] [PubMed] [Google Scholar]
- 11.Yamada SS, Sassaki AL, Fujihara CK, Malheiros DM, de Nucci G, Zatz R. Effect of salt intake and inhibitor dose on arterial hypertension and renal injury induced by chronic nitric oxide blockade. Hypertension. 1996;27:1165–1172. doi: 10.1161/01.hyp.27.5.1165. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Rivas A, Garcia-Estan J, Vargas F. Effects of chronic increased salt intake on nitric oxide synthesis inhibition-induced hypertension. J Hypertens. 1995;13:123–128. [PubMed] [Google Scholar]
- 13.Mattson DL, Bellehumeur TG. Neural nitric oxide synthase in the renal medulla and blood pressure regulation. Hypertension. 1996;28:297–303. doi: 10.1161/01.hyp.28.2.297. [DOI] [PubMed] [Google Scholar]
- 14.Deng AY, Rapp JP. Locus for the inducible, but not a constitutive, nitric oxide synthase cosegregates with blood pressure in the Dahl salt-sensitive rat. J Clin Invest. 1995;95:2170–2177. doi: 10.1172/JCI117906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng AY. Is the nitric oxide system involved in genetic hypertension in Dahl rats? Kidney Int. 1998;53:1501–1511. doi: 10.1046/j.1523-1755.1998.00933.x. [DOI] [PubMed] [Google Scholar]
- 16.Mattson DL, Higgins DJ. Influence of dietary sodium intake on renal medullary nitric oxide synthase. Hypertension. 1996;27:688–692. doi: 10.1161/01.hyp.27.3.688. [DOI] [PubMed] [Google Scholar]
- 17.Mattson DL, Maeda CY, Bachman TD, Cowley AW., Jr Inducible nitric oxide synthase and blood pressure. Hypertension. 1998;31:15–20. doi: 10.1161/01.hyp.31.1.15. [DOI] [PubMed] [Google Scholar]
- 18.Rudd MA, Trolliet M, Hope S, Ward Scribner A, Daumerie G, Toolan G, Cloutier T, Loscalzo J. Salt induced hypertension in Dahl salt resistant and salt sensitive rats with NOS II inhibition. Am J Physiol. 1999;277:H732–H739. doi: 10.1152/ajpheart.1999.277.2.H732. [DOI] [PubMed] [Google Scholar]
- 19.Suto T, Losonczy G, Qiu C, Hill C, Samsell L, Ruby J, Charon N, Venuto R, Baylis C. Acute changes in urinary excretion of nitrite + nitrate do not necessarily predict renal vascular NO production. Kidney Int. 1995;48:1272–1277. doi: 10.1038/ki.1995.411. [DOI] [PubMed] [Google Scholar]
- 20.Qiu C, Muchant D, Beierwaltes WH, Racusen L, Baylis C. Evolution of chronic nitric oxide inhibition hypertension: relationship to renal function. Hypertension. 1998;31:21–26. doi: 10.1161/01.hyp.31.1.21. [DOI] [PubMed] [Google Scholar]
- 21.Xiao S, Erdely A, Wagner L, Baylis C. Uremic levels of BUN do not cause nitric oxide deficiency in rats with normal renal function. Am J Physiol Renal. 2001;280:F996–F1000. doi: 10.1152/ajprenal.2001.280.6.F996. [DOI] [PubMed] [Google Scholar]
- 22.Deng X, Welch WJ, Wilcox CS. Renal vasoconstriction during inhibition of NO synthase: effects of dietary salt. Kidney Int. 1994;46:639–646. doi: 10.1038/ki.1994.316. [DOI] [PubMed] [Google Scholar]
- 23.Baylis C, Vallance P. Measurement of nitrite and nitrate levels in plasma and urine what does this measure tell us about the activity of the endogenous nitric oxide system? [editorial] Curr Opin Nephrol Hypertens. 1998;7:59–62. doi: 10.1097/00041552-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Ni Z, Vaziri ND. Effect of salt loading on nitric oxide synthase expression in normotensive rats. Am J Hypertens. 2001;14:155–163. doi: 10.1016/s0895-7061(00)01234-6. [DOI] [PubMed] [Google Scholar]
- 25.Ni Z, Oveisi F, Vaziri ND. Nitric oxide synthase isotype expression in salt sensitive and salt resistant Dahl rats. Hypertension. 1999;34:552–557. doi: 10.1161/01.hyp.34.4.552. [DOI] [PubMed] [Google Scholar]
- 26.Wolff DJ, Lubeskie A. Aminoguanidine is an isoform-selective, mechanism-based inactivator of nitric oxide synthase. Arch Bio-chem Biophys. 1995;316:290–301. doi: 10.1006/abbi.1995.1040. [DOI] [PubMed] [Google Scholar]
- 27.Laszlo F, Evans SM, Whittle BJ. Aminoguanidine inhibits both constitutive and inducible nitric oxide synthase isoforms in rat intestinal microvasculature in vivo. Eur J Pharmacol. 1995;272:169–175. doi: 10.1016/0014-2999(94)00637-m. [DOI] [PubMed] [Google Scholar]
- 28.Losonczy G, Bloch JF, Samsell L, Schoenl M, Venuto R, Baylis C. Impact of surgery on nitric oxide in rats: evidence for activation of inducible nitric oxide synthase. Kidney Int. 1997;51:1943–1949. doi: 10.1038/ki.1997.265. [DOI] [PubMed] [Google Scholar]
- 29.Waz WR, Van Liew JB, Feld LG. Nitric oxide-inhibitory effect of aminoguanidine on renal function in rats. Kidney Blood Press Res. 1997;20:211–217. doi: 10.1159/000174148. [DOI] [PubMed] [Google Scholar]
- 30.Kitiyakara C, Chabrashvili T, Jose P, Welch WJ, Wilcox CS. Effects of dietary salt intake on plasma arginine. Am J Physiol Regul Comp Integ Physiol. 2001;280:R1069–R1075. doi: 10.1152/ajpregu.2001.280.4.R1069. [DOI] [PubMed] [Google Scholar]