Abstract
It has been suggested that l-arginine availability declines with advanced age, which could contribute to the endothelial dysfunction and decreased nitric oxide (NO) production that are features of aging. l-Arginine is made in the kidney and since the aging kidney develops progressive injury there may be decreased synthesis limiting availability. In this study we investigated the impact of aging on the regulation, at the gene level, of the various enzymes that synthesize l-arginine in the kidney (argininosuccinate synthetase and argininosuccinate lyase) and citrulline, the precursor of l-arginine made in the small intestine (phosphate-dependent glutaminase, carbamyl phosphate synthetase-1 and ornithine transcarbamylase). Studies were in young (3–5 months), middle-aged (11–13 months) and old (18–22 months) male and female Sprague–Dawley rats aged under barrier conditions. The plasma, renal cortical and brain cerebellar levels of l-arginine are unchanged in the old male rat, and expression of the genes involved in renal arginine synthesis and small intestinal citrulline synthesis is unchanged or upregulated with age in both males and females. This study shows that the synthesis of l-arginine is maintained with aging despite developing kidney damage. Therefore, the reduced NO generating capacity that occurs in aging must be due to downstream changes in the NO biosynthesis pathway, such as reduced abundance of NO biosynthetic enzymes.
Keywords: Citrulline, Argininosuccinate synthetase, Argininosuccinate lyase, Phosphate-dependent glutaminase, Carbamyl phosphate synthetase-1, Ornithine transcarbamylase
1. Introduction
A widespread endothelial dysfunction develops with aging which includes blunted nitric oxide (NO) production by the peripheral vasculature; a possible contributor to age-dependent hypertension (Dohi et al., 1990; Tominaga et al., 1994). The total body NO produced also falls with age in the old rat as indicated by declines in the 24 h urinary excretion of NO2 + NO3 (NOX = the stable oxidation products of NO) (Hill et al., 1997; Reckelhoff et al., 1994). In addition, a slowly evolving chronic renal disease develops in aging (Baylis and Corman, 1998) which may also be associated with a declining renal NO production. Indeed, several types of chronic renal disease are associated with a reduced renal NO generating capacity, which may contribute to the progression of kidney injury (Baylis, 2002). There are many possible causes for a decline in NO production which include lack of the substrate arginine, indeed, it has been reported that plasma arginine falls markedly with advancing age in the rat, perhaps leading to substrate deficiency and declines in NO production (Reckelhoff et al., 1994).
l-Arginine is classified as a ‘semi-essential’ or ‘conditionally essential’ amino acid. During periods of high demand, such as maturational growth or following trauma, l-arginine is an essential amino acid, but in the healthy, unstressed adult, endogenous arginine production is adequate for metabolic needs (Barbul, 1986; Beaumier et al., 1996). The two main sites of endogenous arginine synthesis are the liver (where arginine is both synthesized and hydrolyzed within the urea cycle), and the kidney cortex, where most of the synthesized l-arginine is released into the blood and distributed throughout the body (Morris, 1992; Wu and Morris, 1998). The progressive kidney injury that develops during aging (Baylis and Corman, 1998) could lead to a diminished arginine biosynthetic capacity and account for reduced l-arginine availability (Reckelhoff et al., 1994).
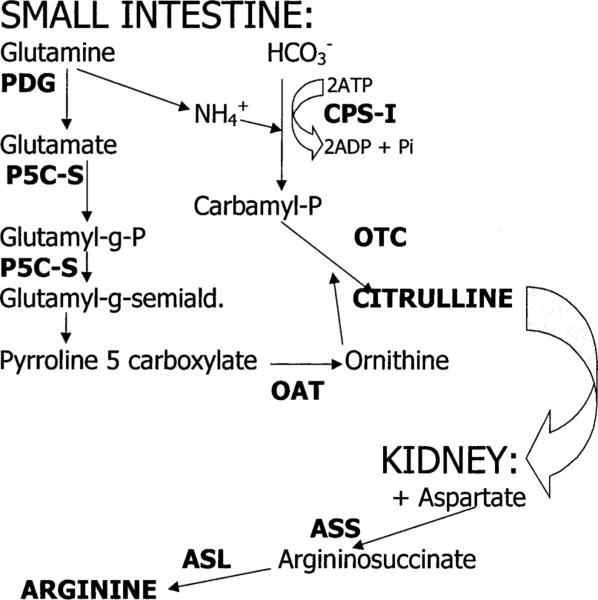
Endogenous arginine synthesis involves a series of enzymatic reactions in the small intestine that lead to formation of citrulline, which is then transported in the blood to the kidney for conversion to l-arginine (Fig. 1) (Wu and Morris, 1998; Windmueller and Spaeth, 1981). The present study was conducted to test the hypothesis that the age-related decline in NO production is secondary to development of an aging defect, at the mRNA level, of key arginine biosynthetic enzymes in the “intestinal–renal” axis. Studies were conducted in young, middle-aged and old male and female Sprague–Dawley (SD) rats.
Fig. 1.
Metabolic pathways in the ‘intestinal–renal axis’ of arginine biosynthesis. Abbreviations not previously defined: P5C-S, pyrroline-5-carboxylate synthase; OAT, ornithine aminotransferase.
2. Materials and methods
2.1. Animals
Studies were conducted on 15 female and 21 male SD rats, purchased from Harlan Sprague–Dawley (Indianapolis) at age 10–12 weeks. Rats were then aged at WVU Animal Facility, maintained throughout their lives under barrier conditions and allowed “ad libitum” access to standard rat chow (~20% protein; 0.4% Na) and tap water, up until sacrifice. Kidney cortex, cerebellum and small intestine (duodenum) were harvested from male and female SD rats aged 3–5 months (young), 11–13 months (middle-aged) and 18–22 months (old) (all n = 5–7). Tissue was harvested on to dry ice, separated into sections, flash frozen in liquid nitrogen and stored at –80 °C. Blood was harvested from some rats and plasma was stored at –20 °C until analysis (see Section 2.2). All protocols have been reviewed and approved by the West Virginia University Animal Care and Use Committee.
2.2. Isolation and Analysis of RNA
RNA was isolated from tissues using TRI reagent (Molecular Research Ctr., Cincinnati, OH) according to the manufacturer's instructions, using full precautions to protect against RNAse contamination. Northern blotting was performed as described previously (Morris et al., 1987), except that all DNA probes were labeled using the Ambion SripEZ DNA kit (Ambion Inc., Austin, TX) to facilitate stripping and rehybridization of Northern blots. Rat cDNA probes were used for the following enzymes: in small intestine, phosphate-dependent glutaminase (PDG) (Shapiro et al., 1991), carbamyl phosphate synthetase-1 (CPS-1) (Adcock and O'Brien, 1984) and ornithine transcarbamylase (OTC) (Nebes and Morris, 1988); in kidney, argininosuccinate synthetase (ASS) (Surh et al., 1988) and argininosuccinate lyase (ASL) (Lambert et al., 1986). The abundance of specific mRNAs on each Northern blot was quantified using a Molecular Dynamics STORM PhosphorImager. After quantifying the mRNA of interest, all membranes were stripped and rehybridized with a probe for 18S rRNA (Katz et al., 1983) to control for minor variations in loading between lanes. To allow comparison between blots, internal standards (IS) of rat liver and kidney total RNA were run as positive controls on every gel and used to normalize mRNA levels. Normalized values of each mRNA within every sample X of total RNA were calculated as (mRNA X/mRNA IS)/(18S rRNA X/ 18S rRNA of IS) and expressed as relative mRNA values, in arbitrary units.
2.3. Quantification of amino acids
Plasma levels of arginine and citrulline were measured on five young and six old male SDs using a reverse phase HPLC method described by us previously (Schmidt et al., 1999). Arginine concentration was also measured in homogenates from kidney cortex and cerebellum using the HPLC method described by us previously (Schmidt et al., 1999) except that the column was run at 41 °C. Tissues were homogenized on ice in 0.9% NaCl (5 × wt. volume), centrifuged cold for 15 min at 14 000 × g in a refrigerated centrifuge and the supernatant was analyzed for protein or filtered through a 10k MWCO centrifuge filter for HPLC analysis.
2.4. Statistical analyses
Changes in mRNA levels with age for males or females were evaluated by one-way ANOVA with Tukey post test, using the utilities in the prism software package (GraphPad Software, San Diego, CA). Pairwise comparisons of males and females of a given age were made by two-tailed t-test. Differences with P < 0.05 were considered to be significant.
3. Results
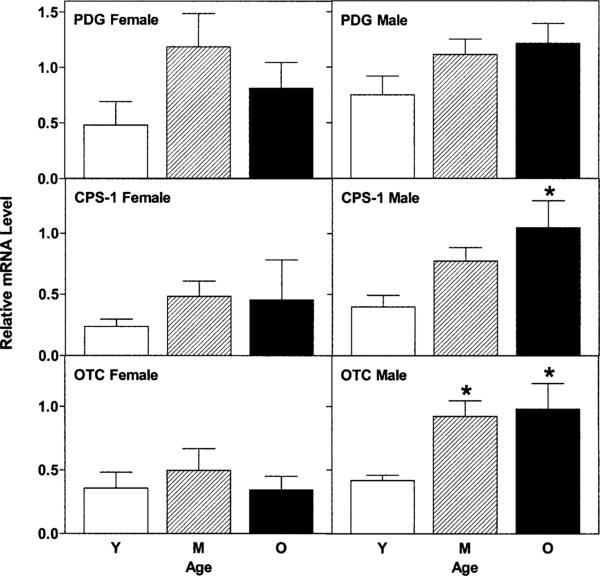
Fig. 2 shows the relative mRNA levels for the small intestinal enzymes involved in citrulline synthesis, for young, middle-aged and old female and male SD rats. There was no age-dependent change in the relative levels of PDG RNA in either males or females. However, relative levels of CPS-1 mRNA were higher in old versus young males and relative levels of OTC mRNA were higher in both middle-aged and old males versus young (all P < 0.05). In contrast, there were no age-dependent changes in relative levels of CPS-1 or OTC mRNAs in females. Gene expression of PDG was lower in small intestine of young female versus males (P < 0.025) as was gene expression of CPS-I in old females versus males (P < 0.025).
Fig. 2.
Relative levels of PDG, CPS-1, and OTC mRNAs in small intestine of female and male rats during aging. Relative mRNA levels were quantified as described in Section 2.2 and are displayed as averages ± S.E.M. Key to abbreviations and symbols: Y = young, M = middle-aged, O = old, * indicates significantly different (P < 0.05) from relative mRNA level in young rats.
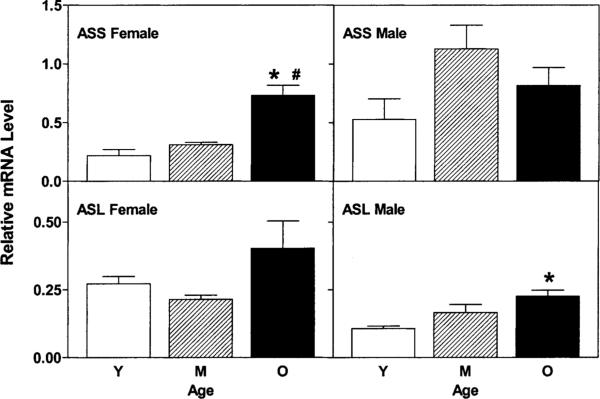
Fig. 3 shows the changes with age in relative level of mRNAs for ASS and ASL, the key enzymes in the renal conversion of citrulline to arginine. In the male, there were no differences in relative levels of ASS mRNA, but relative levels of ASL mRNA were significantly higher in old versus young males (P < 0.05). Conversely, in females the relative level of ASS mRNA was higher in old versus young (P < 0.05), but there was no significant change in ASL mRNA level. Gene expression of ASL was lower in the young female versus male kidney (P < 0.01) and ASS mRNA was lower in the middle aged female versus male (P < 0.05).
Fig. 3.
Relative levels of ASS and ASL mRNAs in kidney of female and male rats during aging. Relative mRNA levels were quantified as described in Section 2.2 and are displayed as averages ± S.E.M. Abbreviations and symbols are as in Fig. 3; in addition, # indicates significantly different (P < 0.05) from relative mRNA level in middle-aged rats.
Plasma levels of l-arginine were similar in young and old male rats (Table 1), whereas plasma citrulline was increased in the old males versus young. The tissue concentration of l-arginine was unaffected by age in both cerebellum and in the renal cortex. Values for females and middle-aged males are not available.
Table 1.
Measured variables in young and old male Sprague–Dawley rats
| Young (n = 5) | Old (n = 6) | |
|---|---|---|
| Plasma citrulline (μM) | 70 ± 8 | 171 ± 23* |
| Plasma arginine (μM) | 164 ± 20 | 208 ± 18 |
| Cerebellar arginine (μmol/g) | 4.4 ± 0.3 | 4.4 ± 0.6 |
| Renal cortex arginine (μmol/g) | 11.5 ± 2.1 | 14.8 ± 1.1 |
P<0.05 young vs. old.
4. Discussion
The focus of the present study was to examine whether age-dependent falls occur in the mRNA of the enzymes that control l-arginine synthesis, which might contribute to the decreased NO synthesis reported in aging rats, via a reduction in substrate availability (Reckelhoff et al., 1994). This is a reasonable hypothesis since when l-arginine deficiency is experimentally produced in rats by feeding an arginine-free diet + surgical resection of the small intestine to compromise citrulline synthesis, total NO production falls and blood pressure increases (Wakabayashi et al., 1994).
To evaluate the ability of the aging rat to produce endogenous l-arginine we measured the gene expression of key enzymes involved in the ‘intestinal–renal’ arginine biosynthetic axis (Fig. 1). Citrulline is the major substrate for endogenous l-arginine synthesis and is derived primarily from glutamine metabolism in the small intestine (Windmueller and Spaeth, 1981). Key enzymes in this pathway include PDG, which generates both ammonia (a substrate for CPS-1) and glutamate (the precursor of ornithine), CPS-1, which catalyzes the committed step in citrulline synthesis, and OTC, which catalyses the production of citrulline from ornithine and carbamyl phosphate. We find that while intestinal mRNA levels for PDG are not altered by aging, mRNAs for both CPS-1 and OTC are upregulated more than twofold with advancing age in male rats but not in females. Although the identity of the limiting step in intestinal citrulline synthesis has not been determined, this increase raises the possibility of an increased citrulline synthetic capacity in the small intestine of old male rats. The plasma concentration of citrulline is increased in old male rats, which may reflect increased citrulline synthesis and/or reduced renal clearance due to age-dependent kidney damage, rather than decreases in levels of kidney ASS and ASL mRNAs (see below).
As shown in Fig. 1, in the kidney the enzymes ASS and ASL are responsible for conversion of citrulline to arginine. Relative levels of ASS and ASL mRNAs in kidney do not decline with age, in fact ASS mRNA increased in old versus young female rats and ASL mRNA increased in old versus young male rats. Coupled with the fact that total kidney weight increases markedly with age in male SD rats from the same vendor (Baylis and Corman, 1998), renal arginine biosynthetic capacity may actually increase with advancing age. This finding, together with the report that intestinal absorption of ingested arginine is also preserved in the old rat (Penzes, 1970), supports the idea that arginine availability is not compromised by normal aging. Although lack of commercially available antibodies has prevented our investigation of the arginine biosynthetic system at the protein level, the present results suggest that the endogenous l-arginine biosynthetic capacity is preserved during advancing age. Our findings are consistent with the hypothesis that regulation of arginine synthesis plays little role in maintenance of arginine homeostasis (Castillo et al., 1993, 1994), although this has not previously been examined in aging.
Although there is some sexual dimorphism in the impact of age on the expression of the various mRNAs in the “renal –intestinal” arginine biosynthetic axis, there is no indication that expression of any of the genes declines with age in either gender. Importantly, the well know susceptibility of the male kidney to age induced damage (Baylis and Corman, 1998) is not likely linked to any deficits in the endogenous synthesis of arginine.
Substantial falls in plasma arginine concentration have been reported in the old male rat (Reckelhoff et al., 1994; Milakofsky et al., 1993); the observation that motivated this study. However, in the present study we found no decline in the plasma level of l-arginine in young versus old male rats, as also reported by others for aging rat (Gross et al., 1991) or man (Moller et al., 1979; Jeevanandam et al., 1990). It may be that the aged rat is more susceptible to stress induced falls in plasma l-arginine, since reductions were observed in the old rat after fasting (Reckelhoff et al., 1994) and several days after surgery and general anesthesia (Milakofsky et al., 1993).
Because arginine must be transported into cells in order to serve as a substrate for NOS and also because other enzymes such as arginase can utilize l-arginine as substrate, the plasma l-arginine level is not the sole determinant of intracellular l-arginine availability (Morris, 1999, 2000). Because of this we also measured intracellular l-arginine concentrations in two locations. In the cerebellum, a high-output source of constitutively generated NO, the intracellular l-arginine concentration presumably reflects the balance between l-arginine synthesis locally, transport into the cell from other sources and utilization by various enzymes. The cerebellar arginine concentrations were similar in young and old male rats, suggesting that l-arginine availability does not become limiting in the old rat cerebellum. In the kidney cortex, l-arginine concentration is much higher, probably due a high rate of localized synthesis of l-arginine by ASS and ASL in the proximal tubules, which exceeds local consumption of l-arginine by various enzymes as well as export from the kidney for utilization elsewhere. Since renal cortical l-arginine concentrations were similar in fed young and old male rats, renal cortical l-arginine production does seem to be well maintained in the old rat; a finding in accord with the maintained/elevated mRNA levels of ASS and ASL.
The present study therefore shows that the availability of the substrate for NO synthesis is maintained in aging rats, despite in vivo and in vitro evidence of reduced NO production (Dohi et al., 1990; Reckelhoff et al., 1994; Tominaga et al., 1994). This finding implicates age-dependent changes in more downstream elements of the NO synthesis system. Recent preliminary observations by us suggest a possible mechanism. In the aging SD male rat, where reduced total NO production is evident, we find that abundance of both endothelial and neuronal NOS is markedly reduced in kidney cortex, as is the in vitro NOS activity. In contrast, in aging females, where total NO production is preserved, renal NOS protein abundance and NOS activity are also maintained (Erdely et al., 2001). Therefore, at least one mechanism for the reduced NO production seen in the aging male is likely to be loss of the NO biosynthetic enzymes. Furthermore, while the male kidney develops significant age-dependent damage, the female is protected (Baylis and Corman, 1998). Thus, aging represents another example where chronic renal disease is associated with a reduced renal NO generating capacity, which may contribute to the progression of kidney damage (Baylis, 2002).
Acknowledgements
These studies were supported by NIH grant # R01 DK45517 (CB) and # R01 GM57384 (SM). We are grateful to Diane Kepka-Lenhart, Lennie Samsell and Chris Stalnaker for technical assistance. No reprint requests please.
References
- Adcock MS, O'Brien WE. Molecular cloning of cDNA for rat and human carbamyl phosphate synthetase I. J. Biol. Chem. 1984;259:13471–13476. [PubMed] [Google Scholar]
- Barbul A. Arginine: Biochemistry, physiology, and therapeutic implications. J. Parenteral and Enteral Nutr. 1986;10:227–238. doi: 10.1177/0148607186010002227. [DOI] [PubMed] [Google Scholar]
- Baylis C. Nitric oxide deficiency; both consequence and cause of chronic renal disease. Hypertonia Nephrol. 2002 in press. [Google Scholar]
- Baylis C, Corman B. The aging kidney: insights from experimental studies. J. Am. Soc. Nephrol. 1998;9:699–709. doi: 10.1681/ASN.V94699. Invited Editorial. [DOI] [PubMed] [Google Scholar]
- Beaumier LL, Castillo YM, Yu A, Ajami M, Young VR. Arginine: New and exciting developments for an ‘old’ amino acid. Biomed. Environ. Sci. 1996;9:296–315. [PubMed] [Google Scholar]
- Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, Vogt J, Young VR. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc. Natl. Acad. Sci. USA. 1993;90:7749–7753. doi: 10.1073/pnas.90.16.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo L, Sanchez M, Chapman TE, Ajami A, Burke JF, Young VR. The plasma flux and oxidation rate of ornithine adaptively decline with restricted arginine intake. Proc. Natl. Acad. Sci. USA. 1994;91:6393–6397. doi: 10.1073/pnas.91.14.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi Y, Thiel MA, Buhler FR, Luscher TF. Activation of endothelial l-arginine pathway in resistance arteries. Hypertension. 1990;15:170–179. doi: 10.1161/01.hyp.16.2.170. [DOI] [PubMed] [Google Scholar]
- Erdely A, Greenfeld Z, Baylis C. Decreased renal nitric oxide synthase (NOS) activity and abundance in kidney of old male rat. J. Am. Soc. Nephrol. 2001;12:675A. [Google Scholar]
- Gross KL, Hartman WJ, Ronnenberg A, Prior RL. Arginine-deficient diets alter plasma and tissue amino acids in young and aged rats. J. Nutr. 1991;121:1591–1599. doi: 10.1093/jn/121.10.1591. [DOI] [PubMed] [Google Scholar]
- Hill C, Lateef AM, Engels K, Samsell L. Basal and stimulated nitric oxide in control of kidney function in the aging rat. Am. J. Physiol. 1997;272:R1747–R1753. doi: 10.1152/ajpregu.1997.272.6.R1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevanandam M, Young DH, Ramias L, Schiller WR. Effect of major trauma on plasma free amino acid concentrations in geriatric patients. Am. J. Clin. Nutr. 1990;51:1040–1045. doi: 10.1093/ajcn/51.6.1040. [DOI] [PubMed] [Google Scholar]
- Katz RA, Mitsialis SA, Guntaka RV. Studies on the methylation of avian sarcoma proviruses in permissive and non-permissive cells. J. Gen. Virol. 1983;64:429–435. doi: 10.1099/0022-1317-64-2-429. [DOI] [PubMed] [Google Scholar]
- Lambert MA, Simard LR, Ray PN, McInnes RR. Molecular cloning of cDNA for rat argininosuccinate lyase and its expression in rat hepatoma cell lines. Mol. Cell. Biol. 1986;6:1722–1728. doi: 10.1128/mcb.6.5.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milakofsky L, Harris N, Vogel WH. Effects of repeated stress on plasma arginine levels in young and old rats. Physiol. Behav. 1993;54:725–728. doi: 10.1016/0031-9384(93)90083-r. [DOI] [PubMed] [Google Scholar]
- Moller P, Bergstrom J, Eriksson S, Furst P, Hellstrom K. Effect of aging on free amino acids and electrolytes in leg skeletal muscle. Clin. Sci. 1979;56:427–432. doi: 10.1042/cs0560427. [DOI] [PubMed] [Google Scholar]
- Morris SM., Jr. Regulation of enzymes of urea and arginine synthesis. Annu. Rev. Nutr. 1992;12:81–101. doi: 10.1146/annurev.nu.12.070192.000501. [DOI] [PubMed] [Google Scholar]
- Morris SM., Jr. Arginine synthesis, metabolism, and transport: Regulators of nitric oxide synthesis. In: Laskin JD, Laskin DL, editors. Cellular and Molecular Biology of Nitric Oxide. Marcel Dekker, Inc; 1999. pp. 57–85. [Google Scholar]
- Morris SM., Jr. Regulation of arginine availability and its impact on NO synthesis. In: Ignarro LJ, editor. Nitric Oxide. Biology and Pathobiology. Academic Press; San Diego: 2000. pp. 187–197. [Google Scholar]
- Morris SM, Jr., Moncman CL, Rand RD, Dizikes GJ, Cederbaum SD, O'Brien WE. Regulation of mRNA levels for five urea cycle enzymes in rat liver by diet, cyclic AMP, and glucocorticoids. Arch. Biochem. Biophys. 1987;256:343–353. doi: 10.1016/0003-9861(87)90455-3. [DOI] [PubMed] [Google Scholar]
- Nebes VL, Morris SM., Jr. Regulation of messenger ribonucleic acid levels for five urea cycle enzymes in cultured rat hepatocytes. Requirements for cyclic adenosine monophosphate, glucocorticoids, and ongoing protein synthesis. Molec. Endocrinol. 1988;2:444–451. doi: 10.1210/mend-2-5-444. [DOI] [PubMed] [Google Scholar]
- Penzes L. Intestinal transfer of l-arginine in relation to age. Exp. Geront. 1970;5:193–201. doi: 10.1016/0531-5565(70)90038-0. [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF, Kellum JA, Blanchard EJ, Bacon EE, Wesley AJ, Kruckeberg WC. Changes in nitric oxide precursor, l-arginine, and metabolites, nitrate and nitrite, with aging. Life Sci. 1994;55:1895–1902. doi: 10.1016/0024-3205(94)00521-4. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Domico J, Samsell L, Yokota S, Tracy T, Sorkin M, Engels K, Baylis C. Indices of activity of the nitric oxide system in patients on hemodialysis. Am. J. Kidney Dis. 1999;34:228–234. doi: 10.1053/AJKD03400228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RA, Farrell L, Srinivasan M, Curthoys NP. Isolation, characterization, and in vitro expression of a cDNA that encodes the kidney isoenzyme of the mitochondrial glutaminase. J. Biol. Chem. 1991;266:18792–18796. [PubMed] [Google Scholar]
- Surh LC, Morris SM, Jr., O'Brien WE, Beaudet AL. Nucleotide sequence of the cDNA encoding the rat argininosuccinate synthetase. Nucleic Acids Res. 1988;16:9352. doi: 10.1093/nar/16.19.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Fujii K, Abe I, Takata Y, Kobayashi K, Fujishima M. Hypertension and aging impair acetylcholine-induced vasodilation in rats. J. Hypertension. 1994;12:259–268. [PubMed] [Google Scholar]
- Wakabayashi Y, Yamada E, Yoshida T, Takahashi H. Deficiency of endogenous arginine synthesis provokes hypertension by exhausting substrate agrinine for nitric oxide synthesis. Biochem. Biophys. Res. Commun. 1994;205:1391–1398. doi: 10.1006/bbrc.1994.2820. [DOI] [PubMed] [Google Scholar]
- Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am. J. Physiol. 1981;241:473–480. doi: 10.1152/ajpendo.1981.241.6.E473. [DOI] [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr. Arginine metabolism: nitric oxide and beyond. Biochem. J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]