Abstract
Recent studies suggest that endogenous endothelin mediates much of the vasoconstrictor activity and vascular fibrotic damage caused by chronic administration of angiotensin II. The present study uses the mixed endothelin-A and endothelin-B receptor antagonist bosentan and the endothelin-A–selective blocker BQ-123 to study the contribution of endogenous endothelin to the pressor and renal action of acutely administered angiotensin II in conscious, chronically catheterized rats. Exposure to angiotensin II at 0.48 pmol 0.5 ng/100 g body weight per min IV (low dose) and 1.91 pmol 2.0 ng/100 g body weight per min IV (high dose) raised mean arterial blood pressure (18±4 mm Hg, P<0.01, and 39±4 mm Hg, P<0.005, respectively) while also increasing renal vascular resistance (4.3±1 mm Hg/mL per min, P<0.001, and 10±1 mm Hg/mL per min, P<0.001, respectively). In the presence of bosentan, pressor and renal vasoconstrictor responses to low-dose angiotensin II were blunted (P<0.02 and P<0.01, respectively), and the results with BQ-123 were similar. In contrast, these parameters were unaffected during high-dose angiotensin II infusion+bosentan, although BQ-123 did selectively reduce the rise in renal vascular resistance, possibly via an endothelin B–mediated nitric oxide effect. In contrast, high-dose angiotensin II caused natriuretic and diuretic effects that were completely prevented by bosentan. These results show that endothelin (via endothelin A) contributes to the pressor and renal vasoconstrictor actions of acutely administered low-dose angiotensin II. Furthermore, our data suggest that the previously described angiotensin II–induced natriuresis and diuresis observed with a high pressor dose of angiotensin II is mediated by endothelin.
Keywords: vascular resistance, glomerular filtration rate, natriuresis, rats, angiotensin II
Angiotensin II (Ang II) is a potent endogenous vasoconstrictor that, when activated, produces increases in blood pressure (BP) and renal vasoconstriction and stimulates cell growth.1,2 Most of these actions of Ang II are mediated by activation of the angiotensin type 1 (AT1) receptor,2 and many of these actions resemble those of another potent endogenous vasoconstrictor, endothelin (ET). Two ET receptor subtypes have been characterized, and activation of ET type A (ETA) receptors on vascular smooth muscle (VSM) causes vasoconstriction and proliferation of cells. ET type B (ETB) receptors are expressed on endothelial cells, in which they cause relaxation of adjacent VSM via nitric oxide and prostacyclin release, and on VSM, in which they cause vasoconstriction.3 Acutely administered ET produces a transient ETB-mediated hypotension, followed by a prolonged hypertension and renal vasoconstriction with consequent decreases in renal blood flow and glomerular filtration rate (GFR) in conscious rats.4
Ang II and ET can interact at several levels. Administered ET has a synergistic effect to augment the pressor actions of Ang II.5 The hypertension and renal vasoconstriction produced by chronic administration of Ang II can be largely prevented by inhibition of endogenous ET,6–8 suggesting that ET mediates much of the vasoconstrictor activity of chronic Ang II. There is increasing evidence that ET mediates some of the vascular fibrotic damage usually attributed to Ang II.9 ET also contributes to the pressor effects of acutely administered, low-dose (LD) Ang II,10 and in vitro, ET mediates some of the vasoconstrictor actions of exogenous Ang II in some parts of the vasculature.11 Stimulation of the AT1 receptor can stimulate ET synthesis and release, and vice versa.6,9 There is also a report of a novel, transmembrane receptor that contains distinct ET and Ang II binding sites, although whether this has any functional role is unclear.12 Finally, it is evident that ET and Ang II signal through several common intracellular pathways.2,3,13
The introduction and use of ETA and ETB receptor antagonists have allowed further investigation into the actions of ET and its interactions with Ang II. In the present study, we have used the mixed ETA and ETB receptor antagonist bosentan14 to investigate the contribution of endogenous ET to the pressor and renal actions of acutely administered Ang II in the conscious, chronically catheterized rat. In a separate series, we used the ETA-selective blocker BQ-12315 to determine its relative importance to the 2 ET receptor subtypes.
Methods
Studies were conducted on 17 male Sprague-Dawley rats, 3 to 6 months of age, obtained from Harlan Sprague Dawley Inc (Indianapolis, Ind). In all rats, a preliminary surgery was conducted in which catheters were placed in the left femoral artery and vein and in the urinary bladder. All surgeries were conducted under general anesthesia with short-acting barbiturate anesthetic (methohexital, Eli Lilly & Co; 176 μmol/kg IP, 17 to 35 μmol/kg IV, as required). At the end of the surgery, vascular catheters and bladder catheters were primed and plugged, and the rats were returned to their individual cages. Full sterile technique was used throughout. Details of this chronic catheterization method have been published previously.15 Rats were allowed free access to rat chow (≈24% protein and ≈0.4% Na) and drinking water and were handled and trained to accustom them to the activity in the laboratory. A period of 7 days elapsed between the surgery and the acute experiments. Two to 3 studies were conducted on each animal, with at least 2 days rest between experiments. All animal procedures were conducted in accordance with institutional guidelines (West Virginia University, Morgantown, WVa).
Renal function studies were conducted as follows: rats were placed in a restraining cage, and the arterial catheter was connected to a pressure transducer and recorder for BP measurement. The arterial line was also used for occasional sampling of blood. An intravenous infusion of 3H inulin (2 to 5 μCi/mL) and paraaminohippuric acid (PAH 1%) was given in 0.9% NaCl at 5 μL/min per 100 g body weight (BW). The bladder pin was removed for collection of urine, and a tube with side arm was attached to the bladder catheter for collection of urine. After 90 minutes of equilibration, two 20-minute control urine collections were made with midpoint arterial blood samples. The bladder catheter was flushed with air immediately before the end of the collection period to ensure complete collection of urine. Midpoint blood samples (≈150 μL) were centrifuged, the plasma was removed for analysis, and the red blood cells were reconstituted with sterile 0.9% NaCl and restored to the rat after the control period.
After completion of control measurements, 1 of the following 7 experiments was conducted. In the first group of animals studied (n=6), rats received LD Ang II (0.48 pmol, 0.5 ng/100 g BW per min IV) alone (Group 1a). Ang II infusion was given for 15 minutes of equilibration and then throughout two 20-minute clearance periods. In a separate experiment, the same rats received the same dose of Ang II in the presence of the mixed ETA/ETB receptor antagonist bosentan (1 mg/100 g BW IV) 10 minutes before starting Ang II. In the second group of rats, a high dose (HD) of Ang II (1.91 pmol, 0.5 ng/100 g BW per min IV) was given alone (Group 2a) and, in separate studies, during combined ETA and ETB blockade (Group 2b). In the third group (n=5), similar LD and HD Ang II infusion studies (Groups 3a and 3b) were conducted during ETA receptor blockade with BQ-123 (1 mg/kg IV bolus and 0.1 mg/kg per h IV infusion). Four of these group 3 rats also received BQ-123 alone in a third experiment (Group 3c). These doses of bosentan and BQ-123 have been previously shown by us to be effective, selective blockers of ETA+ETB and ETA effects, respectively.15 At the end of the final clearance period, red blood cells were reconstituted and restored to the rat. Vascular and bladder catheters were primed and plugged, and rats were returned to their home cages, after which they were either used for additional experiments or euthanized.
The following analyses and calculations were made: urine volume was measured gravimetrically; then urine was analyzed for 3H inulin activity and concentrations of PAH, Na, and K. Blood samples were analyzed for hematocrit, plasma 3H inulin activity, and PAH, Na, and K concentrations. These measurements allow calculation of GFR, renal plasma flow (RPF), renal vascular resistance (RVR), urine flow, urinary excretion of Na and K (UNaV and UKV, respectively), and fractional excretion of Na. Details of these analyses and calculations have been published by us previously.16 When all experiments were completed, rats were euthanized, and the bladder and kidneys were inspected to establish that they were free of infection.
Within group analysis was by paired t test, and between group analysis was by unpaired t test. All data are shown as mean±1 SEM, and P<0.05 is considered to be statistically significant.
Results
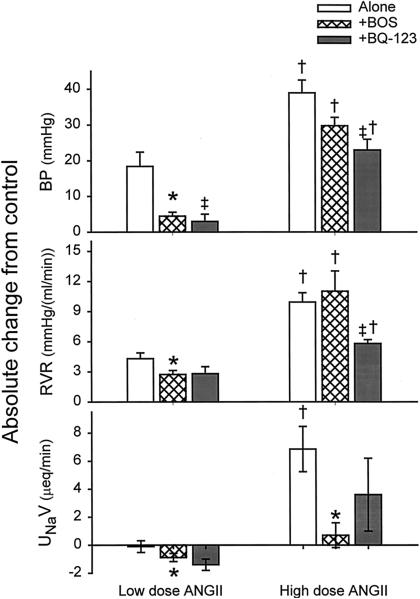
BWs were 420±4 g, 395±13 g, and 375±16 g for groups 1, 2, and 3, respectively. As summarized in the Table, acute systemic LD Ang II (Group 1a) produced a significant rise in blood pressure and renal vasoconstriction (Figure) and falls in RPF and GFR. When the same rats were subjected to the same LD Ang II combined with acute systemic ETA/ETB receptor blockade (Group 1b), the pressor and renal vasoconstrictor responses to LD Ang II were blunted (P<0.02 and P<0.01, respectively) (see Figure and Table). RPF fell substantially with LD Ang II, and the reduction in GFR was proportionally less because of a concomitant rise in filtration fraction (FF). Combined systemic ETA/ETB receptor blockade had no impact on the magnitude of these responses (Table). Although LD Ang II alone did not affect UNaV, there was a significant decrease in UNaV with LD Ang II during ETA/ETB receptor blockade. We did not conduct studies with bosentan alone in this series of experiments, although earlier work by us shows little change on systemic and renal hemodynamics, although sodium excretion increased slightly with bosentan alone.17 In group 3a rats, selective ETA blockade with BQ-123 during LD Ang II produced a similar pattern to that seen with combined bosentan and LD Ang II (Table; Figure). Of particular note, the magnitude of the pressor and renal vasoconstrictor response to Ang II was similar in the rats that received either bosentan or BQ-123, and the UNaV fell similarly in both groups (Figure).
TABLE 1.
Summary of Blood Pressure and Renal Function
| Group | BP, mm Hg | RVR, mm Hg/mL per min | GFR, mL/min | RPF, mL/min | FF | V, μL/min | UNaV, μeq/min | FENa, % |
|---|---|---|---|---|---|---|---|---|
| Group 1a | ||||||||
| Control | 118±2 | 4.7±0.5 | 3.21±0.13 | 14.9±1.6 | 0.226±0.018 | 19±3 | 1.59±0.31 | 0.366±0.078 |
| LD Ang II | 137±4 | 9.0±0.7 | 2.47±0.10 | 8.8±0.7 | 0.287±0.018 | 14±4 | 1.48±0.36 | 0.435±0.105 |
| P | <0.01 | <0.001 | <0.01 | <0.01 | <0.05 | NS | NS | NS |
| Group 1b | ||||||||
| Control | 119±2 | 4.4±0.3 | 2.93±0.09 | 14.9±1.0 | 0.202±0.013 | 14±1 | 1.73±0.28 | 0.438±0.077 |
| LD Ang II+BOS | 123±2* | 7.1±0.3* | 2.47±0.06 | 9.8±0.4 | 0.255±0.012 | 9±1 | 0.85±0.16 | 0.248±0.047 |
| P | <0.01 | <0.005 | <0.001 | <0.005 | <0.05 | NS | <0.05 | NS |
| Group 2a | ||||||||
| Control | 118±2 | 5.9±0.5 | 2.55±0.25 | 11.7±1.2 | 0.221±0.014 | 18±3 | 1.33±0.37 | 0.371±0.097 |
| HD Ang II | 157±3 | 15.8±1.1 | 1.90±0.15 | 6.3±0.6 | 0.338±0.027 | 76±14 | 8.18±1.48 | 3.114±0.480 |
| P | <0.001 | <0.001 | <0.05 | <0.05 | <0.01 | <0.05 | <0.01 | <0.01 |
| Group 2b | ||||||||
| Control | 120±3 | 5.7±0.5 | 2.70±0.28 | 12.6±1.2 | 0.229±0.120 | 22±3 | 1.74±0.37 | 0.501±0.135 |
| HD Ang II+BOS | 150±4 | 16.7±2.1 | 1.80±0.13 | 5.5±0.5 | 0.340±0.021 | 25±6 | 2.44±0.84† | 1.021±0.404† |
| P | <0.001 | <0.01 | <0.01 | <0.01 | <0.05 | NS | NS | NS |
| Group 3a | ||||||||
| Control | 124±4 | 5.1±0.6 | 2.59±0.21 | 13.3±1.1 | 0.196±0.008 | 25±1 | 2.66±0.47 | 0.734±0.080 |
| LD Ang II+BQ123 | 126±4 | 7.9±0.6 | 1.95±0.12 | 8.6±0.5 | 0.226±0.006 | 11±1 | 1.22±0.23 | 0.456±0.089 |
| P | NS | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Group 3b | ||||||||
| Control | 123±2 | 5.4±0.4 | 2.70±0.28 | 12.7±0.8 | 0.214±0.022 | 24±1 | 2.37±0.19 | 0.656±0.071 |
| HD Ang II+BQ123 | 146±3 | 11.2±0.2 | 2.13±0.22 | 7.2±0.08 | 0.298±0.017 | 49±22 | 5.96±2.61 | 1.200±0.860 |
| P | <0.005 | <0.001 | NS | <0.005 | <0.005 | NS | NS | NS |
| Group 3c | ||||||||
| Control | 116±2 | 5.7±0.3 | 2.41±0.26 | 11.2±0.5 | 0.221±0.034 | 15±2 | 1.51±0.22 | 0.493±0.121 |
| +BQ123 | 114±1 | 6.1±0.3 | 2.13±0.25 | 10.4±0.5 | 0.211±0.032 | 12±2 | 1.67±0.46 | 0.630±0.207 |
| P | NS | NS | <0.001 | NS | NS | NS | NS | NS |
Group 1a rats were given LD Ang II (0.5 ng/100 gBW/min, IV). Group 2 rats were given HD Ang II (2 ng/100 gBW/min, IV), in the absence (Group 2a) and the presence (Group 2b) of combined ETA and ETB receptor inhibition with Bosentan. Group 3 rats received either LD or HD Ang II with selective ETA blockade (BQ-123), Groups 3a and 3b, respectively, or BQ-123 alone (Group 3c).
FF indicates filtration fraction; FENa, fractional excretion of sodium; BOS, Bosentan. Values are mean±SE. The P values within the Table give the paired differences of control vs experimental group.
P<0.05 indicates paired difference between Group 1a vs Group 1b
P<0.05, paired difference between Group 2a vs Group 2b.
Absolute change from control in mean arterial BP, RVR, and UNaV during LD and HD Ang II administration with and without bosentan (BOS; ETA+ETB blocker) and during combined Ang II and BQ-123 (ETA-selective blocker). * indicates difference between Ang II alone and Ang II with BOS; †, difference between LD vs HD Ang II, with and without BOS; and ‡, difference between HD Ang II with BOS versus HD Ang II with BQ-123.
Group 2a rats were subjected to acute systemic HD Ang II, which caused large rises in BP and RVR that were significantly greater than the increases seen with LD Ang II (P<0.005 and P<0.001, respectively; Table, Figure). HD Ang II also caused a fall in RPF and a lesser decline in GFR due to increased FF. HD Ang II caused marked natriuretic and diuretic effects not seen with LD Ang II alone. During concomitant acute systemic ETA/ETB receptor blockade in the same rats (Group 2b), the pressor response and renal vasoconstriction were indistinguishable from the responses to HD Ang II alone. The falls in GFR and RPF and the rise in FF were also similar. In contrast, the natriuretic and diuretic effects to HD Ang II were completely prevented by concomitant ETA/ETB receptor blockade. Similarly in group 3b rats, ETA blockade alone had no impact on the pressor response to HD Ang II (Table, Figure). However, ETA blockade alone did blunt the rise in RVR compared with that seen when both ETA and ETB receptors were blocked (Group 2b). This most likely is due to an ETB renal vasodilatory effect that is seen in group 3b but prevented in group 2b. The effects on urine flow and sodium excretion in group 3b rats were very variable, with no significant changes in either variable (Table); however, a rise in 3 of the 5 rats studied was observed. As shown in the Table, BQ-123 alone had no impact on any measured variables in the conscious, chronically catheterized rat.
Discussion
The primary findings in the present study are that blockade of endogenous ETA and ETB receptors attenuates the pressor and renal vasoconstrictor actions of a LD of infused Ang II, but it does not blunt these responses to a HD of Ang II. In contrast, the natriuresis seen with a HD of Ang II alone is abolished by concomitant ET blockade. In regard to the hemodynamic interactions between these vasoconstrictor peptides, there is now a convincing body of evidence to show that in some settings, chronic elevation of Ang II by exogenous infusion stimulates ET release and the vasoconstrictor and/or mitogenic actions of Ang II are partially mediated by endogenous ET.5–9 There is little information on states in which endogenous Ang II levels are elevated. There is controversy over whether ET-receptor blockade does18 or does not19 reduce the hypertension in the Ang II–dependent phases of 2 kidney, 1-clip hypertension. However, there is no evidence for ET mRNA upregulation in aorta or mesenteric arteries during the Ang II–dependent phase of this model of renovascular hypertension.20
There has been less investigation into acute interactions between Ang II and ET. In the present study, we observed that the renal vasoconstrictor and pressor response to the “low” pharmacological dose of acutely administered Ang II is markedly attenuated by combined ETA and ETB receptor blockade. At a higher concentration of Ang II, the ET dependence of the acute pressor and renal vascular responses to Ang II is lost. The ET dependence of Ang II in the lower concentration ranges may have considerable pathophysiological significance. This is the first report that ET mediates the renal vascular responses to acute LD Ang II. On the basis of the magnitude of the reduction in the pressor response to LD Ang II with ET blockade, it is likely that the blunted renal vasoconstriction is at least in part secondary to a reduced renal autoregulatory vasoconstriction. Our observations agree with an earlier study10 in spontaneously hypertensive rats and Wistar-Kyoto normotensive control rats in which ET blockade (with bosentan) profoundly attenuated the pressor effects of acute intravenous infusion of Ang II at 0.3 and 1 ng/100 g BW per min, but it was without impact on the rise in BP caused by higher doses of Ang II. In fact, the exaggerated pressor response to 0.3 ng/100 g BW per min Ang II observed in the spontaneously hypertensive rats versus Wistar-Kyoto rats was abolished by ET inhibition.10 Because ET blockade with bosentan alone had no impact on BP, this strongly suggests that Ang II stimulates activity of the endogenous ET system, which mediates part of the pressor response to LD Ang II. This conclusion is strengthened because bosentan is clearly acting selectively on ET receptors and does not inhibit Ang II receptors.10
Our findings with BQ-123 indicate that ETA blockade is responsible for the blunted pressor and renal vasoconstrictor responses to acute LD Ang II observed with bosentan. Bosentan also blunts the pressor, renal vasoconstrictor, proteinuric, and carotid artery hypertrophic effects of the chronic infusion of HD Ang II (20 ng/100 g BW per min, sc).7 Selective blockade of the ETA receptor also attenuates the pressor response to chronic Ang II8; thus, the ET amplification of the actions of exogenous Ang II is presumably via the ETA receptor. In some settings, the vasoconstrictor actions of chronic Ang II appear to be less ET dependent, whereas the structural injury is clearly ET mediated.9,21 There is also “crosstalk” between the endogenous Ang II and ET systems because combined blockade of Ang II and ET receptors produces enhanced blunting of the high BP in the Ren-2 hypertensive rat22 and the acute hypertension and renal vasoconstriction with systemic nitric oxide synthase (NOS) inhibition.23
We have previously reported that HD Ang II is potently natriuretic in the conscious rat,24 probably due to both inhibition of sodium reabsorption by a direct action of Ang II on the tubule epithelium as well as by a nonspecific pressure natriuresis. In the present study, we observed that the marked natriuresis and diuresis due to HD Ang II were prevented by bosentan. This is remarkable given the fact that the pressor response to HD Ang II was unaffected. In an earlier work using the same conscious, chronically catheterized rat preparation, we found that bosentan also inhibited the natriuretic (but not diuretic) response to a pressor dose of acute systemic NOS inhibition, while only slightly blunting the rise in BP.17 Of note, AT1 receptor blockade with losartan did not attenuate the natriuretic effect of NOS inhibition.25 It is possible, therefore, that ET plays a role in the acute pressure natriuretic response. Controversy over whether ET is natriuretic or antinatriuretic has recently been resolved with the finding that ET-1 evokes a marked ETB-dependent natriuresis that is mediated by local nitric oxide release.26 Thus, the endogenous ET system is likely to exert net natriuretic effects, as indicated by the present study.
The mechanism by which Ang II and ET interact in the present study are unknown. Although Ang II stimulates ET synthesis, this takes several hours,27 and although this probably contributes to the vasoconstrictor and/or fibrotic actions of chronic Ang II administration,9 it is unlikely to participate in the acute responses seen here. The rapidity with which bosentan blunts the acute pressor response to LD Ang II in the study of Balakrishnan et al10 also argues in favor of a nongenomic response as does the immediate in vitro attenuation of Ang II responses in rat mesenteric and tail arteries with ETA blockade.11 However, ET synthesis may not be necessary because in vitro Ang II rapidly leads to ET release from aorta within minutes, implying that preformed ET exists in tissue stores and is under Ang II regulation.28 Chen et al11 suggested that the rapid action of Ang II might be to activate the endothelin-converting enzyme; however, there is no direct evidence to confirm this. Alternatively, because both ET and Ang II signal through multiple common intracellular pathways,2,3,13 the low level of endogenous ET normally present may “prime” the intracellular cascades activated by Ang II. This would certainly explain the dependence of the systemic and renal vasoconstrictor effects of LD Ang II on endogenous ET that we observe. It would also explain the reason hemodynamic actions of HD Ang II are independent of endogenous ET, assuming that high concentrations of Ang II can maximally activate the signaling systems. Finally, it is possible that the novel, transmembrane receptor reported by Ruiz-Opazo and colleagues,12 which contains distinct ET and Ang II binding sites, is functionally active and requires both ET and Ang II ligands for activation.
It seems likely that the vasoconstrictor actions of LD Ang II are mediated primarily through ETA receptors, because we find that the ETA-selective blocker BQ-123 gives similar results to bosentan. The results of blockade of a single receptor subtype have to be interpreted cautiously because there may be amplification of the actions of the unblocked receptor subtype, secondary to loss of receptor-mediated ET clearance. In fact, this is probably the reason that ETA blockade alone is less effective in preventing the rise in RVR with HD Ang II than combined ETA and ETB blockade in the present study. Presumably, the increased ET available results in renal endothelial ETB-mediated release of nitric oxide, which serves to offset the renal vasoconstrictor actions of HD Ang II. The receptor subtype responsible for the natriuretic response to HD Ang II is less clear. On the basis of the report by Hoffman et al,26 we expected to see preservation of the natriuretic and diuretic response during selective ETA blockade; however, the response became more variable and was no longer different from baseline, although it increased numerically. Perhaps both ETA and ETB receptors are involved in the natriuretic response to this very HD of Ang II.
In conclusion, these studies demonstrate that ET makes an important contribution to the renal vasoconstrictor and pressor actions of acutely administered, LD Ang II via an ETA-dependent effect. Furthermore, in the normal conscious rat, endogenous ET is the primary mediator of the natriuresis and diuresis seen with a high pressor dose of Ang II.
Acknowledgments
These studies were funded by National Institutes of Health grant R01 # DK 45517. We thank Dr Martine Clozel (Actelion, Switzerland) for bosentan. The excellent technical assistance of Lennie Samsell and Kevin Engels is gratefully acknowledged.
References
- 1.Menè P, Dunn M. Vascular, glomerular, and tubular effects of angiotensin II, kinins, and prostaglandins. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. Raven Press; New York, NY: 1992. pp. 1205pp. 1214–1215. [Google Scholar]
- 2.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 3.Schiffrin EL, Touyz RM. Vascular biology of endothelin. J Cardiovasc Pharmacol. 1998;32(suppl 3):s2–s13. [PubMed] [Google Scholar]
- 4.Madeddu P, Troffa C, Glorioso N, Pazzola A, Soro A, Manunta P, Tonolo G, Demontis MP, Varoni MV, Anania V. Effect of endothelin on regional hemodynamics and renal function in awake normotensive rats. J Cardiovasc Pharmacol. 1989;14:818–825. doi: 10.1097/00005344-198912000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida K, Yasujima M, Kohzuki M, Kanazawa M, Yoshinaga K, Abe K. Endothelin-1 augments pressor response to angiotensin II infusion in rats. Hypertension. 1992;20:292–297. doi: 10.1161/01.hyp.20.3.292. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG. Role for endothelin-1 in angiotensin II–mediated hypertension. Hypertension. 1997;30:29–34. doi: 10.1161/01.hyp.30.1.29. [DOI] [PubMed] [Google Scholar]
- 7.Herizi A, Jover B, Bouriquet N, Mimran A. Prevention of the cardiovascular and renal effects of angiotensin II by endothelin blockade. Hypertension. 1998;31:10–14. doi: 10.1161/01.hyp.31.1.10. [DOI] [PubMed] [Google Scholar]
- 8.d'Uscio LV, Moreau P, Shaw S, Takase H, Barton M, Lüscher TF. Effects of chronic ETA-receptor blockade in angiotensin II–induced hypertension. Hypertension. 1997;29:435–441. doi: 10.1161/01.hyp.29.1.435. [DOI] [PubMed] [Google Scholar]
- 9.Chatziantoniou C, Dussaule JC. Endothelin and renal vascular fibrosis: of mice and men. Curr Opin Nephrol Hypertens. 2000;9:31–36. doi: 10.1097/00041552-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Balakrishnan SM, Wang HD, Gopalakrishnan V, Wilson TW, McNeill JR. Effect of an endothelin antagonist on hemodynamic responses to angiotensin II. Hypertension. 1996;28:806–809. doi: 10.1161/01.hyp.28.5.806. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, McNeill JR, Wilson TW, Gopalakrishnan V. Heterogeneity in vascular smooth muscle responsiveness to angiotensin II. Hypertension. 1995;26:83–88. doi: 10.1161/01.hyp.26.1.83. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-Opazo N, Hirayama K, Akimoto K, Herrera VL. Molecular characterization of a dual endothelin-1/angiotensin II receptor. Mol Med. 1998;4:96–108. [PMC free article] [PubMed] [Google Scholar]
- 13.van Heugten HA, Eskildsen-Helmond YE, de Jonge HW, Bezstarosti K, Lamers JM. Phosphoinositide-generated messengers in cardiac signal transduction. Mol Cell Biochem. 1996;157:5–14. doi: 10.1007/BF00227875. [DOI] [PubMed] [Google Scholar]
- 14.Clozel M, Breu V, Gray GA, Kalina B, Löffler BM, Burri K, Cassal JM, Hirth G, Müller M, Neidhart W, Ramuz H. Pharmacological characterization of bosentan, a new potent orally active nonpeptide endothelin receptor antagonist. J Pharmacol Exp Ther. 1994;270:228–235. [PubMed] [Google Scholar]
- 15.Qiu C, Samsell L, Baylis C. Actions of endogenous endothelin on glomerular hemodynamics in the rat. Am J Physiol. 1995;38:R469–R473. doi: 10.1152/ajpregu.1995.269.2.R469. [DOI] [PubMed] [Google Scholar]
- 16.Baylis C, Collins RC. Angiotensin II inhibition on blood pressure and renal hemodynamics in pregnant rats. Am J Physiol. 1986;250:F308–F314. doi: 10.1152/ajprenal.1986.250.2.F308. [DOI] [PubMed] [Google Scholar]
- 17.Qiu C, Engels K, Baylis C. Endothelin modulates the pressor actions of acute systemic nitric oxide blockade. J Am Soc Nephrol. 1995;6:1476–1481. doi: 10.1681/ASN.V651476. [DOI] [PubMed] [Google Scholar]
- 18.Schricker K, Scholz H, Hamann M, Clozel M, Kramer BK, Kurtz A. Role of endogenous endothelins in the renin system of normal and 2-kidney, 1 clip rats. Hypertension. 1995;25:1025–1029. doi: 10.1161/01.hyp.25.5.1025. [DOI] [PubMed] [Google Scholar]
- 19.Hocher B, George I, Rebstock J, Bauch A, Schwarz A, Neumayer HH, Bauer C. Endothelin system–dependent cardiac remodelling in renovascular hypertension. Hypertension. 1999;33:816–822. doi: 10.1161/01.hyp.33.3.816. [DOI] [PubMed] [Google Scholar]
- 20.Sventek P, Turgeon A, Garcia R, Schiffrin EL. Vascular and cardiac overexpression of endothelin-1 gene in the one-kidney, one clip Goldblatt hypertensive rats but only in the late phase of two-kidney, one clip Goldblatt hypertension. J Hypertension. 1996;14:57–64. [PubMed] [Google Scholar]
- 21.Casellas D, Bouriquet N, Herizi A. Bosentan prevents preglomerular alterations during angiotensin II hypertension. Hypertension. 1997;30:1613–1620. doi: 10.1161/01.hyp.30.6.1613. [DOI] [PubMed] [Google Scholar]
- 22.Gardiner SM, March JE, Kemp PA, Mullins JJ, Bennett T. Haemodynamic effects of losartan and the endothelin antagonist, SB 209670, in conscious, transgenic ((mRen-2)27), hypertensive rats. Br J Pharmacol. 1995;116:2237–2244. doi: 10.1111/j.1476-5381.1995.tb15059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu C, Baylis C. Endothelin and angiotensin mediate glomerular responses to nitric oxide inhibition. Kidney Int. 1999;55:2390–2396. doi: 10.1046/j.1523-1755.1999.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baylis C. Renal responses to acute angiotensin II (AII) inhibition and administered AII in the aging, conscious chronically catheterized rat. Am J Kid Dis. 1993;22:842–850. doi: 10.1016/s0272-6386(12)70344-x. [DOI] [PubMed] [Google Scholar]
- 25.Baylis C, Engels K, Harton P, Samsell L. The acute effects of endothelial derived relaxing factor (EDRF) blockade in the normal, conscious rat are not due to angiotensin II (AII). Am J Physiol. 1993;264:F74–F78. doi: 10.1152/ajprenal.1993.264.1.F74. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman A, Abassi ZA, Brodsky S, Ramadan R, Winaver J. Mechanisms of big endothelin-1-induced diuresis and natriuresis: role of ETB receptors. Hypertension. 2000;35:732–739. doi: 10.1161/01.hyp.35.3.732. [DOI] [PubMed] [Google Scholar]
- 27.Dohi Y, Hahn AW, Boulanger CM, Buhler FR, Luscher TF. Endothelin stimulated by angiotensin II augments contractility of spontaneously hypertensive rat resistance arteries. Hypertension. 1992;19:131–137. doi: 10.1161/01.hyp.19.2.131. [DOI] [PubMed] [Google Scholar]
- 28.Oriji GK, Keiser HR. Protein kinase C mediates angiotensin II-induced contractions and the release of endothelin and prostacyclin in rat aortic rings. Prostaglandins Leukot Essent Fatty Acids. 1997;57:135–41. doi: 10.1016/s0952-3278(97)90003-x. [DOI] [PubMed] [Google Scholar]