Abstract
Life-long neurogenesis is a characteristic feature of the olfactory pathways of a phylogenetically diverse array of animals. In both vertebrates and invertebrates, the life-long addition of olfactory interneurons in the brain occurs in parallel with the continuous proliferation of olfactory receptor neurons in the olfactory organ. It has been proposed that these two processes are related functionally, with new olfactory interneurons being added to accommodate the new olfactory receptor neurons added in the periphery. While this has not been tested directly because the two processes are not readily separable, this question can be addressed in the olfactory pathway of the crab, Libinia emarginata. Unlike most decapod crustaceans, which moult and grow throughout life, L. emarginata has a terminal, maturational moult after which animals become anecdysic (stop moulting). Because the addition of new receptor neurons in crustaceans is associated with moulting, a comparison of neurogenesis in immature and mature L. emarginata provides an opportunity to examine the interdependence of central and peripheral neurogenesis in the olfactory pathway. This study demonstrates that the continuous addition of olfactory receptor neurons in L. emarginata ceases at the terminal moult but that proliferation and differentiation of olfactory interneurons in the brain continues in mature animals. Contrary to the general assumption, therefore, continuous neurogenesis in the central olfactory pathway of this species does not occur as part of a process involving the coregulation of central and peripheral neurogenesis. These findings suggest that peripheral neurogenesis is not a requirement for continuous neurogenesis in the central olfactory pathway.
Keywords: development, differentiation, Libinia emarginata, olfaction, olfactory receptors
Introduction
Neuronal proliferation and survival within innervating and target neuronal populations in many developing systems are coregulated to achieve numerical or functional balance (Sanes et al., 2000). While most neuronal populations are established during embryogenesis, lifelong neurogenesis amongst interneurons innervating the primary olfactory brain neuropil is a characteristic feature of the brains of a phylogenetically diverse array of taxa. Olfactory interneurons are continually added to the brains of adult decapod crustaceans (Beltz & Sandeman, 2003) as they are in adult fish (Byrd & Brunjes, 2001), reptiles (Font et al., 2001) and mammals (Corotto et al., 1993; Lois & Alvarez-Buylla, 1994). In each of these taxa, this life-long addition of olfactory interneurons occurs in parallel with the continuous proliferation and turnover of olfactory receptor neurons (ORNs) in the olfactory organ (decapods: Sandeman & Sandeman, 1996; Steullet et al., 2000; fish: Byrd & Brunjes, 2001; mammals: Graziadei & Monti-Graziadei, 1978; Farbman, 1992). It has been proposed that these two processes may be related functionally, with new olfactory interneurons being added to accommodate the ingrowing axons of new ORNs (crustaceans: Schmidt, 1997; Sandeman et al., 1998; Harzsch et al., 1999; see review of mammalian studies by: Lledo & Saghatelyan, 2005), though this has yet to be tested directly.
Most decapod crustaceans, such as crayfish and lobsters, have indeterminate growth and continue to moult and grow throughout their lifetimes, which can span several decades (Cooper & Uzmann, 1980). With each moult new olfactory sensilla are added to their olfactory organs (Sandeman & Sandeman, 1996; Steullet et al., 2000; Harrison et al., 2001) and growth of these animals is also accompanied by the continuous addition of both new ORNs and new olfactory interneurons in the brain (Beltz & Sandeman, 2003). While indeterminate growth is characteristic of most decapod crustaceans, individuals of some crab taxa become anecdysic (stop moulting) once they have become mature (Hartnoll, 1963). If, as proposed, the continuous addition of olfactory interneurons is a mechanism by which the central olfactory pathway accommodates the addition of ORNs, it would follow that the proliferation of olfactory interneurons in crabs with determinate growth would not continue beyond the terminal moult when the turnover of ORNs ceases. Such crabs offer, therefore, a unique opportunity to examine the dependency of adult neurogenesis of olfactory interneurons on continuous neurogenesis in the olfactory organ.
In the present study, we addressed this question by examining neurogenesis in the olfactory pathway of immature and mature Libinia emarginata, a crab that undergoes determinate growth and has a terminal, maturational moult accompanied by visible morphological changes (Hinsch, 1972). We demonstrate that although the continuous addition of ORNs ceases at the terminal moult of L. emarginata, the proliferation of olfactory interneurons in the brain continues in mature animals. Life-long neurogenesis in the central olfactory pathway of this species, therefore, does not occur as part of a process involving the coregulation of developing innervating and target populations, demonstrating that continuous proliferation of ORNs is not a requirement for life-long neurogenesis amongst interneurons in the central olfactory pathway.
Materials and methods
Animals
Immature (pre terminal moult) and mature spider crabs, Libinia emarginata (Malacostraca, Decapoda, Brachyura), of both sexes were obtained from the Aquatic Resources Center at the Marine Biological Laboratories (Woods Hole, MA, USA) and maintained at 14 °C in aquaria with circulating artificial seawater and a light: dark cycle of 12: 12 h. Immature spider crabs undergo a terminal, maturational moult following which the glands involved in the synthesis of moulting hormones degenerate (Carlisle, 1957; Chaix et al., 1976; Laufer et al., 1997) and the animals become incapable of further growth. Both male and female L. emarginata undergo pronounced morphological changes during their terminal moults with the claws of the male and the abdomen of the female increasing markedly in size (Hinsch, 1972; Rottlant & Takac, 1999; Laufer et al., 2002). Immature and mature animals are therefore readily distinguishable from one another.
In vivo bromodeoxyuridine labelling
Proliferating neurons within the olfactory pathway were labelled using the substitute nucleoside bromodeoxyuridine (BrdU). BrdU (Sigma, St Louis, MO, USA) was dissolved in crab saline (in mM: NaCl, 440;KCl, 11; CaCl2, 13; MgCl2, 26; glucose, 8; Trizma base, 11; maleic acid, 5; pH 7.4) at a concentration of 3 mg/mL and injected into the haemolymph of the crabs (1 mL per 100 g body weight) via the base of the last walking leg. Crabs received either a single injection or weekly injections over 2–4 months and were killed 24 h after the final injection. To reveal BrdU incorporation, animals were anaesthetized on ice and then decapitated. Brains and first antennae (the olfactory organ) were removed under cold crab saline and fixed for 1–2 days in 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) at 4 °C. Subsequently, preparations were rinsed for 4 h in PB, suspended in 6% Noble agar (DIFCO, Detroit, MI, USA), and sectioned at 100 μm on a vibratome (Technical Products, St Louis, MO, USA). Tissue sections were rinsed in PB containing 0.3% Triton X-100 (PBTx) for 1 h then incubated in 2 n HCl for 20 min. The sections were then rinsed for 1 h in several changes of PBTx, incubated for 150 min at room temperature in a mouse anti-BrdU primary antibody (1: 100; Amersham, Arlington Heights, IL, USA) and then rinsed for 1 h in PBTx. In some preparations, brain sections were then incubated overnight at 4 °C in a mouse anti-Drosophila synapsin primary antibody (SYNORF1, provided by E. Buchner, Universität Würzburg, Germany) diluted 1: 50 in PBTx. All sections were then incubated overnight at 4 °C in a goat antimouse Alexa 488 secondary antibody (Molecular Probes, Eugene, OR, USA) diluted 1: 50 in PBTx. Antennal sections were counterstained with the nuclear stain propidium iodide (25 μg/mL in PB) for 15 min. Subsequently, sections were rinsed for 2 h in PB and mounted in Gelmount (Biømeda, Foster City, CA, USA).
Olfactory interneuron labelling and BrdU immunocytochemistry
In order to determine whether newborn cells in the central olfactory pathways of immature and mature L. emarginata differentiate into neurons, BrdU labelling of proliferating cells was combined with a neuronal marker (crustacean-SIFamide) and neuronal tract-tracing methods (dextran dyes). Crustacean-SIFamide is a newly described neuropeptide (Yasuda et al., 2004) and we have shown that large numbers of newborn cells in adult crayfish Cherax destructor differentiate into olfactory projection neurons expressing this peptide (Sullivan & Beltz, 2005). This differentiation occurs over 4–5 months. Preliminary studies showed that many of the olfactory projection neurons of L. emarginata also express crustacean-SIFamide. Dextran dyes were applied to the main olfactory brain region, the olfactory lobe, to label the interneurons innervating this neuropil (Sullivan & Beltz, 2005).
Immature and mature L. emarginata received two injections of BrdU (3 mg/mL in crab saline; 1 mL per 100 g body weight), 1 week apart, into the base of the last walking leg. Crabs were then placed into aquaria with circulating artifical seawater for 6 months, during which time they were fed thrice weekly with prawns. Subsequently, brains were dissected from the animals in cold crab saline and desheathed in the region surrounding the olfactory lobe. Projection neurons innervating the lobe were labelled using dextran fluorescein, mol. wt. 3000 (micro-emerald; Molecular Probes) applied using the technique of Utting et al. (2000). Briefly, a small dextran crystal was dissolved in 1 μL of distilled water containing 2% bovine serum albumin and left to dry. Upon the reapplication of water to this mixture it develops a paste-like consistency and was used to coat the tips of glass electrodes. The dextran-coated tips were then dipped into molten embedding wax and left to cool. The wax coating prevents the highly soluble dextrans from going into solution before they are applied to the brain. The coated electrode tips were then placed into the olfactory lobe and moved from side to side to dislodge the wax and bring the dextrans into contact with the neuropil. Electrodes were applied at locations distributed throughout the lobe in order to label as many olfactory projection neurons as possible.
Following the dextran applications, brains were incubated in the dark for 150 min at room temperature in L-15 medium (Sigma) altered to be iso-osmotic with crab saline. The brains were then fixed, sectioned, and processed for BrdU immunocytochemistry as described above. Subsequently, tissue sections were then incubated overnight at 4 °C in rabbit anticrustacean-SIFamide (1: 12 000; a generous gift from Dr Akikazu Yasuda, Suntory Institute for Bioorganic Research, Osaka, Japan). Following incubation in the primary antibodies, sections were rinsed for 4 h in PBTx and then incubated overnight at 4 °C in goat antirabbit Alexa 594 (1: 50; Molecular Probes) and goat antimouse Cy5 (1: 100; Jackson ImmunoResearch, West Grove, PA, USA) secondary antibodies. Sections were then rinsed for 2 h in PBTx and mounted in Gelmount.
Confocal microscopy and image processing
Specimens were viewed using a Leica TCS SP laser-scanning confocal microscope equipped with argon, krypton and helium–neon lasers. Serial optical sections were taken at intervals of 0.75–1 μm and saved as both three-dimensional stacks and two-dimensional projections. Individual neurons were considered to be double-labelled when a BrdU-labelled nucleus was observed to be completely surrounded by an immunocytochemically or dextran-labelled soma. Images were processed to adjust brightness and contrast using Adobe Photoshop 7.0 (Adobe Systems).
Results
Organization of the olfactory pathway of the crab
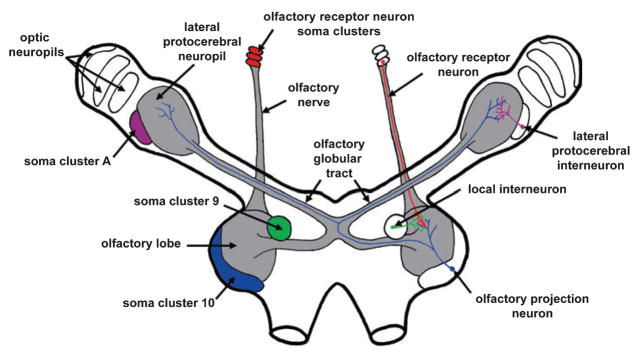
The olfactory pathway of the crab originates in the olfactory organ, which is comprised of specialized sensilla (aesthetascs), located on the first antennae, that are innervated by clusters of olfactory receptor neurons. The axons of the olfactory receptor neurons project ipsilaterally to the brain where they terminate within the glomerular neuropil of the olfactory lobe, the functional homologue of the vertebrate olfactory bulb (Fig. 1). The olfactory lobe glomeruli are sites of synaptic contact between the olfactory receptor neurons, local interneurons and projection neurons. The somata of the local interneurons are found in a cluster medial to the olfactory lobe (soma cluster 9) while those of the projection neurons reside within a densely packed cluster (soma cluster 10) that lies lateral and caudal to the olfactory lobe (Fig. 1; terminology from Sandeman et al., 1992). The main output pathway from the olfactory lobe is provided by the axons of the projection neurons. These axons form a large tract, known as the olfactory globular tract, which bifurcates at the midline of the brain before projecting bilaterally to the lateral protocerebrum (Fig. 1). The lateral protocerebrum contains a higher-order integrative neuropil that is also innervated by a population of local interneurons whose somata reside in a cluster, known as cluster A (Fig. 1; terminology from Blaustein et al., 1988). Previous studies of neurogenesis in the crabs Carcinus maenus and Carcinus pagarus have shown that neurons are added continuously to soma clusters 10 and A in the brains of adult animals (Schmidt, 1997; Schmidt & Harzsch, 1999).
Fig. 1.
Schematic diagram outlining the olfactory pathway of the crab Libinia emarginata. The axons of the olfactory receptor neurons project ipsilaterally to the brain where they terminate within the olfactory lobe. The olfactory lobe is also innervated by populations of local interneurons and projection neurons whose somata reside within soma clusters 9 and 10, respectively. The main output pathway from the olfactory lobe is provided by the projection neurons, whose axons form the olfactory globular tract. The olfactory globular tract bifurcates at the midline of the brain before projecting bilaterally to the lateral protocerebral neuropil in the eyestalk. The lateral protocerebral neuropil is innervated by a large population of local interneurons whose somata reside in a cluster, known as soma cluster A.
Neuronal proliferation in the olfactory pathways of immature crabs
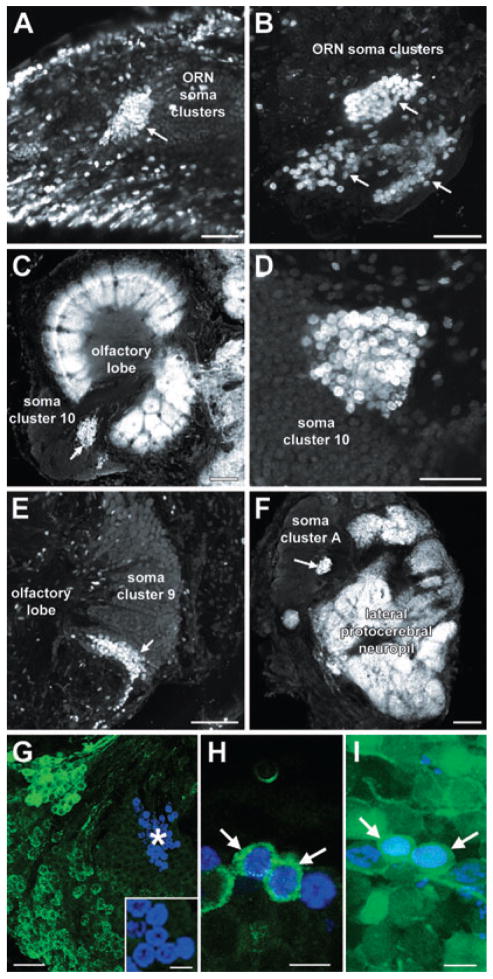
In vivo BrdU labelling in immature L. emarginata revealed the presence of clusters of BrdU-labelled cells in both the olfactory organ and the brain (Fig. 2). In the olfactory organ, elongate clusters of labelled cells were observed at both the proximal and distal margins, suggesting the presence of more than one proliferation zone in the periphery (Fig. 2A and B). Within the brain, clusters of labelled cells were observed within each of the soma clusters associated with the central olfactory pathway: soma clusters 9, 10 and A (Fig. 2C–F). These labelled cells occurred within proliferation zones located in characteristic positions within the soma clusters (Fig. 2C–F). In order to determine whether BrdU-labelled cells within the brain differentiate into neurons, juvenile L. emarginata were injected with BrdU to label proliferating cells and then left for 6 months to enable these cells to differentiate. Subsequent labelling of the brains of these animals for BrdU and crustacean-SIFamide (Fig. 2G and H) and dextran (Fig. 2I) revealed the presence of numerous double-labelled cells, indicating that many of the newborn cells had differentiated into neurons innervating the olfactory lobe.
Fig. 2.
BrdU labelling in the olfactory pathway of immature animals. ORN, olfactory receptor neuron. (A and B) Clusters of BrdU-labelled cells (arrows) at (A) the distal and (B) proximal margins of the olfactory organ. (C–F) Clusters of BrdU-labelled cells (arrows) within (C and D) soma cluster 10, (E) soma cluster 9 and (F) soma cluster A. The olfactory lobe glomeruli (C) and lateral protocerebral neuropil (F) are labelled with an antibody against Drosophila synapsin. (G) BrdU (blue) and crustacean-SIFamide (green) labelling in cluster 10 1 day after the injection of BrdU. Note the absence of crustacean-SIFamide-immunoreactive cells in and around the proliferation zone of cluster 10 (*), indicating that crustacean-SIFamide labelling is specific to mature neurons. The inset shows a higher magnification view of BrdU-labelled cells within the proliferation zone, showing the absence of crustacean-SIFamide labelling in these cells. (H) BrdU (blue) and crustacean-SIFamide (green) labelling in cluster 10, 6 months after the injection of BrdU. The arrows identify neurons double-labelled for BrdU and crustacean-SIFamide. (I) BrdU (blue) and dextran (green) labelling in cluster 10, 6 months after the injection of BrdU. The dextran dye was applied to the olfactory lobe to label the somata of interneurons innervating this neuropil. The arrows identify double-labelled neurons. Scale bars, 50 μm (A, B, D and G), 100 μm (C, E and F), 10 μm (H and I, and inset in G).
Neuronal proliferation in the olfactory pathways of mature crabs
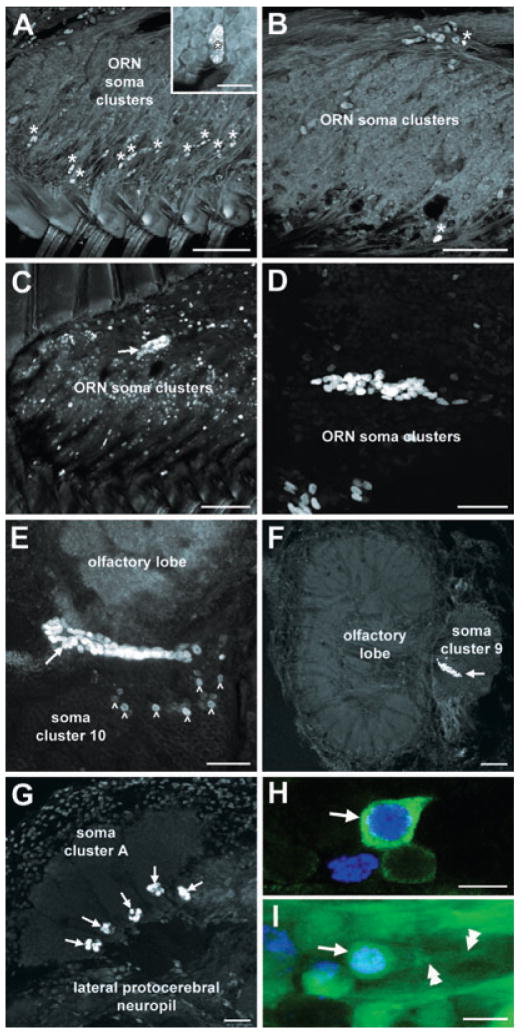
In most of the mature crabs examined (n = 7 of 8), the olfactory organ did not contain clusters of BrdU-labelled cells though numerous labelled glial cell nuclei, distinguishable by their characteristic size, shape and distribution (Harrison et al., 2001; Hollins et al., 2003), could be observed scattered throughout (Fig. 3A and B); the identity of glial cells in the olfactory organ has been confirmed using molecular markers (Hollins et al., 2003). In one mature crab, however, a small cluster of BrdU-labelled cells was observed towards the distal margin of one of the olfactory organs (Fig. 3C). The rarity with which labelled cells were observed within the olfactory epithelia of mature crabs, and the fact that in the one animal in which they were observed they were only present unilaterally, suggests that new olfactory receptor neurons are not added continuously in mature crabs but that the olfactory organ nevertheless retains the capacity to produce new neurons. Localized addition of olfactory receptor neurons has been described in the spiny lobster Panulirus argus as a regenerative response to aesthetasc ablation (Harrison et al., 2004). We therefore decided to examine whether the olfactory organ of mature L. emarginata has a similar capacity. To assess regenerative abilities, the aesthetascs of mature crabs (n = 3) were removed at their bases from both first antennae using fine forceps. The animals were then left for a period of 7–9 days during which they were injected with BrdU on alternate days. The antennae were then removed from the animals and processed for BrdU immunocytochemistry. Clusters of BrdU-labelled cells were observed within the olfactory organ of each of the animals examined, often at sites distant from the sites at which the proliferation zones in the olfactory organs of immature crabs occur (Fig. 3D). Taken together, these observations show that the continuous addition of new olfactory receptor neurons to the olfactory organ ceases at the terminal moult, but that quiescent precursor cells are present in the olfactory organs of mature animals and that they can be activated by damage to the organ.
Fig. 3.
BrdU labelling in the olfactory pathway of mature animals. ORN, olfactory receptor neuron. (A and B) Clusters of BrdU-labelled cells were not observed either (A) distally or (B) proximally within the olfactory organs of mature animals, though numerous labelled glial cells (*) could be observed. The inset in A shows the elongated nucleus of a labelled glial cell (*) surrounded by the smaller, round nuclei of unlabelled olfactory receptor neurons. (C) BrdU-labelled cells observed in the olfactory organ of a mature crab (n = 1 of 8) (D) Cluster of BrdU-labelled cells in the olfactory organ of a mature crab following aesthetasc ablation, a procedure designed to examine regenerative responses. This cluster was located midway along the olfactory organ. (E) BrdU labelling in soma cluster 10 of a mature animal injected with BrdU both 4 months and 24 h before being killed. Cells labelled by the first injection (arrowheads) are present amongst the other cells in the cluster while those labelled by the second injection (arrow) occur within the proliferation zone, adjacent to the olfactory lobe. (F and G) Clusters of BrdU-labelled cells (arrows) in (F) soma cluster 9 and (G) soma cluster A of mature crabs. (H) BrdU (blue) and crustacean-SIFamide (green) labelling in cluster 10 of a mature crab injected 6 months earlier with BrdU. The arrow identifies the soma of a double-labelled neuron. (I) BrdU (blue) and dextran (green) labelling in cluster 10 of a mature animal 6 months after the injection of BrdU. The dextran dye was applied to the olfactory lobe. The soma of a double-labelled neuron is identified by the arrow and its primary neurite by the arrowheads. Scale bars: 100 μm (A–C and G), 50 μm (D–F), 10 μm (H and I), 20 μm (inset in A).
In contrast to the absence of neurogenesis in the proliferation zones of the olfactory organs of mature crabs, robust neurogenesis within the central olfactory pathway was observed in all of the animals examined. Clusters of BrdU-labelled cells were observed in soma clusters 9, 10 and A of mature crabs (Fig. 3E–G), as in immature animals. Experiments examining the differentiation of newborn cells in the brains of mature L. emarginata revealed the presence of numerous cells double-labelled for BrdU and crustacean-SIFamide (Fig. 3H) or BrdU and dextran (Fig. 3I) 6 months after the injection of BrdU. These observations indicate that newborn cells in mature crabs survive to become functional olfactory interneurons. Furthermore, they indicate that neurogenesis in the central olfactory pathway of mature crabs occurs in the absence of neuronal proliferation in the olfactory organ.
Discussion
Although the widespread occurrence of adult neurogenesis in both invertebrate and vertebrate species suggests that this phenomenon plays a fundamental role in adult brain function, the nature of this role remains unclear. The goal of the present study was to test whether one of the primary functions of adult neurogenesis in the central olfactory pathway is to match the continuous proliferation of olfactory receptor neurons that occurs throughout life in most animals. Does proliferation amongst the afferent neurons drive the proliferation of central target neurons? To address this question, we examined peripheral and central neurogenesis in the olfactory pathway of a crab (Libinia emarginata) known to undergo determinate growth (Hinsch, 1972). As the addition of olfactory sensilla (aesthetascs) in crustaceans is associated with moulting (Sandeman & Sandeman, 1996; Steullet et al., 2000; Harrison et al., 2001), a comparison of the patterns of neurogenesis in immature and mature L. emarginata enabled an examination of the interdependence of peripheral and central neurogenesis in the olfactory pathway of this animal. These studies demonstrate that the continuous addition of new olfactory receptor neurons in L. emargi-nata ceases at the terminal, maturational moult but that the proliferation of olfactory interneurons in the brain continues in mature animals. Continuous neurogenesis of olfactory receptor neurons, therefore, is not a requirement in this species for life-long neurogenesis amongst olfactory interneurons in the brain.
Growth in crustaceans is restricted to the period after moulting when the old exoskeleton is shed and the soft, new exoskeleton is able to expand. The addition of new aesthetascs and olfactory receptor neurons in these animals is also associated with the moult cycle (Sandeman & Sandeman, 1996; Steullet et al., 2000; Harrison et al., 2001). The observation that the continuous addition of olfactory receptor neurons in L. emarginata ceases at the terminal moult is consistent therefore with our understanding of crustacean postembry-onic development. Regeneration in crustaceans is also linked with moulting, and anecdysic animals, unlike other crustaceans, are unable to regenerate lost or damaged limbs or cuticular sensilla such as aesthetascs (Skinner, 1985). Therefore, we did not expect that regenerative responses could be induced in the olfactory organs of mature crabs by damage to the aesthetascs. Localized aesthetasc ablation in spiny lobsters, which have indeterminate growth, has also been shown to result in localized proliferation of olfactory receptor neurons within the affected regions (Harrison et al., 2004). Aesthetasc ablation in these animals does not, however, influence the rate of neurogenesis within the proliferation zone of the olfactory organ and the localized proliferation seen following ablation is not observed in undamaged animals, suggesting that it is specific to injury (Harrison et al., 2004). Similarly, clusters of BrdU-labelled cells observed in the olfactory organs of mature L. emarginata following aesthetasc ablation were found in regions outside the sites at which the proliferation zones of immature crabs occur. The rarity with which neurogenesis was observed in the olfactory organs of mature crabs suggests that, as in spiny lobsters, these precursor cells are normally quiescent in undamaged animals and are functionally distinct from those present in the proliferation zones.
Studies of neurogenesis in crustaceans with indeterminate growth have shown that while the numbers of olfactory lobe glomeruli remain constant beyond early postembryonic development (Helluy et al., 1996), as in the mammalian olfactory bulb (Pomeroy et al., 1990; La Mantia et al., 1992), neurogenesis of olfactory interneurons occurs throughout the lifetimes of these animals. These newborn neurons survive for at least a year and become incorporated into the circuitry of the olfactory pathway (Schmidt, 2001; Sullivan & Beltz, 2005), as they do in mammals (Carleń et al., 2002; Winner et al., 2002; Belluzzi et al., 2003). Similarly, pulse-chase BrdU experiments in mature L. emarginata demonstrate that after several months many newborn cells in the central olfactory pathway express the neuropeptide crustacean-SIFamide and have arbors within the olfactory lobe, indicating that these cells have differentiated into functioning neurons. Together, these results suggest that adult neurogenesis in the central olfactory pathway of L. emarginata is the same process as that observed in animals such as crustaceans with indeterminate growth and in vertebrates, in which life-long neurogenesis also occurs in the olfactory organ. We propose, therefore, that the functional independence of central and peripheral neurogenesis observed in L. emarginata may also be a feature of adult neurogenesis in other taxa.
Experimental studies in adult decapod crustaceans and mammals have shown that both the proliferation and survival of olfactory interneurons can be regulated by physiological activity in the olfactory receptor neurons and by their physical presence (decapod crustaceans: Sandeman et al., 1998; Hansen & Schmidt, 2001; insects: Scotto-Lomassese et al., 2002; Cayre et al., 2005; mammals: Corotto et al., 1994; Petreanu & Alvarez-Buylla, 2002; Rochefort et al., 2002; Mandairon et al., 2003). While these studies demonstrate, therefore, that the peripheral olfactory system plays a role in modulating central neurogenesis in adult organisms, the results of the present study suggest that continuous peripheral neurogenesis need not be the factor that drives it.
Acknowledgments
We thank D. Sandeman and J. Benton for critical readings of versions of this article, A. Yasuda and E. Buchner for kindly providing antibodies, and P. Carey and V. Quinan for technical assistance. This study was supported by NSF/IBN 0091092.
Abbreviations
- BrdU
bromodeoxyuridine
- ORN
olfactory receptor neuron
- PB
0.1 M phosphate buffer
- PBTx
0.1 M phosphate buffer containing 0.3% Triton X-100
References
- Belluzzi O, Benedusi M, Ackman J, LoTurco JL. Electro-physiological differentiation of new neurons in the olfactory bulb. J Neurosci. 2003;23:10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz BS, Sandeman DC. Regulation of life-long neurogenesis in the decapod crustacean brain. Arthropod Struct Dev. 2003;32:175–188. doi: 10.1016/S1467-8039(03)00038-0. [DOI] [PubMed] [Google Scholar]
- Blaustein DN, Derby CD, Simmons RB, Beall AC. Structure of the brain and medulla terminalis of the spiny lobster Panulirus argus and the crayfish Procambarus clarkii, with an emphasis on olfactory centers. J Crust Biol. 1988;8:493–519. [Google Scholar]
- Byrd CA, Brunjes PC. Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience. 2001;105:793–801. doi: 10.1016/s0306-4522(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Carlén M, Casssidy RM, Brismar H, Smith GA, Enquist LW, Frisén J. Functional integration of adult-born neurons. Curr Biol. 2002;12:606–608. doi: 10.1016/s0960-9822(02)00771-6. [DOI] [PubMed] [Google Scholar]
- Carlisle DB. On the hormonal inhibition of molting in decapod Crustacea. II Terminal anecdysis in crabs. J Marine Biol Ass UK. 1957;36:291–307. [Google Scholar]
- Cayre M, Malaterre J, Scotto-Lomassese S, Holstein GR, Martinelli GP, Forni C, Nicolas S, Aouane A, Strambi C, Strambi A. A role for nitric oxide in sensory-induced neurogenesis in an adult insect brain. Eur J Neurosci. 2005;21:2893–2902. doi: 10.1111/j.1460-9568.2005.04153.x. [DOI] [PubMed] [Google Scholar]
- Chaix JC, Trilles JP, Vernet G. Dégénérescence de l’organe Y chez les males pubeères d’Acanthonyx lunulatus. C R Acad Sci Paris (D) 1976;283:7–14. [PubMed] [Google Scholar]
- Cooper RL, Uzmann JR. Ecology of juvenile and adult Homarus. In: Cobb JS, Phillips BF, editors. The Biology and Management of Lobsters. Vol. 2. Academic Press; New York: 1980. pp. 97–142. [Google Scholar]
- Corotto FS, Henegar JA, Maruniak JA. Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci Lett. 1993;149:111–114. doi: 10.1016/0304-3940(93)90748-a. [DOI] [PubMed] [Google Scholar]
- Corotto FS, Henegar JR, Maruniak JA. Odor deprivation leads to reduced neurogenesis and reduced neuronal survival in the olfactory bulb of the adult mouse. Neuroscience. 1994;61:739–744. doi: 10.1016/0306-4522(94)90397-2. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Cell Biology of Olfaction. Cambridge University Press; Cambridge: 1992. [Google Scholar]
- Font E, Desfilis E, Pérez-Cañellas MM, García-Verdugo JM. Neurogenesis and neuronal regeneration in the adult reptilian brain. Brain Behav Evol. 2001;58:276–295. doi: 10.1159/000057570. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti-Graziadei GA. Continuous nerve cell renewal in the olfactory system. In: Jacobson M, editor. Handbook of Sensory Physiology, vol 9, Development of Sensory Systems . Springer; New York: 1978. pp. 55–83. [Google Scholar]
- Hansen A, Schmidt M. Neurogenesis in the central olfactory pathway of the adult shore crab Carcinus maenas is controlled by sensory afferents. J Comp Neurol. 2001;441:223–233. doi: 10.1002/cne.1408. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Cate HS, Derby CD. Localized ablation of olfactory receptor neurons induces both localized regeneration and widespread replacement of neurons in spiny lobsters. J Comp Neurol. 2004;471:72–84. doi: 10.1002/cne.20020. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Cate HS, Swanson ES, Derby CD. Postembryonic proliferation in the spiny lobster antennular epithelium: rate of genesis of olfactory receptor neurons is dependent on moult stage. J Neurobiol. 2001;47:51–66. doi: 10.1002/neu.1015. [DOI] [PubMed] [Google Scholar]
- Hartnoll RG. The biology of Manx spider crabs. Proc Zool Lond. 1963;141:423–469. [Google Scholar]
- Harzsch S, Miller J, Benton J, Beltz B. From embryo to adult: persistent neurogenesis and apoptotic cell death shape the lobster deutocerebrum. J Neurosci. 1999;19:3472–3485. doi: 10.1523/JNEUROSCI.19-09-03472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helluy SM, Benton JL, Langworthy KA, Ruchhoeft ML, Beltz BS. Glomerular organization in developing olfactory and accessory lobes of American lobsters: stabilization of numbers and increase in size after metamorphosis. J Neurobiol. 1996;29:459–472. doi: 10.1002/(SICI)1097-4695(199604)29:4<459::AID-NEU4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hinsch GW. Some factors controlling reproduction in the spider crab, Libinia emarginata. Biol Bull. 1972;143:358–366. doi: 10.2307/1540059. [DOI] [PubMed] [Google Scholar]
- Hollins B, Hardin D, Gimelbrant AA, McClintock TS. Olfactory-enriched transcripts are cell-specific markers in the lobster olfactory organ. J Comp Neurol. 2003;455:125–138. doi: 10.1002/cne.10489. [DOI] [PubMed] [Google Scholar]
- La Mantia AS, Pomeroy SL, Purves D. Vital imaging of glomeruli in the mouse olfactory bulb. J Neurosci. 1992;12:976–988. doi: 10.1523/JNEUROSCI.12-03-00976.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer H, Ahl J, Rotllant G, Baclaski B. Evidence that ecdysteroids and methyl farnesoate control allometric growth and differentiation in a crustacean. Insect Biochem Mol Biol. 2002;32:205–210. doi: 10.1016/s0965-1748(01)00104-7. [DOI] [PubMed] [Google Scholar]
- Laufer H, Takac P, Ahl JSB, Laufer MR. Methyl farnesoate and the effect of eyestalk ablation on the morphogenesis of the juvenile female spider crab Libinia emarginata. Invert Reprod Dev. 1997;31:63–68. [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Jourdon F, Didier A. Deprivation of sensory inputs to the olfactory bulb up-regulates cell death and proliferation in the subventricular zone of adult mice. Neuroscience. 2003;119:507–516. doi: 10.1016/s0306-4522(03)00172-6. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy SL, La Mantia AS, Purves D. Postnatal construction of neural circuitry in the mouse olfactory bulb. J Neurosci. 1990;10:1952–1966. doi: 10.1523/JNEUROSCI.10-06-01952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottlant G, Takac P. Ecdysones in the maturational moult of juvenile females of the spider crab, Libinia emarginata Leach, 1815 (Decapoda, Majidae) Crustaceana. 1999;72:221–231. [Google Scholar]
- Sandeman R, Clarke D, Sandeman D, Manly M. Growth-related and antennular amputation-induced changes in the olfactory centers of crayfish brain. J Neurosci. 1998;18:6195–6206. doi: 10.1523/JNEUROSCI.18-16-06195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeman RE, Sandeman DC. Pre- and postembryonic development, growth and turnover of olfactory receptor neurones in crayfish antennules. J Exp Biol. 1996;199:2409–2418. doi: 10.1242/jeb.199.11.2409. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Sandeman R, Derby C, Schmidt M. Morphology of the brain of crayfish, crabs, and spiny lobsters: a common nomenclature for homologous structures. Biol Bull. 1992;183:304–326. doi: 10.2307/1542217. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Reh TA, Harris WA. Development of the Nervous System. Academic Press; San Diego: 2000. [Google Scholar]
- Schmidt M. Continuous neurogenesis in the olfactory brain of adult shore crabs, Carcinus Maenus. Brain Res. 1997;762:131–143. doi: 10.1016/s0006-8993(97)00376-4. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Neuronal differentiation and long-term survival of newly generated cells in the olfactory midbrain of the adult spiny lobster, Panulirus argus. J Neurobiol. 2001;48:181–203. doi: 10.1002/neu.1050. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Harzsch S. Comparative analysis of neurogenesis in the central olfactory pathway of adult decapod crustaceans by in vivo BrdU-labeling. Biol Bull. 1999;196:127–136. doi: 10.2307/1542558. [DOI] [PubMed] [Google Scholar]
- Scotto-Lomassese S, Strambi C, Aouane A, Strambi A, Cayre M. Sensory inputs stimulate progenitor cell proliferation in an adult insect brain. Curr Biol. 2002;12:1001–1005. doi: 10.1016/s0960-9822(02)00889-8. [DOI] [PubMed] [Google Scholar]
- Skinner DM. Molting and reproduction. In: Bliss DE, editor. The Biology of Crustacea, Vol. 9 Integument, Pigments, and Hormonal Processes. Academic Press; Orlando: 1985. pp. 43–146. [Google Scholar]
- Steullet P, Cate HS, Derby CD. A spatio-temporal wave of turnover and functional maturation of olfactory receptor neurons in the spiny lobster, Panulirus argus. J Neurosci. 2000;20:3282–3294. doi: 10.1523/JNEUROSCI.20-09-03282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Newborn cells in the adult crayfish brain differentiate into distinct neuronal types. J Neurobiol. 2005;65:157–170. doi: 10.1002/neu.20195. [DOI] [PubMed] [Google Scholar]
- Utting M, Agricola HJ, Sandeman R, Sandeman D. Central complex in the brain of crayfish and its possible homology with that of insects. J Comp Neurol. 2000;416:245–261. [PubMed] [Google Scholar]
- Winner B, Cooper-Kuhn CM, Aigner R, Winkler J, Kuhn HG. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur J Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- Yasuda A, Yasuda-Kamatani Y, Nozaki M, Nakajima T. Identification of GYRKPPFNGSIFamide (crustacean-SIFamide) in the crayfish Procambarus clarkii by topological mass spectrometry analysis. Gen Comp Endocrinol. 2004;135:391–400. doi: 10.1016/j.ygcen.2003.10.001. [DOI] [PubMed] [Google Scholar]