Abstract
Rosacea is a common and chronic inflammatory skin disease that affects over 10 million Americans. Although the phenotypes of rosacea are clinically heterogeneous, they are all related by the presence of chronic facial skin inflammation. Until recently, the pathophysiology of this disease has been poorly understood and limited to descriptions of factors that exacerbate or improve this disorder. Recent molecular studies suggest that an altered innate immune response is involved in the pathogenesis of the vascular and inflammatory disease seen in patients with rosacea. These findings may help explain the benefits of current treatments and suggest new therapeutic strategies helpful for alleviating this disease. This article discusses the possible molecular mechanisms for the pathogenesis of rosacea from current clinical observations and laboratory research.
Introduction
Most individuals affected by rosacea are of northern European origin and up to 1/3 have a family history of the disorder [1]. The disease affects mostly facial skin and is characterized by flushing, nontransient erythema, papules, pustules, inflammatory nodules and telangiectasia. Secondary features that often occur include burning and stinging of the face, occasional dermatitis or scaling of the face, and edema. In many sufferers, rosacea can be worsened or triggered by factors that initiate flushing, such as exercise, emotion, menopause and alcohol [2]. In 2002, the National Rosacea Society Expert Committee created a standard classification system for rosacea [3] and grading system in 2004 [4]. The purpose of the committee is to develop a standard system that can serve as an instrument to investigate the manifestation of rosacea for both clinician and researchers.
Since the phenotypes of rosacea are clinically heterogeneous, rosacea studies were diversely conducted based on the findings in clinical manifestations, histology, and factors exacerbating the skin disorder. From the diverse findings, the pathology of rosacea was thought be ‘unknown’ and was expected to be from multiple factors. We recently reported findings of a consistently aberrant innate immune response in rosacea. The multiple factors that lead to a trigger of the innate immune system would explain the diverse findings on rosacea etiology and help to understand why the current therapies are effective. This article attempts to organize the possible pathology of rosacea by connecting proposed mechanisms through the window of the innate immune system. We categorized pathological mechanisms of rosacea in a) innate immunity, b) vascular changes, c) reactive oxygen species, d) ultra violet radiation, and e) microbes. These molecular events can now be linked to each other with our current knowledge of innate immunity.
Innate immunity
We have proposed the hypothesis that a dysregulation of the innate immune system in patients with rosacea could unify current clinical observations. In innate immunity, the pattern recognition system, which includes the TLR (toll-like receptor) and NLR (nucleotide-binding domain and leucine-rich repeat-containing) families, respond to environmental stimuli such as UV, microbes, physical and chemical trauma. Triggering the innate immune system normally leads to a controlled increase in cytokines and antimicrobial molecules in the skin [5, 6]. One of these antimicrobial molecules is a peptide known as cathelicidin [7]. Some forms of cathelicidin peptides were known to have a unique capacity to be both vasoactive and proinflammatory. Therefore, given the potential for a single molecule to affect both of the events that describe rosacea, we began an analysis of cathelicidin in rosacea. Individuals with rosacea expressed abnormally high levels of cathelicidin [8]. Importantly, the cathelicidin peptide forms found in rosacea were not only more abundant but were different from those in normal individuals. These forms of cathelicidin peptides promote and regulate leukocyte chemotaxis [9], angiogenesis [10], and expression of extracellular matrix components [11]. The presence of the vasoactive and inflammatory cathelicidin peptides in rosacea was subsequently explained by abnormal production of local protease kallikrein 5 (KLK5), which controls the production of cathelicidin peptides in epidermis [8, 12]. To confirm the importance of these observations and test the hypothesis that abnormal cathelicidin could induce the signs of rosacea, we injected these peptides or the enzymes that produce cathelicidin into the skin of mice. This rapidly resulted in skin inflammation resembling pathological changes in rosacea, therefore confirming our hypothesis [8]. Combined, these findings indicated that an exacerbated innate immune response induces abnormal cathelicidin, and that this then leads to the clinical findings.
Normally, the innate immune system of the skin is programmed to detect microbes, tissue damage such as UV-induced apoptosis, or damage of the extracellular matrix [13, 14]. As described above, sun exposure, dermal matrix changes and microbes have been shown to be triggers of rosacea. Our preliminary data showed that TLR2 expression is altered in rosacea skin, which enhances skin susceptiblity to innate immune stimuli and leads to increased cathelicidin and kallikrein production [15]. Interestingly, TLR2 involvement in other disorders is also suggested by the clinical findings in glucocorticoid inducing rosacea-like dermatitis, so-called perioral dermatitis [16-19]. Although the precise molecular mechanisms of the steroid-induced dermatitis is not determined, Shibata et al recently reported that glucocorticoid increases TLR2 expression in epidermal keratinocytes, and that P. acnes enhanced glucocorticoid-dependent TLR2 induction [20]. Thus, our new findings and accumulated knowledge on rosacea suggest the innate immune response in rosacea has gone awry. For a variety of reasons these patients are more susceptible to stimuli that do not cause inflammatory reactions in normal patients. Innate immunity is triggered by the events previously associated with worsening of the disease.
Vascular changes
Much of the previous work on the pathophysiology of rosacea has focused on attempts to make sense of associations between triggers of the disease and its clinical manifestations. Most patients report flushing episodes, thus leading to a common hypothesis that vascular hyperreactivity and increased blood play a role in the susceptibility to this disease. A few studies have demonstrated a measurable increase in blood flow in skin lesions of patients with rosacea [21, 22]. Some factors that trigger flushing such as emotional stress, spicy food, hot beverages, high environmental temperatures and menopause worsen rosacea [23], thus supporting this hypothesis. Resolution of erythema and flushing by topical α1-adrenergic receptor agonist application also supports the hypothesis that vascular hyperreactivity is major factor of rosacea pathology [24].
Elevated expression of vascular endothelial growth factor (VEGF), CD31, and lymphatic endothelium maker D2-40 are observed in the skin of patients with rosacea [25]. VEGF proliferate vascular endothelial cells as well as increase permeability of vessels. CD31 is platelet/endothelial cell adhesion molecule (PECAM1 in gene symbol), and anti-CD31 antibody recognizes the endothelial cells. Anti-D2-40 monoclonal antibody identifies a 40 kDa O-linked sialoglycoprotein and has also been demonstrated to label lymphatic endothelium whereas it is unreactive with vascular endothelium. Elevated expression of VEGF, CD31 and D2-40 in rosacea suggests rosacea skins have more stimulants for vascular and lymaphtic endothelial cells and increase endothelia cells. As discussed later, UV irradiation induces VEGF in human keratinocytes and skin [26], which could be involved in the molecular mechanism of rosacea exacerbation after sun and UV exposure. From the aspect of innate immunity, cathelicidin would be one of triggers of hyper vascularity in rosacea. Injection of cathelicidin peptides LL-37 in mouse skin induced vasodilatation [8], and application of LL-37 resulted in neovascularization in a rabbit model of hind-limb ischemia [10]. The angiogenesis by LL-37 is mediated by formyl peptide receptor-like 1 (FPRL1), a G-protein coupled receptor expressed on endothelial cells [10]. LL-37 transactivates epidermal growth factor receptor (EGFR) and downstream signaling in epithelial cells [27, 28], and EGFR signaling induces VEGF in epidermal keratinocytes [29]. Thus, cathelicidin induces endothelial cell changes through several signaling pathways, and could be a common explanation for some vascular effects.
Reactive oxygen species (ROS)
ROS involvement in rosacea pathology has been discussed as explanation for the action of medicines for rosacea treatment. Inhibition of ROS generation in neutrophils by tetracyclins [30], azelaic acid [31], metronidazole [32], and retinoids [33], which are used for rosacea treatment, provokes the hypothesis of ROS involvement in rosacea pathology. Erythromycin and azithromycin, the other effective medicine for rosacea treatment, have been shown to have antioxidant effects [34, 35]. ROS levels were examined in skin biopsy samples from rosacea and healthy individuals, and confirmed higher ROS activity in rosacea lesional skin than healthy controls [36, 37]. The decrease of ROS in rosacea skin was also observed after azithromycin treatment [36], suggesting rosacea treatments affect ROS activity and supporting the hypothesis of ROS involvement in rosacea pathology. Although the precise localization of ROS activities is not determined in rosacea skin, the source of ROS would be infiltrated leukocytes and epidermal keratinocytes.
ROS activates cellular signaling. UV radiation generates ROS and activates cellular signaling in keratinocytes [37, 38]. ROS mediates cytokine induction by TNFα in human keratinocytes [39] and chemokine production by TLR2 stimuli in monocytes [40, 41]. ROS simulate fibroblast and alters matrix metalloproteinases (MMP) and tissue inhibitor of metalloproteinases (TIMP) expression. UVA radiation increases MMP-1, and ROS increase MMP-2 mRNA and suppressed TIMP-1 in human dermal fibroblast [42]. In a three-dimensional culture, normal human dermal fibroblasts increase MMP-1 and MMP-2 mRNA expression by ROS, whereas both proalpha1(I) and proalpha1(III) collagen mRNA production were suppressed by ROS. Thus, increased ROS activity in skin would enhance inflammatory reactions and degenerate collagens and matrix in dermis.
Ultra violet radiation
UV and sun exposure are known to cause a flushing response and appears to worsen the clinical symptoms of rosacea [23]. Mechanistically, in mice, UV-B induces cutaneous angiogenesis that is histologically similar to the telangiectasia seen in rosacea histopathology [43]. In skin, epidermal keratinocytes are a major source of angiogenic factor VEGF (vascular endothelial growth factor) and FGF2 (fibroblast growth factor 2, also know as basic FGF) [29, 44]. UV-B increases VEGF and FGF2 secretion from human keratinocytes and expression in mouse epidermis [26, 43, 45]. As discussed previously, UV irradiation also produces ROS, which cause vascular and dermal matrix damage via upregulation of matrix metalloproteinases [46-48]. The abnormal and damaged dermal matrix may permit leakage and accumulation of inflammatory mediators and prolonged retention of inflammatory cells, factors which could lead to inflammation in the disease. Thus UV irradiation could induce erythema in the skin by increasing expression of angiogenic factors and by degenerating extracellular matrix.
Recent publication suggests involvement of innate immune molecules in UV-mediated cytokine and matrix metalloproteinases expression in keratinocytes. Myeloid Differentiation Factor 88 (MyD88), an essential adaptor molecule for Toll-like receptors (TLR) family signaling, increases expression in UV-irradiated human cultures keratinocytes as well as photo-aged human skin [49]. Overexpression of dominant negative form of MyD88 prevented UV-induced expressions of IL-6 and MMP-1 in human keratinocytes, whereas overexpression of dominant positive form of MyD88 increases IL-6 and MMP-1 expression. Combining with ROS involvement in chemokines production by TLR2 stimuli [40, 41], TLRs/MyD88 signaling would be the part of the link between UV irradiation to skin inflammation. The future studies of photo-aging in animals lacking TLRs signaling molecules will be of great interest.
Proteases
Although proteases involvements have not been discussed well in rosacea pathology, protease actions would be responsible for a part of rosacea histology. We identified serine protease kallikrein 5 (KLK5, also know as stratum corneum tryptic enzyme SCTE) as the processing enzyme of cathelicidin and found high KLK5 expression in rosacea skin [8, 12]. KLK5 expresses in upper epidermis (granular to cornfield cell layer) in normal skin, and rosacea skin expresses KLK5 in the entire epidermis. KLK5 digest corneodesmosome proteins desmocollin 1 and desmoglein 1 in epidermis, and suppose to affect desquamation of epidermal keratinocytes [50, 51]. KLK5 also efficiently digest the extracellular matrix components, collagens type I, II, III, and IV, fibronectin, and laminin [52]. Considering the high KLK5 expression in basal cells of rosacea epidermis, KLK5 could play a part of skin inflammatory reactions in rosacea by affecting dermal matrix and vascular remodeling.
The other supports for hypothesis of proteases involvement in rosacea pathology is evidence that tetracyclines inhibit several matrix metalloproteinases and serine proteases [53-55]. MMPs digest dermal matrixes such as collagens, fibronectin, elastin etc, and balances of MMPs and their inhibitor TIMPs dictate dermal components and vascular remodeling [56]. Although MMPs expressions in rosacea skin were not reported so far, MMPs are inducible by UV irradiation and ROS stimulation in keratinocytes and fibroblasts [42, 49], and MMP-8 (collagenase 2) and MMP-9 (gelatinase B) activities are higher in the fluid of ocular rosacea than normal subjects [54, 55]. Thus, the effective rosacea treatments might be partially dependent on their anti-protease properties.
Microbes
Two microbes have been discussed in rosacea pathology: Demodex folliculorum and Helicobacter pylori. D. folliculorum, a mite that lives within sebaceous follicles, has been implicated as a trigger of rosacea since histological studies revealed inflammation of the pilosebaceous follicle units. Studies have shown increased density of the mites in patients with rosacea compared with control patients [57-59]. Lacey et al isolated Bacillus oleronius from D. folliculorum and identified the antigens reacting to sera from rosacea individuals but not from control individuals [60]. The extracts of the B. oleronius stimulate proliferation of mononuclear cells from patients with rosacea suggesting that rosacea individuals are exposed to the B. oleronius molecules and that B. oleronius from D. folliculorum induces inflammatory in rosacea. Interestingly, they identified heat shock proteins (HSP) and a lipoprotein in the antigenic molecules of B. oleronius. HSP and lipoproteins from microbes are also known to be stimulant for TLRs [61, 62]. This report supports the hypothesis of mite and pilosebaceaous unit involvement in rosacea. Further studies are required to examine if these B. oleronius molecules evokes innate immune reaction or if rosacea is caused by adaptive immune reaction against B. oleronius and D. folliculorum.
Correlation of H. pylori infection and rosacea is controversial and inconsistent among clinical observation [63-67]. Several reports showed seropositivity to H. pylori in rosacea individuals [68]. Eradication therapy for gastric H. pylori infection showed preferable outcome for rosacea symptoms though it is not clear if the improve of rosacea is due to H. pylori eradication [69-71]. H. pylori produces ROS [72-74] and rosacea individuals showed higher ROS including NO (nitric oxide) in plasma than controls [68, 75]. H. pylori induce cytokine release through TLR2 and TLR4 in gastric epithelial cells [76, 77]. Thus ROS and cytokines released by TLRs stimuli in organs other than skin may be mediators that worsen rosacea by H. pylori infection. However, concrete molecular evidence is still required to support the involvement of H. pylori in rosacea pathology.
Summary
The newly discovered role of Cathelicidin in promotion of inflammation in rosacea creates new and exciting questions about the origins of this disease. The factors that promote cathelicidin production include innate immune molecules that to connect clinical and molecular observations (Figure). Microbes and environmental changes, such as sun and UV exposure, would be sensed by innate immune systems through pattern recognition molecules. The innate immune systems would enhance and be enhanced by cytokine, ROS, antimicrobial peptide, and proteases, which lead histological changes observed in rosacea. The multiple factors may heap up to cause rosacea clinical manifestations, while individual susceptibility to the factors is highly counted to cause rosacea. These new associations give us clues to further our understanding of the mechanisms responsible for the disease. Importantly, these advances also provide informed strategies for the optimal treatment of the clinical findings. While much work needs to be done, we hope this article facilitates future progress in basic research for the diagnosis and treatment of rosacea.
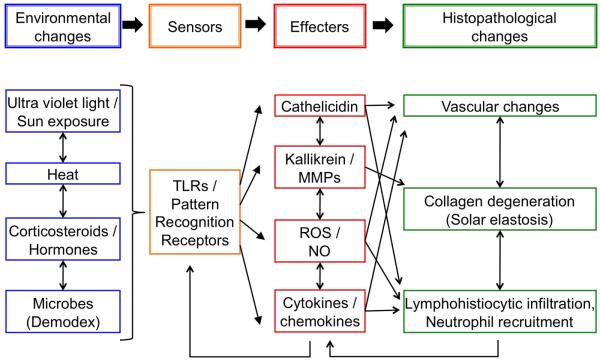
Figure. The possible molecular mechanisms for the pathogenesis of rosacea.
Environmental changes, altered hormone balances and microbe challenges are sensed by TLRs (Toll-like receptors) and other pattern recognition receptors. TLRs signaling induce effecter molecules: cathelicidin, kallikrein, MMPs (matrix metalloproteinases), ROS (reactive oxygen species), NO (nitric oxides), cytokines, and chemokines. These effectors modify the dermal structure by vascular changes and collagen degeneration accompanied with inflammatory cells recruitments. Infiltrated neutrophils and lymphocytes will be the further source of effecter molecules, which activate TLRs directly and indirectly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest in the descriptions and findings from this manuscript.
References
- 1.Del Rosso JQ. Update on rosacea pathogenesis and correlation with medical therapeutic agents. Cutis. 2006;78:97–100. [PubMed] [Google Scholar]
- 2.Crawford GH, Pelle MT, James WD. Rosacea: I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327–41. doi: 10.1016/j.jaad.2004.03.030. quiz 42-4. [DOI] [PubMed] [Google Scholar]
- 3.Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, et al. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584–7. doi: 10.1067/mjd.2002.120625. [DOI] [PubMed] [Google Scholar]
- 4.Wilkin J, Dahl M, Detmar M, Drake L, Liang MH, Odom R, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907–12. doi: 10.1016/j.jaad.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 5.Takeda K, Kaisho T, Akira S. TOLL-LIKE RECEPTORS. Annual Review of Immunology. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 6.Meylan E, Tschopp J.r., Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 7.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–7. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 8.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–80. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 9.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, et al. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci U S A. 1994;91:11035–9. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. Faseb J. 2006;20:2068–80. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 13.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–6. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 14.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–75. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 15.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sneddon I. Perioral dermatitis. Br J Dermatol. 1972;87:430–4. doi: 10.1111/j.1365-2133.1972.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 17.Weber G. Rosacea-like dermatitis: contraindication or intolerance reaction to strong steroids. Br J Dermatol. 1972;86:253–9. doi: 10.1111/j.1365-2133.1972.tb02225.x. [DOI] [PubMed] [Google Scholar]
- 18.Cotterill JA. Perioral dermatitis. Br J Dermatol. 1979;101:259–62. doi: 10.1111/j.1365-2133.1979.tb05617.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson DS, Kirton V, Wilkinson JD. Perioral dermatitis: a 12-year review. Br J Dermatol. 1979;101:245–57. doi: 10.1111/j.1365-2133.1979.tb05616.x. [DOI] [PubMed] [Google Scholar]
- 20.Shibata M, Katsuyama M, Onodera T, Ehama R, Hosoi J, Tagami H. Glucocorticoids Enhance Toll-Like Receptor 2 Expression in Human Keratinocytes Stimulated with Propionibacterium acnes or Proinflammatory Cytokines. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.237. [DOI] [PubMed] [Google Scholar]
- 21.Sibenge S, Gawkrodger DJ. Rosacea: a study of clinical patterns, blood flow, and the role of Demodex folliculorum. J Am Acad Dermatol. 1992;26:590–3. doi: 10.1016/0190-9622(92)70086-u. [DOI] [PubMed] [Google Scholar]
- 22.Guzman-Sanchez DA, Ishiuji Y, Patel T, Fountain J, Chan YH, Yosipovitch G. Enhanced skin blood flow and sensitivity to noxious heat stimuli in papulopustular rosacea. J Am Acad Dermatol. 2007 doi: 10.1016/j.jaad.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Buechner SA. Rosacea: an update. Dermatology. 2005;210:100–8. doi: 10.1159/000082564. [DOI] [PubMed] [Google Scholar]
- 24.Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369–71. doi: 10.1001/archderm.143.11.1369. [DOI] [PubMed] [Google Scholar]
- 25.Gomaa AH, Yaar M, Eyada MM, Bhawan J. Lymphangiogenesis and angiogenesis in non-phymatous rosacea. J Cutan Pathol. 2007;34:748–53. doi: 10.1111/j.1600-0560.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 26.Brauchle M, Funk JO, Kind P, Werner S. Ultraviolet B and H2O2 are potent inducers of vascular endothelial growth factor expression in cultured keratinocytes. J Biol Chem. 1996;271:21793–7. doi: 10.1074/jbc.271.36.21793. [DOI] [PubMed] [Google Scholar]
- 27.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–8. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 28.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, et al. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 29.Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, et al. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–6. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyachi Y, Yoshioka A, Imamura S, Niwa Y. Effect of antibiotics on the generation of reactive oxygen species. J Invest Dermatol. 1986;86:449–53. doi: 10.1111/1523-1747.ep12285793. [DOI] [PubMed] [Google Scholar]
- 31.Akamatsu H, Komura J, Asada Y, Miyachi Y, Niwa Y. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283:162–6. doi: 10.1007/BF00372056. [DOI] [PubMed] [Google Scholar]
- 32.Akamatsu H, Oguchi M, Nishijima S, Asada Y, Takahashi M, Ushijima T, et al. The inhibition of free radical generation by human neutrophils through the synergistic effects of metronidazole with palmitoleic acid: a possible mechanism of action of metronidazole in rosacea and acne. Arch Dermatol Res. 1990;282:449–54. doi: 10.1007/BF00402621. [DOI] [PubMed] [Google Scholar]
- 33.Yoshioka A, Miyachi Y, Imamura S, Niwa Y. Anti-oxidant effects of retinoids on inflammatory skin diseases. Arch Dermatol Res. 1986;278:177–83. doi: 10.1007/BF00412920. [DOI] [PubMed] [Google Scholar]
- 34.Bakar O, Demircay Z, Yuksel M, Haklar G, Sanisoglu Y. The effect of azithromycin on reactive oxygen species in rosacea. Clin Exp Dermatol. 2007;32:197–200. doi: 10.1111/j.1365-2230.2006.02322.x. [DOI] [PubMed] [Google Scholar]
- 35.Jain A, Sangal L, Basal E, Kaushal GP, Agarwal SK. Anti-inflammatory effects of erythromycin and tetracycline on Propionibacterium acnes induced production of chemotactic factors and reactive oxygen species by human neutrophils. Dermatol Online J. 2002;8:2. [PubMed] [Google Scholar]
- 36.Bakar Ö, D. Z, Yuksel M, Haklar G, Sanisoglu Y. The effect of azithromycin on reactive oxygen species in rosacea. Clinical and Experimental Dermatology. 2007;32:197–200. doi: 10.1111/j.1365-2230.2006.02322.x. [DOI] [PubMed] [Google Scholar]
- 37.Peus D, Vasa RA, Beyerle A, Meves A, Krautmacher C, Pittelkow MR. UVB activates ERK1/2 and p38 signaling pathways via reactive oxygen species in cultured keratinocytes. J Invest Dermatol. 1999;112:751–6. doi: 10.1046/j.1523-1747.1999.00584.x. [DOI] [PubMed] [Google Scholar]
- 38.Peus D, Vasa RA, Meves A, Pott M, Beyerle A, Squillace K, et al. H2O2 is an important mediator of UVB-induced EGF-receptor phosphorylation in cultured keratinocytes. J Invest Dermatol. 1998;110:966–71. doi: 10.1046/j.1523-1747.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 39.Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd SL, Terlecky SR. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol. 2008;128:2606–14. doi: 10.1038/jid.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HM, Shin DM, Kim KK, Lee JS, Paik TH, Jo EK. Roles of Reactive Oxygen Species in CXCL8 and CCL2 Expression in Response to the 30-kDa Antigen of Mycobacterium tuberculosis. J Clin Immunol. 2009;29:46–56. doi: 10.1007/s10875-008-9222-3. [DOI] [PubMed] [Google Scholar]
- 41.Yang CS, Shin DM, Lee HM, Son JW, Lee SJ, Akira S, et al. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell Microbiol. 2008;10:741–54. doi: 10.1111/j.1462-5822.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- 42.Kawaguchi Y, Tanaka H, Okada T, Konishi H, Takahashi M, Ito M, et al. The effects of ultraviolet A and reactive oxygen species on the mRNA expression of 72-kDa type IV collagenase and its tissue inhibitor in cultured human dermal fibroblasts. Arch Dermatol Res. 1996;288:39–44. doi: 10.1007/BF02505041. [DOI] [PubMed] [Google Scholar]
- 43.Bielenberg DR, Bucana CD, Sanchez R, Donawho CK, Kripke ML, Fidler IJ. Molecular regulation of UVB-induced cutaneous angiogenesis. J Invest Dermatol. 1998;111:864–72. doi: 10.1046/j.1523-1747.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 44.Ballaun C, Weninger W, Uthman A, Weich H, Tschachler E. Human keratinocytes express the three major splice forms of vascular endothelial growth factor. J Invest Dermatol. 1995;104:7–10. doi: 10.1111/1523-1747.ep12613450. [DOI] [PubMed] [Google Scholar]
- 45.Longuet-Perret I, Schmitt D, Viac J. Tumour necrosis factor-alpha is involved in the contrasting effects of ultraviolet B and ultraviolet A1 radiation on the release by normal human keratinocytes of vascular permeability factor. Br J Dermatol. 1998;138:221–4. doi: 10.1046/j.1365-2133.1998.02064.x. [DOI] [PubMed] [Google Scholar]
- 46.Naru E, Suzuki T, Moriyama M, Inomata K, Hayashi A, Arakane K, et al. Functional changes induced by chronic UVA irradiation to cultured human dermal fibroblasts. Br J Dermatol. 2005;153(Suppl 2):6–12. doi: 10.1111/j.1365-2133.2005.06964.x. [DOI] [PubMed] [Google Scholar]
- 47.Wlaschek M, Briviba K, Stricklin GP, Sies H, Scharffetter-Kochanek K. Singlet oxygen may mediate the ultraviolet A-induced synthesis of interstitial collagenase. J Invest Dermatol. 1995;104:194–8. doi: 10.1111/1523-1747.ep12612751. [DOI] [PubMed] [Google Scholar]
- 48.Scharffetter-Kochanek K, Wlaschek M, Briviba K, Sies H. Singlet oxygen induces collagenase expression in human skin fibroblasts. FEBS Lett. 1993;331:304–6. doi: 10.1016/0014-5793(93)80357-z. [DOI] [PubMed] [Google Scholar]
- 49.Lee Y, Kim H, Kim S, Shin MH, Kim YK, Kim KH, et al. Myeloid Differentiation Factor 88 Regulates Basal and UV-Induced Expressions of IL-6 and MMP-1 in Human Epidermal Keratinocytes. J Invest Dermatol. 2009;129:460–7. doi: 10.1038/jid.2008.261. [DOI] [PubMed] [Google Scholar]
- 50.Caubet C, Jonca N, Brattsand M, Guerrin M, Bernard D, Schmidt R, et al. Degradation of Corneodesmosome Proteins by Two Serine Proteases of the Kallikrein Family, SCTE//KLK5//hK5 and SCCE//KLK7//hK7. J Investig Dermatol. 2004;122:1235–44. doi: 10.1111/j.0022-202X.2004.22512.x. [DOI] [PubMed] [Google Scholar]
- 51.Descargues P, Deraison C, Prost C, Fraitag S, Mazereeuw-Hautier J, D'Alessio M, et al. Corneodesmosomal Cadherins Are Preferential Targets of Stratum Corneum Trypsin- and Chymotrypsin-like Hyperactivity in Netherton Syndrome. J Invest Dermatol. 2006;126:1622–32. doi: 10.1038/sj.jid.5700284. [DOI] [PubMed] [Google Scholar]
- 52.Michael IP, Sotiropoulou G, Pampalakis G, Magklara A, Ghosh M, Wasney G, et al. Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. J Biol Chem. 2005;280:14628–35. doi: 10.1074/jbc.M408132200. [DOI] [PubMed] [Google Scholar]
- 53.Acharya MR, Venitz J, Figg WD, Sparreboom A. Chemically modified tetracyclines as inhibitors of matrix metalloproteinases. Drug Resist Updat. 2004;7:195–208. doi: 10.1016/j.drup.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–65. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Sorsa T, Lindy O, Konttinen YT, Suomalainen K, Ingman T, Saari H, et al. Doxycycline in the protection of serum alpha-1-antitrypsin from human neutrophil collagenase and gelatinase. Antimicrob Agents Chemother. 1993;37:592–4. doi: 10.1128/aac.37.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rundhaug Joyce E. Matrix metalloproteinases and angiogenesis. Journal of Cellular and Molecular Medicine. 2005;9:267–85. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonnar E, Eustace P, Powell FC. The Demodex mite population in rosacea. J Am Acad Dermatol. 1993;28:443–8. doi: 10.1016/0190-9622(93)70065-2. [DOI] [PubMed] [Google Scholar]
- 58.Forton F, Seys B. Density of Demodex folliculorum in rosacea: a case-control study using standardized skin-surface biopsy. Br J Dermatol. 1993;128:650–9. doi: 10.1111/j.1365-2133.1993.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 59.Erbagci Z, Ozgoztasi O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421–5. doi: 10.1046/j.1365-4362.1998.00218.x. [DOI] [PubMed] [Google Scholar]
- 60.Lacey N, Delaney S, Kavanagh K, Powell FC. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474–81. doi: 10.1111/j.1365-2133.2007.08028.x. [DOI] [PubMed] [Google Scholar]
- 61.Costa CP, Kirschning CJ, Busch D, Durr S, Jennen L, Heinzmann U, et al. Role of chlamydial heat shock protein 60 in the stimulation of innate immune cells by Chlamydia pneumoniae. Eur J Immunol. 2002;32:2460–70. doi: 10.1002/1521-4141(200209)32:9<2460::AID-IMMU2460>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 62.Gobert AP, Bambou JC, Werts C, Balloy V, Chignard M, Moran AP, et al. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a toll-like receptor (TLR)-2-, TLR-4-, and myeloid differentiation factor 88-independent mechanism. J Biol Chem. 2004;279:245–50. doi: 10.1074/jbc.M307858200. [DOI] [PubMed] [Google Scholar]
- 63.Rebora A, Drago F, Picciotto A. Helicobacter pylori in patients with rosacea. Am J Gastroenterol. 1994;89:1603–4. [PubMed] [Google Scholar]
- 64.Diaz C, O'Callaghan CJ, Khan A, Ilchyshyn A. Rosacea: a cutaneous marker of Helicobacter pylori infection? Results of a pilot study. Acta Derm Venereol. 2003;83:282–6. doi: 10.1080/00015550310016544. [DOI] [PubMed] [Google Scholar]
- 65.Argenziano G, Donnarumma G, Iovene MR, Arnese P, Baldassarre MA, Baroni A. Incidence of anti-Helicobacter pylori and anti-CagA antibodies in rosacea patients. Int J Dermatol. 2003;42:601–4. doi: 10.1046/j.1365-4362.2003.01817.x. [DOI] [PubMed] [Google Scholar]
- 66.Szlachcic A. The link between Helicobacter pylori infection and rosacea. J Eur Acad Dermatol Venereol. 2002;16:328–33. doi: 10.1046/j.1468-3083.2002.00497.x. [DOI] [PubMed] [Google Scholar]
- 67.Jones MP, Knable AL, Jr., White MJ, Durning SJ. Helicobacter pylori in rosacea: lack of an association. Arch Dermatol. 1998;134:511. doi: 10.1001/archderm.134.4.511. [DOI] [PubMed] [Google Scholar]
- 68.Gurer MA, Erel A, Erbas D, Caglar K, Atahan C. The seroprevalence of Helicobacter pylori and nitric oxide in acne rosacea. Int J Dermatol. 2002;41:768–70. doi: 10.1046/j.1365-4362.2002.01452.x. [DOI] [PubMed] [Google Scholar]
- 69.Utas S, Ozbakir O, Turasan A, Utas C. Helicobacter pylori eradication treatment reduces the severity of rosacea. J Am Acad Dermatol. 1999;40:433–5. doi: 10.1016/s0190-9622(99)70493-7. [DOI] [PubMed] [Google Scholar]
- 70.Gedik GK, Karaduman A, Sivri B, Caner B. Has Helicobacter pylori eradication therapy any effect on severity of rosacea symptoms? J Eur Acad Dermatol Venereol. 2005;19:398–9. doi: 10.1111/j.1468-3083.2005.01144.x. [DOI] [PubMed] [Google Scholar]
- 71.Boixeda de Miquel D, Vazquez Romero M, Vazquez Sequeiros E, Foruny Olcina JR, Boixeda de Miquel P, Lopez San Roman A, et al. Effect of Helicobacter pylori eradication therapy in rosacea patients. Rev Esp Enferm Dig. 2006;98:501–9. doi: 10.4321/s1130-01082006000700003. [DOI] [PubMed] [Google Scholar]
- 72.Ding SZ, Minohara Y, Fan XJ, Wang J, Reyes VE, Patel J, et al. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect Immun. 2007;75:4030–9. doi: 10.1128/IAI.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mashimo M, Nishikawa M, Higuchi K, Hirose M, Wei Q, Haque A, et al. Production of reactive oxygen species in peripheral blood is increased in individuals with Helicobacter pylori infection and decreased after its eradication. Helicobacter. 2006;11:266–71. doi: 10.1111/j.1523-5378.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 74.Bagchi D, Bhattacharya G, Stohs SJ. Production of reactive oxygen species by gastric cells in association with Helicobacter pylori. Free Radic Res. 1996;24:439–50. doi: 10.3109/10715769609088043. [DOI] [PubMed] [Google Scholar]
- 75.Baz K, Cimen MY, Kokturk A, Aslan G, Ikizoglu G, Demirseren DD, et al. Plasma reactive oxygen species activity and antioxidant potential levels in rosacea patients: correlation with seropositivity to Helicobacter pylori. Int J Dermatol. 2004;43:494–7. doi: 10.1111/j.1365-4632.2004.02137.x. [DOI] [PubMed] [Google Scholar]
- 76.Kawahara T, Kuwano Y, Teshima-Kondo S, Kawai T, Nikawa T, Kishi K, et al. Toll-like receptor 4 regulates gastric pit cell responses to Helicobacter pylori infection. J Med Invest. 2001;48:190–7. [PubMed] [Google Scholar]
- 77.Smith MF, Jr., Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, et al. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem. 2003;278:32552–60. doi: 10.1074/jbc.M305536200. [DOI] [PubMed] [Google Scholar]