Abstract
The authors describe a new approach to measuring DNA hybridization based on surface plasmon-coupled emission (SPCE). SPCE is the resonance coupling of excited fluorophores with electron motions in thin metal films, resulting in efficient transfer of energy through the film and radiation into the glass substrate. The authors evaluated the use of SPCE for detection of DNA hybridization. An unlabeled capture biotinylated oligonucleotide was attached near the surface of a thin (50 nm) silver film using streptavidin. The authors then measured the emission intensity of single-stranded Cy5-labeled DNA upon binding to a complementary oligomer attached to a silver film. Hybridization could be detected by an increase in SPCE, which appeared as light radiated into the substrate at a sharply defined angle near 73 degrees from the normal. The largest signals were observed when the excitation angle of incidence equaled the surface plasmon wavelength, but directional emission was also observed without excitation by the surface plasmon evanescent field. The increased intensity is due to proximity to the metal surface, so that hybridization can be detected without a change in the quantum yield of the fluorophore. These results indicate that SPCE can provide highly sensitive real-time measurement of DNA hybridization.
Keywords: PDNA microarrays, DNA hybridization, fluorescence, surface plasmons, surface plasmon resonance, directional emission
Introduction
Fluorescence detection of dna hybridization is widely used in biomedical research and diagnostics. Most methods to detect hybridization typically depend on a change in the quantum yield of the fluorophore upon intercalation between the base pairs or binding to the minor groove of DNA.1-4 In several recent reports, we described a new phenomenon called surface plasmon-coupled emission (SPCE).5-7 If an excited fluorophore is located within about 200 nm of a thin (∼ 50 nm) silver or gold film, the dipole can couple with electron motions in the metal film. These electron motions are called surface plasmons. This coupling results in efficient transfer of energy through the film and into the glass substrate. Remarkably, the energy radiates at a sharply defined angle equal to the surface plasmon angle at the emission wavelength.
The phenomenon of SPCE is closely related to surface plasmon resonance (SPR). In SPR, one measures the reflectivity of light from a thin metal surface, typically gold, for varying angles of incidence. A sharp decrease in reflectivity occurs when the incident light excites surface plasmons at the metal-sample interface. For SPR to occur, the incident light must impinge on the metal film through a higher dielectric constant medium, typically glass. SPR only occurs for p-polarized incident light, that is, when the electric vector is parallel to the plane of incidence.
SPCE is in a sense the reverse process to SPR.5-7 Excited fluorophores near the metal surface create surface plasmons. These plasmons radiate into the glass substrate at the surface plasmon angle for the emission wavelength, and the SPCE is p-polarized. SPCE occurs for fluorophores in the sample phase within about 200 nm of the metal surface.
In the present report, we explore the use of SPCE to measure DNA hybridization. This was accomplished using a biotinylated DNA oligomer (ss DNA-biotin) as the capture probe and a Cy5–labeled oligomer (ss Cy5-DNA) as the complementary strand. Specificity of binding was tested using a noncomplementary Cy5–labeled oligomer (Fig. 1).
FIG. 1.
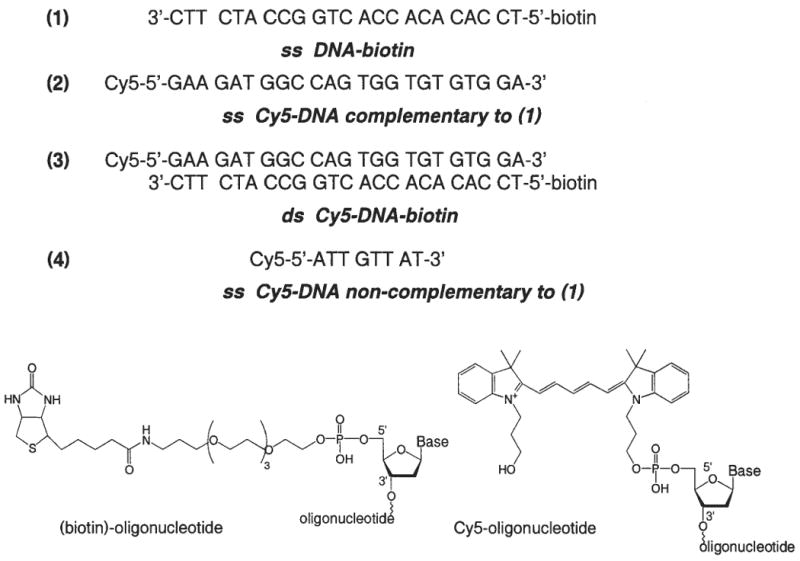
Structures of DNA oligomers.
Materials and Methods
Sample preparation
All samples were prepared on quartz slides, which were smooth, ungrooved parts of 0.2 mm-demountable cuvettes (12.5 mm × 45 mm; Starna Cell, Inc., Atascadero, CA). This cuvette is basically a thin cuvette with 1 removable side. Oligonucleotides labeled with biotin (1) or Cy5 (N,N′-(dipropyl)-tetramethylindodicarbocyanine) (2) and (4) (see Fig. 1) were obtained from the Biopolymer Core Facility at the University of Maryland, School of Medicine. Nanopure H2O (18.0 MΩ), purified using Millipore Milli-Q Gradient System, was used for all experiments. Buffer components were purchased from Sigma-Aldrich (St. Louis, MO).
Each quartz slide was half covered with a continuous 50-nm thick silver film, which was vapor deposited by EMF Corp. (Ithaca, NY). For improved adhesion, the quartz was coated with 2 nm chromium prior to coating with silver. The silvered surface was then covered with 1000 μl of an aqueous solution of 10 μM BSA-biotin (Sigma, St. Louis, MO) and placed in a humid chamber for 20 h at 5 °C. After being washed 3 times with water, the slides were again placed in a humid chamber and 1000 μl of 5 μM streptavidin (Molecular Probes, Eugene, OR) in a 0.1 × PBS buffer was deposited on each BSA-biotin-coated surface for 40 min (room temperature). The slides were then washed 3 times with 0.1 × PBS buffer, and 1000 μl of biotinylated DNA samples in 5 mM HEPES (pH 7.5), 0.1 M KCl, and 0.25 mM EDTA were deposited for 1 h at room temperature. After washing with the same buffer, and covering with second part of demountable cuvette, samples that consist of DNA-streptavidin-BSA layer on silver film were ready to use.
To determine the conditions needed to observe SPCE, we used the double-stranded Cy5-DNA-biotin (3) deposited on protein monolayer by incubation with 1000 μl of 2 μM Cy5-DNA-biotin previously hybridized by mixing complementary oligos (1) and (2) in 5 mM HEPES (pH 7.5), 0.1 M KCl, and 0.25 mM EDTA.
For hybridization experiments, the protein layer was covered with 1000 μl of 2 μM single-stranded biotinylated oligo (ss DNA-biotin) in buffer solution. The 0.2-mm cuvette was then filled with a hybridization buffer containing 50 nM complementary ss Cy5-DNA (2) or 50 nM noncomplementary ss Cy5-DNA (4).
Fluorescence measurements
The labeled slides were attached with index-matching fluid to a hemicylindrical prism made of BK7 glass and positioned on a precise rotary stage.6 The stage was equipped with an arm about 15 cm long for fiber optic detection, which allowed observation at any angle relative to the incident angle. For collection of the angle dependent emission intensity, a 200-μm air slit was placed on the fiber input. The output of the fiber was directed to the SLM 8000 spectrofluorometer. For measurement of the emission spectra, the 200-μm slit was removed from the fiber and the fiber input was positioned as close as possible to the sample. The 605-nm excitation was obtained from a cavity-dumped rhodamine 6G dye laser, 3.8 MHz repetition rate, 10 ps pulse width. Scattered incident light at 605-nm was suppressed on observation using a combination 630 nm long pass filter and a 665 interference filter. The indicated incident light was polarized horizontally in the laboratory axis, that is, p-polarized relative to the plane of incidence.
Theory
We do not have a general theory for SPCE. However, SPCE can be understood in terms of the concepts underlying SPR8,9 and the physics of surface plasmons.10,11 SPR is observed as a decrease in reflectivity of a thin metal film at a specific angle of incidence (θSP) through a glass prism. This occurs when the wavevector of the incident light matches the wavevector of the surface plasmons. The wavevector of the incident light is given by
where np is the refractive index of the prism, λ is the wavelength in the prism, ω is the frequency in radians/sec, and k0 is the wavevector in free space. Calculation of the wavevector for the surface plasmon is more difficult. The dielectric constant (∈) of a metal (m) is a complex quantity given by
where and the subscripts indicate the real (r) and imaginary (im) components. For a metal, the wavevector for the surface plasmon at the metal-sample interface can be approximated by
where ∈r and ∈s are the real parts of the dielectric constants of the metal (m) and sample (s), respectively.
The incident light interacts with the surface plasmon when its x-axis component equals the wavevector for the surface plasmons. The wavevector for the incident light in the prism along the sample-metal interface is given by
where θI is the incidence angle in the prism. The conditions for SPR absorption is satisfied when
SPR only occurs for p-polarized incident light.12,13 In reality, SPR does not occur only at a single angle, but over a relatively narrow range of angles determined by the optical constants and resonance response of the metal.
SPCE is similar to SPR except that the surface plasmons are created by interactions with the excited fluorophores. These plasmons then emit into the glass prism, which is possible because the wavevector of the plasmons can be matched with far-filed radiation according to eq. 5. For SPCE, the wavelength is now the emission wavelength, which radiates at an angle θ F.
Results
The use of SPCE to measure DNA hybridization is shown in Figure 2. Upon hybridization, the labeled oligomers become localized near the silver surface. The excited fluorophores couple with the surface plasmons, and the energy radiates into the glass at an angle θ F, where θ F is the surface plasmon angle for the emission wavelength. The fluorophores more distant from the silver emit mostly isotropically into space and do not contribute significantly to the SPCE. Based on results to date, we believe fluorophores within about 200 nm of the metal surface result in SPCE5-6 When using metal films, there are 2 modes of excitation and 2 modes of observation (Fig. 3). The excitation can be from the aqueous (lower refractive index) phase, which is called the reverse Kretschmann (RK) configuration. In this case, the incident light cannot excite surface plasmons in the metal.5 The fluorophores are excited mostly uniformly through the sample thickness. The 2nd mode of excitation is through the glass substrate (higher refractive index) at the surface plasmon angle, which is called the Kretschmann (KR) configuration. This results in excitation of electrons oscillations and an approximate 20-fold enhanced evanescent field in the aqueous phase.13-14 That is, the evanescent field due to resonance excitation of surface plasmons is about 20-fold more intense than the evanescent field from total internal reflectance. In this configuration, fluorophores near the silver film are selectively excited. In addition to these modes of excitation, there are 2 modes of observation. The free space (FS) emission can be observed at any angle on the water side of the samples. Alternatively, one can observe the SPCE at an angle θ F on the substrate side of the sample. These 4 experimental configurations are summarized in Figure 3.
FIG. 2.
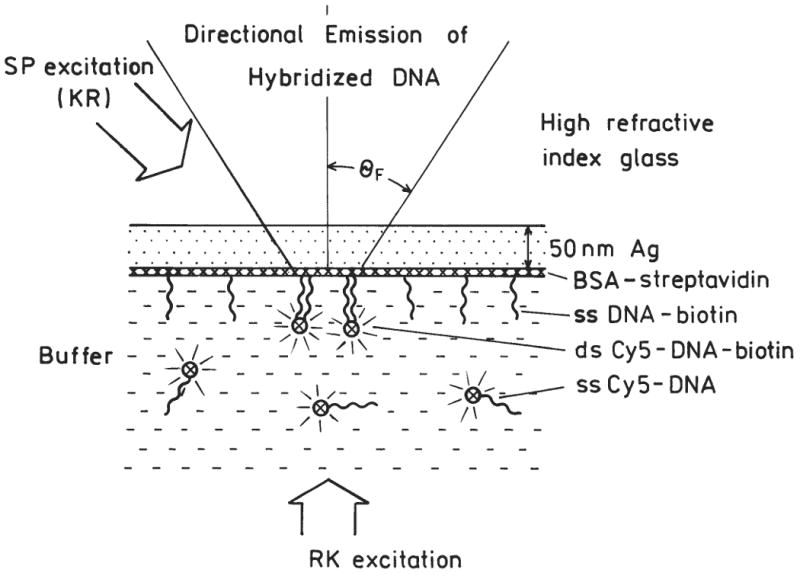
Intuitive description of directional surface plasmon-coupled emission (SPCE) from hybridized DNA. Figure not drawn to scale, BSA-streptavidin Ł 90, Cy5-DNA Ł 90. The excitation is either direct (RK) or through the coupling prism (KR).
FIG. 3.
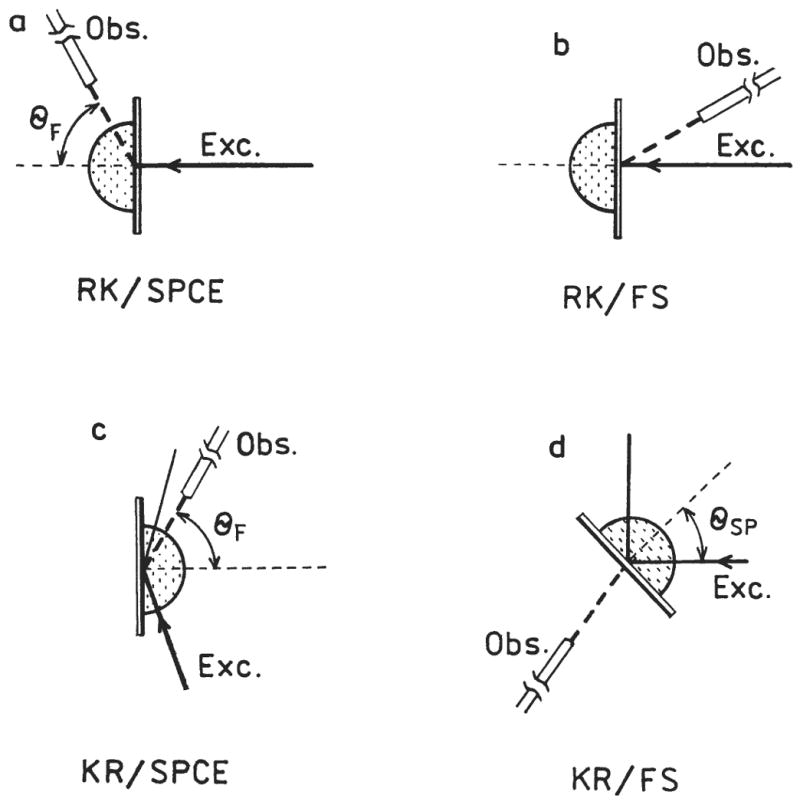
Optical configurations used to study Cy5-DNA hybridization.
We first established the conditions to detect SPCE. This was accomplished by binding prehybridized ds Cy5-DNA-biotin to a streptavidin-coated surface. Initially, the sample was excited using the RK configuration, where the incident light could not excite surface plasmons. The emission intensity of Cy5 was measured at all observable angles through the hemicylindrical prism (Fig. 4, top). The emission was found to be sharply distributed at ±73° relative to the normal axis through the prism. Free space emission was also observed. Integration of the intensity at all angles indicates that about 30% of the total emission occurred as SPCE. The emission observed at ±73° and the free space displayed polarizations of 0.95 and 0.1, respectively. The high polarization at ±73° proves that this signal is SPCE. To be more explicit, the maximum polarization for photoluminescence from a random distribution of fluorophores is 0.5. The low polarization of the free space emission is a result of depolarization factors such as rotational freedom of the probe. We believe the high polarization for SPCE occurs because the emission is due to radiating surface plasmons, and only p-polarized emission can satisfy the necessary match of wavevectors.
FIG. 4.
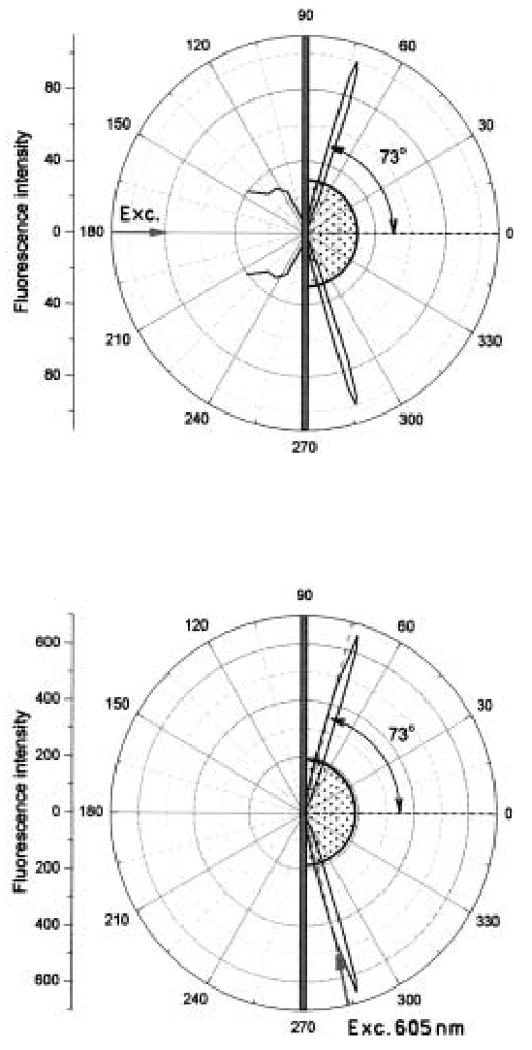
Angular distribution of directional fluorescence emission from Cy5-DNA-biotin-streptavidin-biotin-BSA on a 50-nm Ag mirror observed in RK configuration (top) and in KR configuration (bottom). The excitation was at 605 nm (p-polarized) and observation was at 665 nm (p-polarized).
The sample was then excited using the KR configuration (Fig. 4, bottom). In this case, the excitation incidence angle was at the reflectivity minimum near 74 degrees. Once again, we observed directional emission at ±73°. However, there was much less free-space emission. With KR excitation, we estimate that 90% of the total emission occurs as directional emission. This percentage seems high but is theoretically possible. For dipoles oriented perpendicular to the surface, about 150 nm from the surface, the coupling efficiency can be as high as 93%.15 For a random distribution of orientations, the coupling efficiency can be about 65% for dipoles about 30 nm from the surface.15 We note that there is considerable uncertainty in the 90% estimate, but in any event a large fraction of the total emission couples through the metal surface.
We examined the intensity of the free-space emission with various excitation angles of incidence (Fig. 5). The emission occurred for a narrow range of incidence angles near 74°. This angle is in reasonable agreement with the calculated reflectivity minimum for a thin silver film,12-13,16 which supports our conclusion that SPCE is similar to surface plasmon resonance (SPR) except the light is coupling out of the metal film rather than being absorbed.5
FIG. 5.
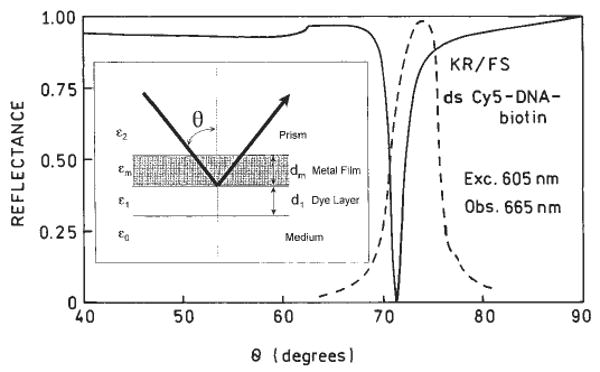
Angle dependent reflectivity (solid line) of a Ag mirror calculated according to [11-12].The assumed values were dielectric constant ∈2 = 2.29 for the coupling prism, ∈m = −17 + 0.6i for silver, ∈1 = 2.07 for the protein/DNA layer and ∈0 = 1.79 for the buffer, thickness: dm = 50 nm for silver and d1 = 15 nm for the protein/DNA layer. The dashed line is emission intensities of ds Cy5-DNA-biotin fluorescence observed in the KR/FS configuration with various incident angles (Fig. 3d).
Next we used the various optical configurations to measure time-dependent hybridization. We used slides coated with BSA-streptavidin and ssDNA-biotin. We then added the Cy5-labeled complementary oligomer to the slides with surface-bound capture oligomer. Using KR excitation and measuring the SPCE, we observed a rapid increase in Cy5 emission, which we attribute to hybridization (Fig. 6, top). It is important to note that this intensity increase does not require a change in quantum yield of the probe, but only a change in probe localization. This conclusion is supported by the absence of a time-dependent change in intensity upon addition of the noncomplementary Cy5-oligomer, which does not localize near the metal surface. We also observed a SPCE–intensity increase using RK excitation (Fig. 6, bottom), but the magnitude of the signal and the percentage change was much less than with KR excitation. The emission spectra were all characteristic of Cy5 (Fig. 7). Little free-space emission could be observed with RK excitation. In total, our results suggest the use of Kretschmann excitation and observation of the SPCE for maximal sensitivity.
FIG. 6.
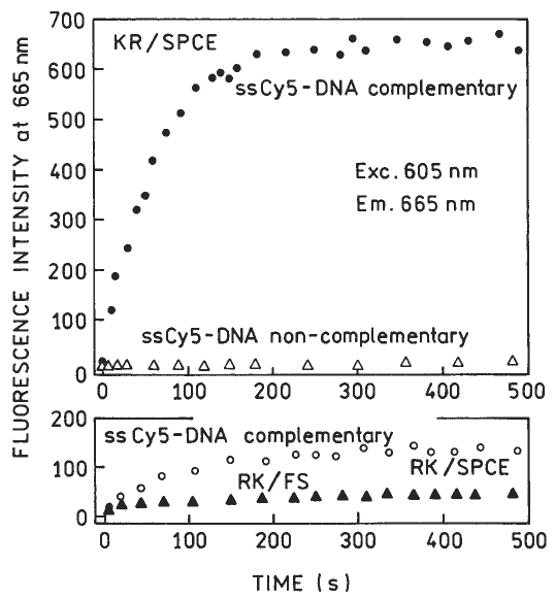
Fluorescence intensities upon injection of Cy5-DNA. Top panel: KR configuration. Bottom panel: RK configuration. RK/FS is the free-space fluorescence observed in RK configuration at 30° (Fig. 4, top). Cy5-DNA was complementary to DNA deposited on the Ag surface. In a separate experiment, noncomplementary Cy5-DNA was used (top panel).
FIG. 7.
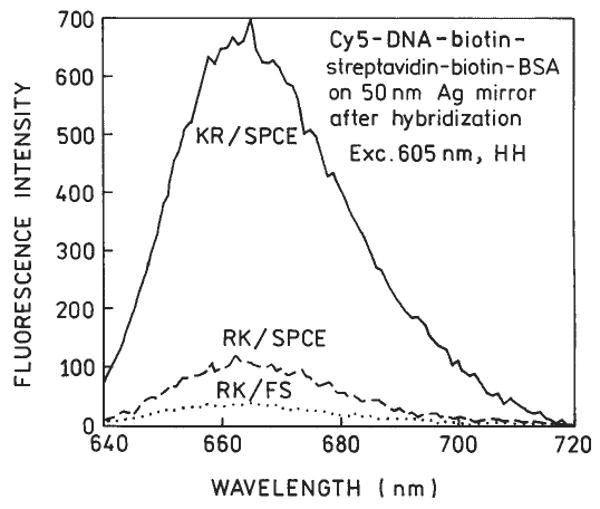
Fluorescence spectra of Cy5-DNA observed in different configurations after complete hybridization.
Discussion
The use of SPCE offers several advantages when used to measure DNA hybridization or other bioaffinity reactions on surfaces. The binding reaction can be detected without a change in probe quantum yield because proximity to these metal surfaces results in SPCE. Additionally, unwanted autofluorescence from the sample is suppressed when using SPCE. Background suppression occurs because only fluorophores near the metal film couple with the surface plasmons. Typically the molecules responsible for the background emission will not bind to the surface and thus contribute little to the SPCE. Additionally, illumination using the Kretschmann configuration results in an amplified evanescent field near the metal, localizing the excitation to regions containing the molecules of interest.
Another favorable feature of SPCE is its technical simplicity. The only requirement is for a thin metal surface. The assays can be performed without separation steps. Silver is somewhat reactive but can be protected with polymers, self-assembled monolayers, or inorganic coating. Gold is less reactive, requires little if any protection, and we have recently shown that SPCE can be observed with thin gold films.17 The fact that SPCE occurs with gold demonstrates that coupling occurs over distances much larger than the Forster distances for resonance energy transfer, which are near 5 nm. Hence, in contrast to surface-enhanced Raman scattering (SERS), SPCE is not dependent upon the detailed molecular interactions between the analyte and the metal surfaces. These attributes of SPCE make it readily applicable for use in many bioaffinity assays.
It should be noted that SPCE offers an efficient collection of the signal. The directional fluorescence appears as a hollow cone of emission.6 In the described experiments, we utilized only a fraction of this cone (5%-10%), which was enough to comfortably detect 1 pmole/cm2 collection device such as a parabolic or elliptical reflector will easily increase detection sensitivity by an order of magnitude.
Acknowledgments
This work was supported by the NIH National Center for Research Resources, RR-08119, HG-002655, EB-000682, and EB-00981. Zygmunt Gryczynski thanks Philip Morris USA, Inc., for financial support.
References
- 1.Le Pecq JB, Le Bret M, Barbet J, Roques B. DNA polyintercalating drugs: DNA binding of diacridine derivatives. Proc Natl Acad Sci. 1975;72(8):2915–2919. doi: 10.1073/pnas.72.8.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rye HS, Yue S, Wemmer DE, et al. Stable fluorescent complexes of double-stranded DNA with bis-intercalating asymmetric cyanine dyes: properties and applications. Nucleic Acids Res. 1992;20(11):2803–2812. doi: 10.1093/nar/20.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glazer AN, Peck K, Mathies RA. A stable double-stranded DNA ethidium homodimer complex: application to picogram fluorescence detection of DNA in agarose gels. Proc Natl Acad Sci USA. 1990;87:3851–3855. doi: 10.1073/pnas.87.10.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson SC, Mathies RA, Glazer AN. Heterodimeric DNA-binding dyes designed for energy transfer: stability and applications of the DNA complexes. Nucleic Acids Res. 1993;21:5720–5726. doi: 10.1093/nar/21.24.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakowicz JR. Radiative decay engineering 3. Surface plasmon coupled directional emission. Anal Biochem. 2004;324(2):153–169. doi: 10.1016/j.ab.2003.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gryczynski I, Malicka J, Gryczynski Z, Lakowicz JR. Radiative decay engineering 4. Experimental studies of surface plasmon coupled directional emission. Anal Biochem. 2004;324(2):170–182. doi: 10.1016/j.ab.2003.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakowicz JR, Malicka J, Gryczynski I, Gryczynski Z. Directional surface plasmon-coupled emission: a new method for high sensitivity detection. BBRC. 2003;307:435–439. doi: 10.1016/S0006-291X(03)01214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurihara K, Suzuki K. Theoretical understanding of an absorption-based surface plasmon resonance sensor based on Kretschmann's theory. Anal Chem. 2002;74:696–701. doi: 10.1021/ac010820+. [DOI] [PubMed] [Google Scholar]
- 9.Salamon Z, Macleod HA, Tollin G. Surface plasmon resonance spectroscopy as a tool for investigating the biochemical and biophysical properties of membrane protein systems. I. Theoretical principles. Biochem et Biophys Acta. 1997;1331:117–129. doi: 10.1016/s0304-4157(97)00004-x. [DOI] [PubMed] [Google Scholar]
- 10.Raether H. Surface Plasmons on Smooth and Rough Surfaces and on Gratings. New York: Springer Verlag; 1988. [Google Scholar]
- 11.Forstmann F, Gerhardts RR. Metal Optics Near the Plasma Frequency. Vol. 109 New York: Springer-Verlag; 1986. [Google Scholar]
- 12.Frutos AG, Corn RM. SPR of ultra thin organic films. Anal Chem. 1999:449A–455A. [Google Scholar]
- 13.Neumann T, Johansson ML, Kambhampati D, Knoll W. Surface-plasmon fluorescence spectroscopy. Adv Funct Mater. 2002;12(9):575–586. [Google Scholar]
- 14.Liebermann T, Knoll W. Surface-plasmon field-enhanced fluorescence spectroscopy. Colloids Surfaces. 2000;171:115–130. [Google Scholar]
- 15.Weber WH, Eagen CF. Energy transfer from an excited dye molecule to the surface plasmons of an adjacent metal. Optics Letts. 1979;4(8):236–238. doi: 10.1364/ol.4.000236. [DOI] [PubMed] [Google Scholar]
- 16.Kolomenskii AA, Gershon PD, Schuessler HA. Sensitivity and detection limit of concentration and adsorption measurements by laser-induced surface-plasmon resonance. Appl Optics. 1997;36(25):6539–6547. doi: 10.1364/ao.36.006539. [DOI] [PubMed] [Google Scholar]
- 17.Gryczynski I, Malicka J, Gryczynski Z, Lakowicz JR. Surface plasmon-coupled emission using gold films. J Phys Chem B. doi: 10.1021/jp040221h. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]


