Abstract
Objective
Increased circulating levels of lipopolysaccharide (LPS) have been demonstrated in HIV-1-infected progressors. We investigated the effect of antiretroviral therapy (ART) interruptions on plasma LPS levels.
Design and methods
Overall, 77 individuals participated in this study (51 HIV-positive and 26 healthy). Ten out of 51 HIV-positive participants were viremic ART-naive patients and 41 out of 51 were chronically suppressed patients on ART (three or more drugs, CD4 cell count more than 400 cells/µl, HIV-1 RNA less than 500 copies/ml for more than 8 months, less than 50 copies/ml at recruitment) undergoing therapy interruption. The limulus amebocyte assay was used to measure plasma LPS levels; enzyme-linked immunosorbent assay to measure plasma levels of endotoxin-core antibodies (EndoCAb), soluble (s)CD14, LPS-binding protein and IFN-α; Immunoblotting to measure plasma gelsolin levels; and same day whole blood flow cytometry to measure levels of T-cell-activation markers (CD8+/CD38+, CD8+/HLA-DR+ and CD3+/CD95+).
Results
Increases in viremia and T-cell-activation markers were observed during therapy interruptions. During short-term therapy interruptions of less than 12 weeks, no change in LPS levels was found, whereas negative associations between viral load and LPS levels (Spearman’s Rho= −0.612, P = 0.0152), viral load and EndoCAb change (ΔEndoCAb, correlation = −0.502, P = 0.0204), and between ΔLPS and ΔEndoCAb (correlation = −0.851, P = 0.0073) were observed. In contrast, increased LPS (P = 0.0171) and sCD14 (P < 0.0001) levels were observed during long-term therapy interruption of more than 12 weeks compared with levels during ART, together with no association between LPS and viral load or EndoCAb. No association between immune activation and LPS was evident at any time point.
Conclusion
Increased plasma LPS levels were observed only after more than 12 weeks of ART interruption, despite presence of LPS-controlling host mechanisms.
Keywords: HIV-1, lipopolysaccharide, lipopolysaccharide-binding molecules, microbial translocation, therapy interruption
Introduction
During established HIV-1 infection, an increase in plasma bacterial lipopolysaccharide (LPS) levels has been proposed to reflect microbial translocation from the gut serving as a potential inflammatory stimulus accelerating disease pathogenesis [1]. The level and effect of circulating LPS can be modulated by LPS-binding protein (LBP) [2], soluble CD14 (sCD14) [3–5], naturally occurring antibodies to the LPS core oligosaccharide [endotoxin-core antibodies (EndoCAb)] [6,7] and plasma gelsolin (which can bind actin and LPS) [8–12]. The interplay between antiretroviral therapy (ART) interruption-mediated viremic episodes and changes in plasma levels of LPS and its host-derived ligands remains unknown and is the subject of this study.
Patients and methods
Participants
Cryopreserved plasma from 26 uninfected and 51 HIV-positive patients was analyzed. Ten out of the 51 HIV-positive participants were viremic ART-naive [median, 25th-75th interquartile range (IQR) plasma HIV-1 RNA (viral load)=19601 copies/ml (11545, 69954); median (IQR) CD4 cell count = 273.5 cells/µl (191.25, 347)] and 41 of 51 HIV-positive participants were chronically suppressed patients participating in a parent study [recruitment characteristics: age ≥18 years, presence of ART (three or more drugs), CD4 cell count >400 cells/µl with a history of nadir CD4 cell count ≥100 cells/µl, and viral load <50 copies/ml with a >6 months history of < 500 copies/ml] and followed under continuous therapy (n = 20) or repeated therapy interruptions (n = 21) before undergoing an open-ended therapy interruption. Patient demographics for the parent study are described in our prior publications [13,14].
Samples from uninfected and viremic ART-naive HIV-positive patients were analyzed at a single visit, whereas available samples from the parent study were analyzed as follows: at baseline (viral load < 50 copies/ml on ART, n = 41), on continuous ART for a 40-week follow-up (n = 16), during a 6-week therapy interruption (n = 21), and during open-ended therapy interruption: after patients reached viral set point (average viral load of the first three consecutive measurements of viral load with <0.5 log variation, n = 21) and at the last available viremic time point of the open-ended therapy interruption (n = 9).
Informed consent was obtained according to the Human Experimentation Guidelines of the US Department of Health and Human Services and of the authors’ institutions. The study protocol was approved by the Institutional Review Boards of the Wistar Institute and Philadelphia FIGHT.
Lipopolysaccharide levels and immune activation
LPS levels were determined in duplicate by the limulus amebocyte assay according to the manufacturer’s protocol (Cambrex Bioscience, Walkersville, Maryland, USA) in plasma samples diluted 1/100 (dilution determined by product inhibition test) with endotoxin-free water and heated to 70°C for 10 min to inactivate plasma proteins. Plasma levels of sCD14 (R&D, Minneapolis, Minnesota, USA), LBP (Cell Sciences, Canton, Massachusetts, USA), IgM EndoCAb (Cell Sciences), and IFN-α (PBL Biomedical Laboratories, Piscataway, New Jersey, USA) were determined by enzyme-linked immunosorbent assay (ELISA) as per manufacturer’s specifications. Measurements were run in duplicate on a kinetic absorbance reader at 450 nm (Rainbow Reader; SLT-Lab Instruments, Grodig/Salzburg, Austria). Lower limits for LPS, sCD14, LBP, EndoCAb and IFN-α were 0.1 EU/ml, 250 pg/ml, 781.25 pg/ml, 0.054 MMU/ml and 12.5 pg/ml, respectively. Plasma levels of gelsolin were determined by immunoblotting using monoclonal antihuman gelsolin antibody (G4896; Sigma, St Louis, Missouri, USA) as described [11]. Whole blood flow cytometry was used for assessment of T-cell activation (CD8+/CD38+, CD8+/HLA-DR+ and CD3+/CD95+) as described [15].
Statistical analysis
Data are presented as medians with 25th–75th IQR in parenthesis. Variable distributions were analyzed for normality using the Shapiro–Wilk W test (P > 0.05). Depending on data distribution, between groups comparisons were performed by t-test or the Wilcoxon/Kruskal–Wallis test (rank sums), whereas between time points comparisons were performed using nonparametric Wilcoxon sign-rank test or paired t-tests. Associations between variables were assessed using Spearman or pairwise correlation tests. All statistical tests were performed using JMP4 (SAS Institute, Cary, North Carolina, USA).
Results
Increased plasma lipopolysaccharide levels observed only after long-term HIV-1 replication
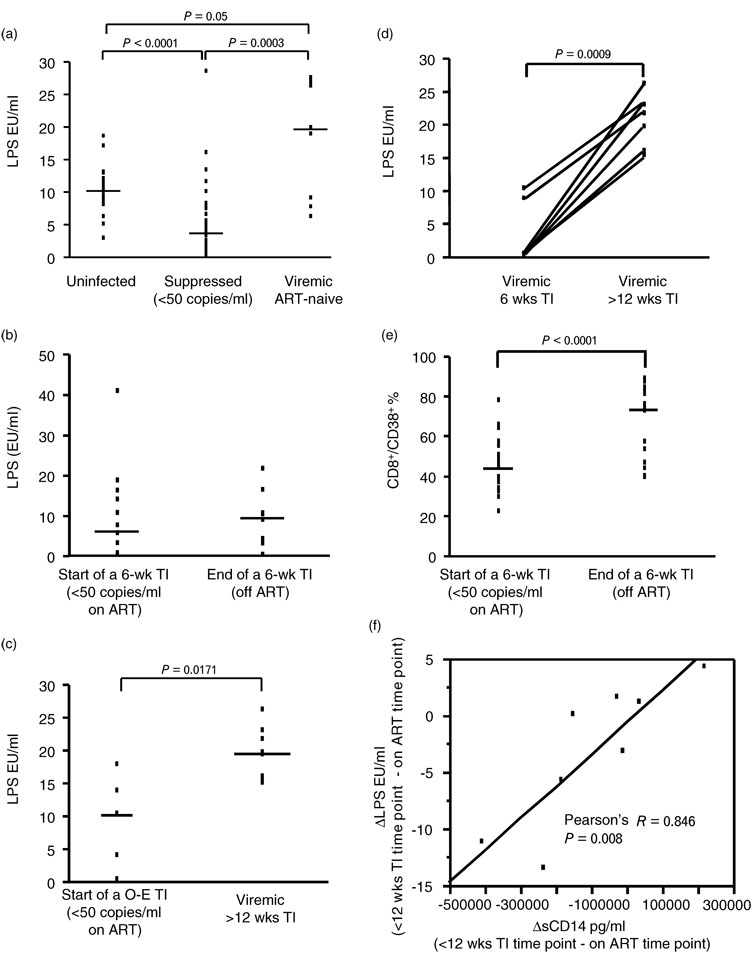
Initial cross-sectional analysis showed that ART suppressed HIV-1-positive patients had lower LPS levels compared with uninfected participants (P < 0.0001), whereas ART-naive viremic patients had higher LPS levels compared with ART-suppressed HIV-1-positive patients (P = 0.0003) (Fig. 1a).
Fig. 1. Increased plasma lipopolysaccharide levels during viremia of >12 weeks and lack of association between plasma lipopolysaccharide levels and T-cell activation.
Plasma lipopolysaccharide (LPS) levels are shown: (a) in uninfected subjects, HIV-positive suppressed and HIV-positive viremic ART-naive patients, (b) at start (viral load <50 copies/ml) and end of a 6-week therapy interruption, (c) at start (viral load <50 copies/ml) and last available viremic time point during open-ended (O-E) therapy interruption (TI), and (d) at 6-week and more than 12 weeks TI. (e) Percentages (%) of CD8+/CD38+ T cells at start (viral load <50 copies/ml) and end of a 6-week TI. (f) The correlation between the increase in plasma LPS levels (ΔLPS, EU/ml) and the increase in plasma soluble CD14 (ΔsCD14, pg/ml) during less than 12 weeks TI. Data are shown as individual subject data (dots) with median (lines) of the cohort distribution. Significant P values are shown on the top of each graph. Data in panel f are plotted along with the regression line, correlation coefficient and P value.
Longitudinal analysis showed no change in LPS levels during continuous ART, as well as during therapy interruptions of less than 12 weeks, including analysis during a 6-week therapy interruption [median (IQR) viral load = 10745 copies/ml (2527, 61874), Fig. 1b] and during open-ended therapy interruption when viral set point was reached [median (IQR) duration = 9 weeks (8, 12); median (IQR) viral load = 11 067 copies/ml (2851, 26259)]. In contrast, steady-state viremia for more than 12 weeks resulted in significantly increased LPS levels (P = 0.0171, Fig. 1c), as shown by higher LPS levels at the last available viremic time point during open-ended therapy interruption [median (IQR) duration = 19 weeks (12, 35); median (IQR) viral load = 43 748 copies/ml (23192, 101044)] compared with levels at start of the open-ended therapy interruption (viral load <50 copies/ml). This differential effect of less than 12 versus more than 12 weeks of viral replication on LPS levels was observed despite a lack of significant difference in viral load. The impact of prolonged viremia was also supported by the longitudinal change of LPS in patients followed over sequential 6 week to more than 12 weeks therapy interruptions (P = 0.0009, n = 7, Fig. 1d) and by cross-sectional analysis showing higher LPS levels in patients with steady-state viremia for more than 12 weeks therapy interruption compared with uninfected participants (P < 0.0001) or ART-suppressed HIV-1-positive patients (P = 0.031).
Lack of association between onset of immune activation and plasma lipopolysaccharide levels
In agreement with previous studies, we observed a significant rise in the frequency of CD3+/CD95+ (P = 0.0022, n = 21), CD8+/CD38+ (P < 0.0001, n = 20, Fig. 1e) and CD8+/HLA-DR+ (P = 0.001, n = 20) T cells concurrent with the onset of viral replication during the 6-week therapy interruption. Change in the frequency of activated T cells after therapy interruption was observed despite lack of changes in LPS levels (Fig. 1b). No correlation between LPS levels and T-cell activation or plasma levels of IFN-α following therapy interruption was observed at any time point analyzed.
Binding of lipopolysaccharide by endotoxin-core antibodies and sCD14 during therapy interruption-mediated viral rebound
To investigate potential reasons for a lack of change in LPS levels after short-term viremia, plasma levels of LPS-binding molecules (EndoCAb, sCD14, LBP, gelsolin) were measured on the same samples.
A constitutive role for EndoCAb in modulating LPS levels was supported by a negative correlation between LPS and EndoCAb levels in uninfected participants (correlation = −0.430, P = 0.0282). Activation of an effective LPS clearance response by circulating EndoCAb during less than 12 weeks therapy interruption was suggested by a negative association between viral load at week 6 of therapy interruption (correlation = −0.502, P = 0.0204) and the change of EndoCAb (ΔEndoCAb) from start to end of the 6-week therapy interruption (Fig. 2a). A negative association between viral load and LPS levels at week 6 of therapy interruption (Spearman’s Rho = −0.612, P = 0.0152) was also observed (Fig. 2a) concurrent with a lack of rise in LPS (Fig. 1). A strong negative association between ΔEndoCAb and ΔLPS (correlation = −0.851, P = 0.0073) was present from time on therapy to a subsequent steady-state viremic time point during less than 12 weeks therapy interruption (Fig. 2b), whereas no correlation between EndoCAb and LPS was observed after more than 12 weeks therapy interruption.
Fig. 2. Binding and clearance of lipopolysaccharide by endotoxin-core antibodies during short-term viremia.
(a) Correlation of plasma HIV-1 RNA at 6-week therapy interruption (Tl) with the change in plasma endotoxin-core antibodies (EndoCAb) levels (ΔEndoCAb) from start to end of the 6-week Tl (top panel) and with plasma lipopolysaccharide (LPS) levels at week 6 of Tl (bottom panel), (b) Correlation between the change in plasma LPS levels (ΔLPS) and the change in plasma EndoCAb levels (ΔEndoCab) from time on therapy (viral load <50 copies/ml) to a subsequent steady viremic time point less than 12 weeks of Tl. Data are shown along with the regression lines, correlation coefficients and P values.
In contrast to EndoCAb, sCD14 showed a positive association with LPS (Fig. 1f). In addition, sCD14 showed changes similar to those observed for LPS, such as higher levels in steady-state viremic patients for more than 12 weeks therapy interruption (P = 0.02) or ART-naive patients (P = 0.0006) compared with uninfected participants and in ART-naive patients (P < 0.0001) compared with ART-suppressed HIV-1-positive patients (data not shown). Levels of sCD14 did not change during continuous ART or a 6-week therapy interruption, but increased during long-term therapy interruption for more than 12 weeks compared with levels during ART (P < 0.0001), further suggesting sCD14 to be a correlate to LPS levels. A negative correlation between ΔsCD14 and ΔEndoCAb (correlation = −0.896, P = 0.0025) during a 6-week therapy interruption was also observed.
Unexpectedly, no difference among groups or change during viremia was demonstrated for LBP or gelsolin, beside lower levels of LBP in steady-state viremic patients after more than 12 weeks therapy interruption compared with uninfected participants (P = 0.01).
Discussion
We found different effects of viral replication on LPS plasma levels possibly dependent on the duration of the viremic episode and the degree of plasma LPS clearance by its ligands over time. No association between plasma LPS levels and the onset of immune activation during therapy interruption-associated viral replication was observed.
Increased EndoCAb IgM levels are common in conditions of chronic microbial translocation in the presence of functional B-cell responses [16], whereas acute microbial translocation coincides with lower levels of EndoCAb [7,17,18]. Decreases in EndoCAb levels observed in our study during viral replication of less than 12 weeks suggest that clearance of LPS from the circulation during short-term viremia is mediated by EndoCAb. A rise in LPS levels during long-term viremia of more than 12 weeks is likely attributed to EndoCAb saturation by excess of LPS or to inadequate B-cell function [19].
Consistent with previous studies showing increased levels of sCD14 in trauma [20], sepsis [21,22], autoimmune diseases [23,24], and HIV-1 infection [1,25,26], we observed a rise of sCD14 plasma levels in the presence of viral replication. In contrast to EndoCAb, no negative association with LPS was found, suggesting an inactivating role for sCD14 in the presence of LPS. In contrast to EndoCAb or sCD14, no changes in plasma levels of LBP and gelsolin were observed at any time point. These findings could be explained by differences in affinity, production, or clearance of these other LPS-binding molecules, or perhaps by the low molar ratio of LPS to these relatively abundant ligands.
Brenchley et al. [1] reported decreased LPS levels in the presence of ART and a positive association between immune activation and plasma LPS levels in chronically HIV-infected patients and in patients with AIDS. We not only confirmed their finding in patients with HIV viremia, but also observed lower LPS levels in suppressed patients compared with uninfected participants. This last counterintuitive finding may potentially reflect independent effect of ART on LPS levels or gut flora. Use of antibiotic prophylaxis by our suppressed patients could have contributed to this finding, and this type of information should be collected in targeted future studies to address this possibility. In addition to effective inactivation of LPS effects by EndoCAb in the short term, the observed lack of association between LPS levels and initial changes in immune activation following therapy interruption may be attributable to characteristics of our cohort such as lack of progression to AIDS, prior exposure to ART resulting in control of immune activation during suppression, or limited exposure to viral replication. Importantly, our data do not exclude the fact that T-cell activation during therapy interruption-mediated viremia may be affected by microbial translocation in addition to direct HIV-1 effects, as this study did not address measurement of other microbial products or their direct effects on T cells [27,28] or both.
Taken together, our data indicate that control of plasma microbial product translocation is only transiently maintained in the face of de-novo viral replication following therapy interruption and is dependent on the duration of the viremic episode and EndoCAb levels. This finding may help explain the lack of a positive association between LPS levels and viral load observed by others and us. The mechanism and capacity of LPS clearance achievable during therapy interruption following immune reconstitution remain to be further elucidated.
Acknowledgements
We would like to thank the uninfected participants and the HIV-1-positive patients who participated in the study and their providers, Cecile Gallo and Agnieszka Mackiewicz for study assistance, Jane Shull and the Board and Staff of Philadelphia FIGHT for providing patients samples, and Anne Meibohm for manuscript review. This work was primarily supported by a grant to L.J.M. by the National Institute of Allergy and Infectious Disease NIH AI48398. Additional support was provided by The Philadelphia Foundation (Robert I. Jacobs Fund), The Stengel–Miller family, AIDS funds from the Commonwealth of Pennsylvania, and from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health, as well as by the Cancer Center Grant (P30 CA10815). P.A.J. and R.B. began a sponsored research agreement with Critical Biologics Inc in May 2008 involving measurements of plasma gelsolin levels, but not otherwise related to the present study. This study was established before the SRA was initiated, and no support from it enabled any of the work presented in this manuscript. More than 7 years ago, M.J.DiN. served as a paid consultant to Biogen that was developing aerosolized rhGSN for the treatment of lung disease in patients with cystic fibrosis. He has more recently advised Critical Biologics Corporation (which has acquired the rights to rhGSN) regarding potential diagnostic and therapeutic uses of pGSN on an informal basis and without reimbursement. He is currently an employee of Merck Research Laboratories, which has no past or present ties to the development of gelsolin.
Footnotes
Data presented previously at 15th Conference on retroviruses and opportunistic infections, Boston, USA, February 3-February 6 2008, and published as abstract (number 299) in the conference’s abstract book (page 162).
References
- 1.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 2.Hailman E, Lichenstein HS, Wurfel MM, Miller DS, Johnson DA, Kelley M, et al. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frey EA, Miller DS, Jahr TG, Sundan A, Bazil V, Espevik T, et al. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11:225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 5.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 6.Cohen IR, Norins LC. Natural human antibodies to gram-negative bacteria: immunoglobulins G, A, and M. Science. 1966;152:1257–1259. doi: 10.1126/science.152.3726.1257. [DOI] [PubMed] [Google Scholar]
- 7.Strutz F, Heller G, Krasemann K, Krone B, Muller GA. Relationship of antibodies to endotoxin core to mortality in medical patients with sepsis syndrome. Intensive Care Med. 1999;25:435–444. doi: 10.1007/s001340050877. [DOI] [PubMed] [Google Scholar]
- 8.Bucki R, Georges PC, Espinassous Q, Funaki M, Pastore JJ, Chaby R, Janmey PA. Inactivation of endotoxin by human plasma gelsolin. Biochemistry. 2005;44:9590–9597. doi: 10.1021/bi0503504. [DOI] [PubMed] [Google Scholar]
- 9.DiNubile MJ. Plasma gelsolin: in search of its raison d’etre. Focus on “Modifications of cellular responses to Iysophospha-tidic acid and platelet-activating factor by plasma gelsolin”. Am J Physiol Cell Physiol. 2007;292:C1240–C1242. doi: 10.1152/ajpcell.00007.2007. [DOI] [PubMed] [Google Scholar]
- 10.Goetzl EJ, Lee H, Azuma T, Stossel TP, Turck CW, Karliner JS. Gelsolin binding and cellular presentation of lysophosphatidic acid. J Biol Chem. 2000;275:14573–14578. doi: 10.1074/jbc.275.19.14573. [DOI] [PubMed] [Google Scholar]
- 11.Mounzer KC, Moncure M, Smith YR, Dinubile MJ. Relationship of admission plasma gelsolin levels to clinical outcomes in patients after major trauma. Am J Respir Crit Care Med. 1999;160:1673–1681. doi: 10.1164/ajrccm.160.5.9807137. [DOI] [PubMed] [Google Scholar]
- 12.Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999;274:33179–33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 13.Papasavvas E, Azzoni L, Pistilli M, Hancock A, Reynolds G, Gallo C, et al. Increased soluble vascular cell adhesion mole-cule-1 plasma levels and soluble intercellular adhesion mole-cule-1 during antiretroviral therapy interruption and retention of elevated soluble vascular cellular adhesion molecule-1 levels following resumption of antiretroviral therapy. AIDS. 2008;22:1153–1161. doi: 10.1097/QAD.0b013e328303be2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papasavvas E, Kostman JR, Mounzer K, Grant RM, Gross R, Gallo C, et al. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med. 2004;1:e64. doi: 10.1371/journal.pmed.0010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papasavvas E, Kostman JR, Thiel B, Pistilli M, Mackiewicz A, Foulkes A, et al. HIV-1-specific CD4+ T cell responses in chronically HIV-1 infected blippers on antiretroviral therapy in relation to viral replication following treatment interruption. J Clin Immunol. 2006;26:40–54. doi: 10.1007/s10875-006-7518-8. [DOI] [PubMed] [Google Scholar]
- 16.Barclay GR. Endogenous endotoxin-core antibody (EndoCAb) as a marker of endotoxin exposure and a prognostic indicator: a review. Prog Clin Biol Res. 1995;392:263–272. [PubMed] [Google Scholar]
- 17.Kivilaakso E, Valtonen VV, Malkamaki M, Palmu A, Schroder T, Nikki P, et al. Endotoxaemia and acute pancreatitis: correlation between the severity of the disease and the antienter-obacterial common antigen antibody titre. Gut. 1984;25:1065–1070. doi: 10.1136/gut.25.10.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Windsor JA, Fearon KC, Ross JA, Barclay GR, Smyth E, Poxton I, et al. Role of serum endotoxin and antiendotoxin core antibody levels in predicting the development of multiple organ failure in acute pancreatitis. Br J Surg. 1993;80:1042–1046. doi: 10.1002/bjs.1800800840. [DOI] [PubMed] [Google Scholar]
- 19.Titanji K, De Milito A, Cagigi A, Thorstensson R, Grutzmeier S, Atlas A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 20.Kruger C, Schutt C, Obertacke U, Joka T, Muller FE, Knoller J, et al. Serum CD14 levels in polytraumatized and severely burned patients. Clin Exp Immunol. 1991;85:297–301. doi: 10.1111/j.1365-2249.1991.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanco A, Solis G, Arranz E, Coto GD, Ramos A, Telleria J. Serum levels of CD14 in neonatal sepsis by Gram-positive and Gram-negative bacteria. Acta Paediatr. 1996;85:728–732. doi: 10.1111/j.1651-2227.1996.tb14135.x. [DOI] [PubMed] [Google Scholar]
- 22.Landmann R, Zimmerli W, Sansano S, Link S, Hahn A, Glauser MP, Calandra T. Increased circulating soluble CD14 is associated with high mortality in gram-negative septic shock. J Infect Dis. 1995;171:639–644. doi: 10.1093/infdis/171.3.639. [DOI] [PubMed] [Google Scholar]
- 23.Horneff G, Sack U, Kalden JR, Emmrich F, Burmester GR. Reduction of monocyte-macrophage activation markers upon anti-CD4 treatment. Decreased levels of IL-1, IL-6, neopterin and soluble CD14 in patients with rheumatoid arthritis. Clin Exp Immunol. 1993;91:207–213. doi: 10.1111/j.1365-2249.1993.tb05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nockher WA, Wigand R, Schoeppe W, Scherberich JE. Elevated levels of soluble CD14 in serum of patients with systemic lupus erythematosus. Clin Exp Immunol. 1994;96:15–19. doi: 10.1111/j.1365-2249.1994.tb06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lien E, Aukrust P, Sundan A, Muller F, Froland SS, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: correlation to disease progression and clinical events. Blood. 1998;92:2084–2092. [PubMed] [Google Scholar]
- 26.Nockher WA, Bergmann L, Scherberich JE. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clin Exp Immunol. 1994;98:369–374. doi: 10.1111/j.1365-2249.1994.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funderburg N, Luciano AA, Jiang W, Rodriguez B, Sieg SF, Lederman MM. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS ONE. 2008;3:e1915. doi: 10.1371/journal.pone.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]