Abstract
Objective
Sepsis-induced organ dysfunctions remain prevalent and account for >50% of intensive care unit admissions for acute renal failure with a mortality rate nearing 75%. In addition to the fact that the mechanisms underlying the pathophysiology of sepsis-related acute renal failure are unclear, the impact on septic-induced acute renal failure of either norepinephrine, a gold-standard vasopressor, and arginine vasopressin, a candidate alternative, are not well understood.
Design
Randomized and controlled in vivo study.
Setting
Research laboratory and animal facilities.
Subjects
Adult rats treated with endotoxin (lipopolysaccharide) and/or vasopressors.
Interventions
Rats were intraperitoneally injected with lipopolysaccharide (12 mg/kg) or saline and then infused with either saline, 0.375 μg/μL arginine vasopressin, or 32.5 μg/μL norepinephrine for 18 hrs. These vasopressor rates yielded respective targeted blood levels observed in human septic shock.
Measurements and Main Results
Renal function, including glomerular filtration rate and fraction, renal blood flow, aquaporin-2, and arginine vasopressin-2 (V2 receptor) networking, water and salt handling, and urinary protein excretion, were evaluated. After lipopolysaccharide challenge arginine vasopressin infusion: 1) impaired creatinine clearance without affecting renal blood flow, glomerular filtration rate, and fraction but reduced free-water clearance, both of which being partially restored by the V2 receptor antagonist SR-121463B; 2) decreased the recognized ability of arginine vasopressin alone to recruit aquaporin-2 to the apical membrane increase its mRNA expression and urinary release; 3) increased urinary protein content but decreased specific kidney injury molecule-1, and Clara cell protein-16 release (p < 0.05 vs. lipopolysaccharide alone). Conversely, norepinephrine infusion did not add to lipopolysaccharide-induced alteration of urine biochemistry, except for improved creatinine clearance and increased microalbuminuria.
Conclusion
In this endotoxic model, dose-targeted arginine vasopressin infusion increased lipopolysaccharide-induced renal dysfunction without affecting renal blood flow and glomerular function, but with particular disruption of aquaporin-2/V2 receptor networking, consecutive decreased salt and water handling ability. This is in clear contrast with norepinephrine infusion and suggests specific arginine vasopressin-induced “tubular epithelial dysfunction.”
Keywords: AQP-2, V2R, kidney dysfunction, tubular injury, endotoxin (lipopolysaccharide)
Severe sepsis is commonly associated with kidney dysfunction and accounts for >50% of intensive care unit admissions for acute renal failure (ARF) with a mortality rate of 75%, when compared with 45% in intensive care unit patients without sepsis (1, 2). Septic shock can lead to altered renal blood flow (RBF) with afferent arteriolar vasoconstriction, resulting in ischemic kidneys, although this is still under debate. To compare outcomes, new RIFLE and AKIN criteria have been published as to acute kidney injury and ARF definitions, including creatinine, urine output (UO), and/or glomerular filtration rate (GFR) assessments (3, 4). In parallel, hyperdynamic septic shock animal models have revealed increased RBF with a reduction in vascular resistance (5). Endotoxemia per se causes specific alterations in intra-RBF distribution, inducing an imbalance between “dilators” (nitric oxide, prostaglandins) and “constrictors” (endothelin, angiotensin II, norepinephrine [NE]) (5).
Sepsis treatment guidelines recommend the use of vasopressors in septic shock resistant to fluid resuscitation (i.e., sustained life-threatening low systemic blood pressure) (6). Indeed, infusion of NE is a standard of care (6) but at least 20% of septic shock requires high doses of NE (i.e., above 0.5 μg/kg/min) reaching blood concentrations at which the beneficial to harmful effect ratio of catecholamines can often be deleterious. Meanwhile, rescue molecules, alternatives to NE, have recently been considered and are still currently under investigation. Arginine vasopressin (AVP) is one such example and has been shown to be a valuable catecholamine-sparer in extreme conditions (7). AVP acts through at least three known differentially located G-protein (V1a, V2, and V1b) receptors (8). V1aR triggers AVP-related vasoconstrictive activity, whereas V2R regulates water resorption in the kidney collecting tubes through a specific water channel: i.e., aquaporin (AQP)-2 (8). V1b, mainly expressed in the anterior pituitary gland, regulates some of the adrenocorticotropic hormone releasing activity.
NE was first thought to worsen renal function by extreme nonselective vascular constriction in septic shock, combining both afferent and efferent effects (9). However, NE at “relevant dosing” can help maintain RBF, GFR, and UO; restore blood pressure; and preserve medullary flow (9-11). AVP, on the other hand, preferentially induces vasoconstriction of efferent, more than afferent arterioles, and decreases medullary flow with equivocal impact on RBF and GFR (12-14). Whether UO and creatinine clearance (CCr) are ultimately improved by using AVP in septic conditions still remains a matter of debate (8, 15-17).
The primary objective of this work was to investigate the impact of “dose-targeted” NE vs. AVP infusions on renal function, modulation of AQP-2/V2R expression and tubular injury, in a subacute endotoxic model. A second objective was to analyze the influence of a V2R antagonist (V2Ra) on AVP- and lipopolysaccharide (LPS)-induced renal dysfunction. Both objectives were aimed at testing the main hypothesis that, at relevant dosing, AVP differentially modulates LPS-induced AQP-2/V2R dysregulation with distinctive renal functional impact.
METHODS
Experimental Animal Models
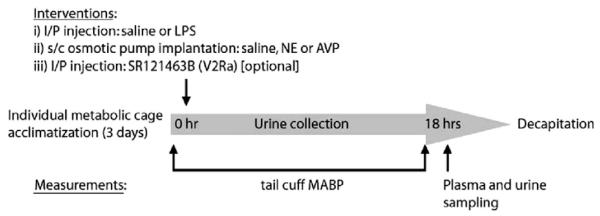
Pathogenfree male Wistar rats (350 g) were purchased from Charles River Laboratories (Wilmington, MA) and received care in compliance with the National Institutes of Health and Canadian Council Guides for the Care and Use of Laboratory Animals. The following protocols were approved by our institution’s Ethics Committee for animal care and experimentation. A cuff with a pneumatic sensor (IITC Life Science; Woodland Hills, CA) was attached to rat’s tail at baseline and at the end of the experimental challenge, for mean arterial blood pressure measurements using a sphygmomanometer (Mabis; Waukegan, IL). An intraperitoneal injection of 1 mL saline with or without 12 mg/kg of Escherichia coli 055:B5 LPS (Sigma, St-Constant, PQ, Canada) was given and thereafter, osmotic minipumps (Alzet® osmotic pumps, model 2001, Cupertino, CA) were implanted subcutaneously in the dorsal neck of isoflurane-anesthetized rats. Pumps contained either saline, or 0.375 μg/μL AVP in saline, or 32.5 μg/μL NE in 2 μg/μL ascorbic acid/saline. These dosages targeted representative concentrations achieved during blood pressure-supporting vasopressor infusion in human septic shock (i.e., 3000–6000 pg/mL for NE and 100–300 pg/mL for AVP) (18, 19). After this short anesthesia (i.e., <5 mins), rats were individually placed in metabolic cages with free access to food and water, to collect urine on ice for 18 hrs. At this end point, urine was collected, rats were quickly decapitated for trunk blood collection, and kidney medulla was snap frozen and stored at -80°C. Six groups with eight rats in each group were compared: 1) control (saline); 2) NE; 3) AVP; 4) LPS; 5) LPS and NE; 6) LPS and AVP. Specific supplemental experiments were conducted with similar design in the AVP and LPS-AVP groups, using SR-121463B[1-[4-(N-tert-butylcarbamoyl)-2-methoxybenzenesulfonyl]-5-ethoxy-3-spiro [4[(2morpholinoethoxy)cyclohexane]indoline-2-one, phosphate monohydrate cis-isomer], a selective V2R non-peptide antagonist, 10 mg/kg intraperitoneally at the same time as LPS injection (gift of Dr C. Serradeil-Le Gal, Sanofi-Synthelabo, Toulouse, France) (20) (Fig. 1).
Figure 1.
Study design; LPS, lipopolysaccharide; NE, norepinephrine; AVP, arginine vasopressin.
Analysis of Plasma and Urine Biochemistry
Measurements of plasma and urinary concentrations of sodium and creatinine were determined by a Vitros 750 XRC analyzer (Johnson-Johnson Clinical Diagnostics; Rochester, NY). Electrolyte free-water clearance (CH2O) was calculated as UV × (1 - [UrNa]) + [UrK]/[Na plasma] (20). CCr was calculated from the clearance formula UV/P × 1/1440 min, where U and V are urine concentration and volume, and P is plasma concentration, normalized to rat weight. Fractional sodium excretion (FeNa+) was calculated as clearance of Na+/clearance of creatinine. Analysis of urinary osmolality was carried out by freezing point depression. At the end of the experimental period, main renal hemodynamic parameters were calculated using inulin and PAH clearances, as markers of GFR and effective renal plasma flow, respectively. Hematocrit was measured and standard calculation formulas were used to determine RBF (RBF = [effective renal plasma flow/0.73]/[1-hematocrit]), and filtration fraction (FF) (FF = GFR/effective renal plasma flow/0.73) (21). Kidney injury molecule (Kim)-1 protein in urine was measured using Microsphere-based Luminex xMAP technology with monoclonal antibodies raised against rat Kim-1 in the Vaidya/Bonventre laboratory. This technique is an adaptation of the recently developed and validated sandwich enzyme-linked immunosorbent assay described previously (22, 23). Measurement of urinary CC-16 (also called Clara cell protein or urinary protein-1) concentrations was determined by an automated latex immunoassay (24). AVP plasma concentration was quantified according to a commercially available enzyme immunosorbent assay kit (Assay Designs, Ann Arbor, MI), with a measurement range of 4.1–1000 pg/mL. After alumina extraction, NE plasma concentration was quantified by high-performance liquid chromatography with electrochemical detection.
Western and Dot Blottings
Protein extracts from kidney medulla were obtained as described previously (25), with additional centrifugation for plasma membrane proteins. For V2R dot blot analysis, 1 μg of plasma membrane protein was dropped onto nitrocellulose membranes. Membranes were incubated overnight with rabbit anti-AQP-2 (1:2000; Calbiochem, La Jolla, CA) or anti-V2R (1:1000; gift of Werner Muller-Esterl, JW Goethe University, Germany) and then with horseradish peroxidase-labeled goat anti-rabbit (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA), before enhanced chemiluminescence detection system revelation (Amersham ECL, GE Healthcare, Chalfont St. Giles, UK).
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
Total RNA of medullary samples were extracted with TRIzol (Invitrogen Life Technologies, Burlington, ON). Real-time polymerase chain reaction reactions were carried out using the real-time one step RNA polymerase chain reaction kit (Takara, Madison, WI) combined with SyBRGreen, and analyzed with the Rotor-Gene apparatus (Corbett Research, Montreal Biotech, Kirkland, QC), using 18S as housekeeping gene. AQP-2 primers sequences were designed based on published sequences (26): forward, 5′-CTGGTGCTGTGCATCTTTGC-3′; reverse, 5′-ATGGAGCAACCGGTGAAAT-3′. V2R primer sequences were designed using Primer Express software (Applied Biosystems, Foster City, CA): forward, 5′-TAGCATACCGCCATGGA-3′; reverse, 5′-CAAAGATGAAGAGCTGAGGCA-3′.
Immunofluorescence
Five-micrometer kidney medulla slices were labeled with polyclonal rabbit anti-AQP-2 (1:100; Calbiochem), lectin from Dolichos biflorus TRITC conjugated (0.0125 mg/mL; Sigma) in 1.5% goat serum. Anti-AQP-2 staining was revealed with FITC-conjugated goat anti-rabbit IgG (1:50; Santa Cruz) and 2% goat serum, and nuclear contrast with To-PRO-3 staining (Invitrogen).
Quantitative Confocal Laser Scanning Microscopy
Kidneys were examined with a scanning confocal microscope (FV1000, Olympus, Tokyo, Japan) coupled to an inverted microscope with a 63× oil immersion objective (Olympus). Images of tubule segments (15–25/image), co-labeled with AQP-2 and lectin, were selected in the kidney medullary region (region of interest) of each rat. For FITC/TRITC merged fluorescence images, dot fluorograms were obtained by plotting pixel values of each component toward horizontal and vertical axes, respectively.
Statistical Analysis
Results are expressed as mean ± SD. A one-way analysis of variance was used to evaluate within-group differences. Inter-group difference was tested using a two-way analysis of variance (with treatment as independent variable) and a Kruskal-Wallis test (nonparametric analysis of variance) for CC-16 measurements. For real-time polymerase chain reaction experiments, data were first transformed into log units and then compared using ratio paired Student’s t-tests. The p values <0.05 were considered as the threshold for significance.
RESULTS
Implanted vasopressor-containing osmotic pumps resulted in targeted blood concentrations described in human septic shock for both AVP and NE (Table 1). In parallel, rat mean arterial blood pressure remained essentially similar in all LPS-challenged groups at the end-point (64 ± 8.5 mm Hg, 61 ± 5 mm Hg, and 67.5 ± 9 mm Hg, for LPS-saline, LPS-AVP, and LPS-NE, respectively), being slightly decreased in comparison with the control group (86 ± 11 mm Hg, not significant), whereas generally unchanged with AVP or NE infusions alone (76 ± 8 mm Hg and 84 ± 17 mm Hg, respectively, not significant). End point hematocrit measurements were similar except for LPS-saline and LPS-AVP groups (0.41 ± 0.12 vs. 0.44 ± 0.08, respectively, p < 0.01, controls: 0.42 ± 0.07).
Table 1.
Plasma vasopressin and norepinephrine after 18 hr-osmotic pump infusion
| Experimental Groups |
Plasma AVP (pg/mL) |
Plasma NE (nmol/L) |
|---|---|---|
| Saline | 4.33 ± 0.6 | 240 ± 2 |
| NE | 12.4 ± 4.3a | 3466 ± 45b |
| AVP | 269 ± 34c | 494 ± 33a |
| LPS Saline | 5.5 ± 1.3 | 839 ± 24a |
| LPS NE | 15.7 ± 6.9a | 4835 ± 128b |
| LPS AVP | 213.7 ± 61c | 262 ± 5 |
AVP, arginine vasopressin; NE, norepinephrine; LPS, lipopolysaccharide.
The values are expressed as mean ± SD (n = 8).
p < 0.05
p < 0.001 vs. saline (control)
p < 0.01
Impact of LPS on Renal Function and AQP-2/V2R Expression
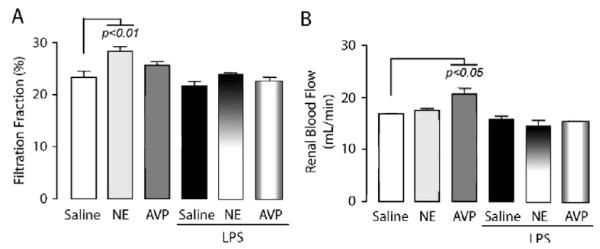
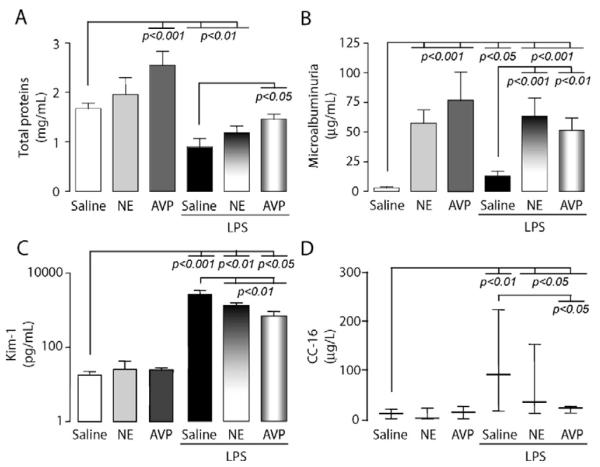
UO was enhanced 18 hrs after endotoxin challenge (717 ± 50 μL/hr vs. 445 ± 65 μL/hr, p < 0.05), exhibiting reduced osmolality, FeNa+, as well as creatinine and H2O clearances (Fig. 2A—D). GFR, as well as FF and RBF, were not significantly affected by LPS (2.09 ± 0.29 vs. 2.32 ± 1.15) (Fig. 3A and B). The concentrations of proximal epithelial-derived tubular proteins, Kim-1 and CC-16, by contrast to glomerular microalbumin, were significantly increased in urine in response to LPS (Fig. 4C and D). Apical membrane AQP-2 insertion increased without alteration of mRNA or urinary release (Fig. 5D and E), whereas V2R protein and mRNA were decreased in the inner medulla (Fig. 6A and B).
Figure 2.
Modulation of renal function after endotoxin (lipopolysaccharide, LPS) challenge in the presence or absence of vasopressors (arginine vasopressin [AVP] and norepinephrine [NE]). Rats (n = 8) were challenged according to the methodology described under Methods section. The bar charts represent analysis (mean ± SD) of A, creatinine clearance (CCr) established by the following formula (UV/P × 1/1440 min); B, urine osmolality; C, fractional sodium excretion (FeNa+) established by the following formula (C Na+/CCr); D, Electrolyte- free-water clearance (CH2O) calculated by the following formula (UV × (1 - [UrNa]) + [UrK]/[Na plasma]). Data are representative of trunk blood and total 18-hr urine from control (saline, white bar), norepinephrine (NE, gray bar), arginine vasopressin (AVP, dark-gray bar), LPS plus saline (black bar), LPS plus NE (horizontal shading gradient bar), and LPS plus AVP (vertical shading gradient bar)-treated rats. The p values are indicative of significant difference(s) vs. control or vs. LPS.
Figure 3.
Modulation of renal filtration fraction (FF) and renal blood flow (RBF) after endotoxin (lipopolysaccharide, LPS) challenge in the presence or absence of vasopressors (arginine vasopressin [AVP] and norepinephrine [NE]). The bar charts represent measurements (mean ± SD) of A, FF(%) and B, RBF, calculated as described in “Methods” section. Data are representative of total 18 hr-urine from groups with similar bar assignment (n = 8), as described in Fig 2. The p values are indicative of significant difference(s) vs. control.
Figure 4.
Modulation of urinary biomarkers after endotoxin (lipopolysaccharide, LPS) challenge in the presence or absence of vasopressors (arginine vasopressin [AVP] and norepinephrine [NE]). The bar charts represent measurements in urine of A, total proteins; B, microalbumin; C, kidney injury molecule-1 (Kim-1) (log scale on y-axis) (mean ± SD); D, Clara cell protein (CC-16) (median, 25th—75th percentiles). Data are representative of total 18-hr urine from groups with similar bar assignment (n = 8), as described in Fig 2. The p values are indicative of significant difference(s) vs. control or vs. LPS.
Figure 5.
Modulation of renal aquaporin (AQP)-2 apical membrane translocation, mRNA expression and urinary release after endotoxin (lipopolysaccharide, LPS) challenge in the presence or absence of vasopressors (arginine vasopressin [AVP] and norepinephrine [NE]). A, AQP-2 expression in kidney inner medulla (green fluorescence) with nuclear To-Pro-3 contrast (red fluorescence) (low magnification, 100×, bar represents 200 μm). The white-lined square delineates the region of interest for subsequent laser confocal double labeling. B, Internal medulla collecting ducts (IMCD) AQP-2 and lectin double-labeled confocal image analysis with pixel fluorograms. The left portion represents typical fluorograms obtained from the medulla of control, AVP, and LPS plus AVP-treated rats (respectively from top to bottom). Dot fluorograms were obtained by plotting AQP-2 (FITC pixel values) towards the horizontal axis and lectin from Dolichos biflorus (TRITC pixel values) towards the vertical axis. Quadrant markers were placed forming background (lower left), lectin-only (upper left), AQP-2-only (lower right), and overlapping lectin-AQP-2 areas (upper right). The right portion represents typical merged images consistent with fluorogram quantitative analysis. The overlapping signals of green-labeled AQP-2 and red-labeled lectin from Dolichos biflorus appear as yellow (magnification: 630×; scale bar represents 5 μm). C, Quantitation of AQP-2 apical membrane expression in IMCD as described above. D, Urinary release of nonglycosylated AQP-2: representative duplicates of both glycosylated and nonglycosylated (ng) forms run on Western blots are shown, but only ng forms were scan-analyzed. E, Real-time polymerase chain reaction quantification normalized over the housekeeping gene 18S. For all relevant panels, bar assignment (n = 8) is similar to that described in Fig 2. The p values are indicative of significant difference(s) vs. control or vs. LPS.
Figure 6.
Modulation of renal V2 receptor (V2R) protein and mRNA expression after endotoxin (lipopolysaccharide, LPS) challenge in the presence or absence of vasopressors (arginine vasopressin [AVP] and norepinephrine [NE]) The bar charts represent quantification (mean ± SD) of A, V2R protein membrane and B, real-time polymerase chain reaction of V2R mRNA expression normalized over the housekeeping gene 18S. Data are representative of kidney medulla with similar bar assignment (n = 8) as that shown in Fig 2. The p values are indicative of significant difference(s) vs. control or vs. LPS.
Influence of Vasopressors (AVP vs. NE) on Renal Function and AQP-2/V2R Expression in Rats Exposed or Not Exposed to LPS
AVP sole infusion induced previously reported changes on renal functions: i.e., 1) decreased UO (275 ± 63 μL/hr vs. 445 ± 65 μL/hr, p < 0.05), 2) increased urine osmolality (Fig. 2B), 3) decreased creatinine and H2O clearances (Fig. 2A and D), 4) enhanced RBF but not FF (Fig. 3A and B), 5) increased urinary total protein and microalbuminuria but not urinary Kim-1 or CC-16 (Fig. 4A and D), and 6) decreased V2R with enhanced AQP-2 protein apical membrane expression and AQP-2 urinary release (Figs. 5 and 6).
However, LPS challenge together with AVP infusion altered the above profile: 1) restored UO (595 ± 78 μL/hr vs. 275 ± 63 μL/hr, p < 0.05), 2) collapsed urine osmolality (Fig. 2B), 3) further decreased CCr with enhanced FeNa+ and reduced CH2O (Fig. 2A, C, E) without further affecting GFR, FF, or RBF (Fig. 3A and B), 4) increased urinary Kim-1 release (Fig. 4C), and 5) suppressed enhanced-AQP-2 apical membrane protein recruitment/accumulation and urinary release, and blunted transcriptional level (Fig. 5).
NE sole infusion offered a distinctive pattern with increased UO (688 ± 159 μL/hr vs. 275 ± 63 μL/hr for AVP, p < 0.05) and FF (Fig. 3A), without changes in CCr, FeNa+, urinary osmolality and CH2O (Fig. 2) with no increase in AQP-2 urinary release (Fig. 5B). The combination of LPS challenge together with NE resulted in decreases in both osmolality and FeNa+ and reduced CH2O (Fig. 2B—D), increased Kim-1 and CC-16 urinary release (Fig. 4C and D), and reduced V2R mRNA (Fig. 6B) but there was an increase in CCr over LPS alone and equivalent changes in GFR, FF, RBF, osmolality, FeNa+, and CH2O compared with LPS alone (Figs. 2B—D,3A and B).
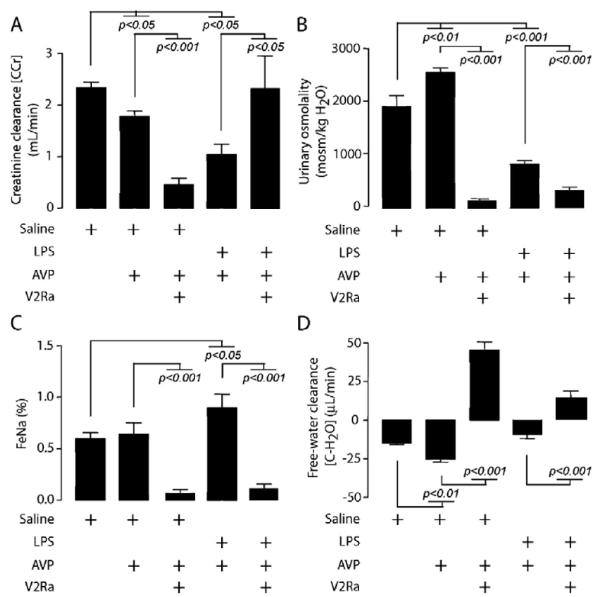
Impact of V2Ra on LPS- and AVP-LPS-Induced Renal Dysfunction
V2Ra injection completely converted AVP-induced relative oliguria into a severe polyuria (7040 ± 900 μL/hr vs. 288 ± 60 μL/hr in the AVP group and 445 ± 65 μL/hr in the saline group, p < 0.05). The effect was aquaretic with collapsed osmolarity and FeNa+, decreased CCr, and enhanced CH2O (Fig. 7). V2Ra co-administration, together with LPS challenge and AVP infusion, further increased diuresis (1800 ± 400 μL/hr vs. 590 ± 80 μL/hr in the LPS-AVP group, p < 0.05), restored CCr, further reduced urine osmolality and FeNa+, and inversed CH2O from negative to positive (Fig. 7).
Figure 7.
Effect of V2R antagonist (V2Ra) SR-121463B on lipopolysaccharide (LPS)-induced renal dysfunction with or without arginine vasopressin (AVP) infusion. The bar charts represent analysis (mean ± SD) of A, creatinine clearance (CCr) established by the formula (UV/P × 1/1440 min); B, urine osmolality; C, fractional sodium excretion (FeNa+) established by the formula (C Na+/CCr); D, Electrolyte free-water clearance (CH2O) calculated by the formula (UV × (1 - [UrNa]) + [UrK]/[Na plasma]). Data are representative of trunk blood and total 18-hr urine from (left to right bar) control (saline), AVP and V2Ra (SR-121463B), LPS plus AVP, and LPS plus AVP and V2Ra (SR-121463B)-treated rats (n = 8). The p values are indicative of significant difference(s) vs. control or respective group without V2Ra.
DISCUSSION
In the present normotensive experimental endotoxic model, there was evidence of renal dysfunction without macrocirculatory RBF and filtration disability but with disturbed AQP-2 exocytosis processes and impaired ability for water resorption after LPS challenge. More specifically, LPS not only impaired AVP’s ability to boost AQP-2 exocytosis but also probably induced functional alterations, as suggested by the relative dissociation between urinary osmolality and AQP-2 membrane availability.
The pathogenesis of ARF in sepsis remains incompletely understood (27). Most human reports have focused on renal vasoconstriction with a subsequent impact on GFR. Endotoxemia causes alterations in intrarenal distribution of blood flow which affects the medullary level earlier (14, 28). This medulla-to-cortex microvascular redistribution is not necessarily apparent by measuring total RBF, and the associated reactivity to vasoconstrictor agents is either normal or increased (29-31). Several hours after challenge, endotoxemia in rats often causes polyuria (31, 32, 34). Indeed, as much as one third of sepsis-induced ARF are polyuric at bedside (33, 34), and when oliguric, acute tubular necrosis is generally suspected (5, 34). However, acute tubular necrosis is generally lacking at autopsy (5) and minimal or no histologic alterations have been described in a series of patients deceased from sepsis—including half with ARF—and in septic sheep exhibiting ARF (35). It may be, however, that the histologic tests for necrosis are not sensitive enough and a marked increase in Kim-1 has been shown to be a very sensitive marker of early injury (22, 23).
AQP-2 Exocytosis Network After LPS Challenge: Water- and Pump-Excretion and Salt Handling
Fractional water excretion has already been described as elevated with short-term intravenous endotoxin challenge, in spite of moderately increased AVP plasma concentration (∼20 pg/mL) (32). In this study, an increased UO and water clearance were clearly observed using an intraperitoneal endotoxin challenge despite an AVP osmotic pump infusion, which induced a tenfold higher induced AVP plasma concentration. Importantly, this model demonstrated increased kidney AQP-2 apical membrane expression after LPS challenge, contrary to that observed in three studies (31, 32, 34), especially that of Versteilen et al. (34) who localized reduced lumen AQP-2, but in a short-term intravenous and fluid-resuscitated model. In addition, AVP-induced enhanced daily urinary excretion of AQP-2, which represents nearly 4% of total kidney expression at baseline (36), was almost completely abolished by LPS challenge. In Jonassen’s study (31), there was no clear explanation regarding the discordance between high water release and unchanged AQP-2 expression. In this study, this discrepancy was obvious with LPS- and even more so with AVP-LPS-induced enhanced modulation of AQP-2 expression. The inability of AVP to recruit AQP-2 apically after LPS challenge was also observed by using an V2R selective agonist (34). Indeed, the end-exocytic process of AQP-2 was clearly abortive with membrane pump accumulation and urine release down-regulation, indicating a “functional” impairment of distal tubular resorption. The mechanisms underlying this functional alteration remain unexplained. On the other hand, AQP-2 gene expression together with V2R protein and gene expression were decreased, suggesting an adaptive process which may no longer be sustained.
In this study, urinary osmolality dramatically decreased with LPS challenge as observed by some but not all studies (27, 31), together with collapsed FeNa+. AVP completely reversed the LPS-induced drop in FeNa+, and this effect is more likely related to decreased CCr and impaired tubular Na reabsorptive capacity provoked by LPS and AVP co-exposure. In spite of a relative increase in sodium clearance, urine osmolality remained low, presumably related to a greater increase in water clearance with AQP-2 dysfunction.
Differential Effects of Vasopressors on Renal Function After LPS Challenge
During the past 20 years, NE has often been accused of inducing a disproportionate/excessive reduction of renal afferent arteriole flow with consequent reduction in GFR and UO. In actual fact, NE at clinically relevant doses increases RBF and medullary flow (11, 37), and can markedly increase UO. AVP, on the other hand, has been shown to improve or preserve RBF in animal models (7, 14), in particular in the cortex, suggesting potential beneficial effects on UO in this context. Indeed, in this model, NE on the contrary to AVP infusion did not affect RBF but increased renal FF, whereas both drugs did not further influence these two parameters after LPS challenge. In fact, UO rather increased after LPS challenge in this study, and was unchanged by AVP infusion, although no specific fluid resuscitation was done and in spite of a decline in CCr. Nonetheless, recent human reports on septic shock have reported that AVP or terlipressin, a synthetic analog with longer half-life and higher V1R selectivity, can increase UO after 4- to 8-hrs treatment (15, 38), but improved CCr is not clearly sustained with longer exposure (16, 17).
Impact of a V2Ra
Nonpeptide V2Ra is known to reduce urine osmolality by increasing water excretion (i.e., aquaretic effect). This is likely related to a sustained reduction in AQP-2 molecular expression and apical membrane translocation (39). In this study, SR-121463B, a V2Ra, in combination with AVP, in effect induced hypoosmolar water diuresis and reversed LPS-induced decrease in CCr while maintaining aquadiuretic trend. Whether CCr should be considered as the gold standard of renal function in this context is disputable.
Proteins in Urine After LPS Challenge
Microalbuminuria is considered as a sensitive marker of increased glomerular endothelium permeability, well correlated with systemic permeability and subsequent organ failure (40). However, only minor focal injury of glomeruli have been observed histologically in patients with sepsis and in experimental settings (32, 41). These data confirmed that LPS, per se, does not clearly provoke microalbumin leakage to the levels seen with AVP or NE, although dDAVP, a V2R agonist, has been shown to increase proteinuria (42). Of course, altered regional perfusion with vasopressor infusion cannot be discarded as cause of the protein urinary release from proximal tubules after LPS challenge. Indeed, peritubular capillary dysfunction as an early event in tubular stress and renal injury has been recently highlighted (43). Specific protein measurements in urine have been advocated as useful and sensitive markers for the early detection of acute kidney injury (42), such as released Kim-1 or secreted CC-16 (or UP-1) (22, 44, 45) from proximal tubule segments. Herein, Kim-1 and CC-1, but not microalbumin, were massively released in urine after LPS challenge. Mechanisms and locations of these protein/peptide’s release are clearly distinctive and might explain this observation (21-24). Vasopressor’s infusion reduced both LPS-induced Kim-1 and CC-16 release although the Kim-1 level remained quite elevated.
Limitations of this Study
This study describes the renal impact of an experimental endotoxic model without serial blood pressure measurements (leaving aside the end point measurement) and without specific fluid resuscitation. Even with the recent VASST data release (17), which used a fixed low-dose of AVP in septic patients and found no benefit/no harm on the kidney function, more knowledge is still mandatory, and our data should not be directly translated to human septic shock before further validation (7, 15-17, 21, 38). Also, not all AQP-2 “in and out” trafficking was studied, especially with regard to the endocytotic component of pump cycling (46).
CONCLUSION
Selection of vasopressors has a major impact on the regulation of AVP receptor (V2R) and AQP-2 expression (as well as in exocytosis for the latter) in the kidney after endotoxin injection. After LPS challenge and at similar systemic and renal blood perfusion pressures, exogenous AVP has greater effects on water and salt handling as well as urinary protein and pump excretion than does NE, suggesting that AVP’s effects on the vasculature and/or impaired exocytosis and possibly dysfunctional AQP-2 activity contribute to its effects seen in sepsis.
ACKNOWLEDGMENTS
The authors thank Lucie Chouinard, Angele Chainey, and Xavier Dumont for their technical expertises, Leonid Volkov for his expertise and availability during confocal microscopy assays, and Marc Letellier from the Department of Biochemistry for NE measurements.
Supported in part by the Département de Médecine du CHUS; NIH grants DK39773, DK72381, and DK74099 (JVB); and Scientist Development grant 0535492T from the American Heart Association (VSV). Olivier Lesur is a research scholar of the FRSQ.
Footnotes
See also Vasopressin and the kidney: Two false friends? Ertmer, D; Morelli, A; Westphal M. Critical Care Medicine, November 2008, Vol. 36 (11), 3111-3112 (doi: 10.1097/CCM.0b013e318187b7b3).
The authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.DeMendonca A, Vincent JL, Suter PM, et al. Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915–921. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- 2.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 3.Hoste EAJ, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan L, Bellomo R, Di Giantomasso D, et al. The pathogenesis of septic acute renal failure. Cur Opin Crit Care. 2003;9:496–502. doi: 10.1097/00075198-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Dellinger RP, Carlet JM, Masur H, et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 7.Holmes CL, Walley KR. Vasopressin in the ICU. Curr Opin Crit Care. 2004;10:442–448. doi: 10.1097/01.ccx.0000144769.19213.0c. [DOI] [PubMed] [Google Scholar]
- 8.Dibas AI, Mia AJ, Yorio T. Aquaporins (water channels): Role in vasopressin-activated water transport. Proc Soc Exp Biol Med. 1998;219:183–199. doi: 10.3181/00379727-219-44332. [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R. Noradrenaline: Friend or foe? Heart Lung Circ. 2003;12(Suppl 2):S42–S48. doi: 10.1046/j.1443-9506.2003.t01-7-.x. [DOI] [PubMed] [Google Scholar]
- 10.Farand P, Hamel M, Lauzier F, et al. Effects of norepinephrine and vasopressin on sepsis-induced organ perfusion/permeability. Can J Anaesth. 2006;53:934–946. doi: 10.1007/BF03022837. [DOI] [PubMed] [Google Scholar]
- 11.Di Giantomasso D, May CN, Bellomo R. Norepinephrine and vital organ blood flow during experimental hyperdynamic sepsis. Intensive Care Med. 2003;29:1774–1781. doi: 10.1007/s00134-003-1736-9. [DOI] [PubMed] [Google Scholar]
- 12.Bourgoin A, Leone M, Delmas A, et al. Increasing mean arterial pressure in patients with septic shock: Effects on oxygen variables and renal function. Crit Care Med. 2005;33:780–786. doi: 10.1097/01.ccm.0000157788.20591.23. [DOI] [PubMed] [Google Scholar]
- 13.Medina P, Vila JM, Martinez MC, et al. Effects of vasopressin on human renal arteries. Eur J Clin Invest. 1996;26:966–972. doi: 10.1046/j.1365-2362.1996.2310543.x. [DOI] [PubMed] [Google Scholar]
- 14.Albert M, Losser M-R, Hayon D, et al. Systemic and renal macro- and microcirculatory responses to arginine vasopressin in endotoxic rabbits. Crit Care Med. 2004;32:1891–1898. doi: 10.1097/01.ccm.0000139708.10718.e3. [DOI] [PubMed] [Google Scholar]
- 15.Lauzier F, Levy B, Lamarre P, et al. Vasopressin versus norepinephrine in early septic shock: A randomized controlled pilot trial. Intensive Care Med. 2006;32:1782–1789. doi: 10.1007/s00134-006-0378-0. [DOI] [PubMed] [Google Scholar]
- 16.Albanese J, Leone M, Delmas A, et al. Terlipressin or norepinephrine in hyperdynamic septic shock: A prospective, randomized study. Crit Care Med. 2005;33:1897–1902. doi: 10.1097/01.ccm.0000178182.37639.d6. [DOI] [PubMed] [Google Scholar]
- 17.Russell JA, Walley KR, Singer J, et al. Vasopressin versus Norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MD, Smith PG, Mills E, et al. Paradoxical elevation of sympathetic activity during catecholamine infusion in rats. J Pharmacol Exp Ther. 1983;227:254–259. [PubMed] [Google Scholar]
- 19.Hauger RL, Aguilera G. Regulation of pituitary corticotropin releasing hormone (CRH) receptors by CRH: Interaction with vasopressin. Endocrinology. 1993;133:1708–1714. doi: 10.1210/endo.133.4.8404613. [DOI] [PubMed] [Google Scholar]
- 20.Huang D-Y, Plaff I, Serradeil-Le Gal C, et al. Acute renal response to the non-peptide vasopressin V2-receptor antagonist SR121463B in anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:201–207. doi: 10.1007/s002100000282. [DOI] [PubMed] [Google Scholar]
- 21.Levy B, Vallée C, Lauzier F, et al. Comparative effects of vasopressin, norepinephrine, and L-concanavanine, a selective inhibitor of inducible nitric oxide synthase, in endotoxic shock. Am J Physiol Heart Circ Physiol. 2004;287:H209–H215. doi: 10.1152/ajpheart.00946.2003. [DOI] [PubMed] [Google Scholar]
- 22.Vaidya VS, Ramirez V, Bobadilla NA, et al. A microfluidics based assay to measure Kidney Injury Molecule-1 (Kim-1) in the urine as a biomarker for early diagnosis of acute kidney injury. J Am Soc Nephrol. 2005;16:192A. [Google Scholar]
- 23.Vaidya VS, Ramirez V, Ichimura T, et al. Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 24.Halatek T, Hermans C, Broeckaert F, et al. Quantification of Clara Cell Protein in rat and mouse biological fluids using a sensitive immunoassay. Eur Respir J. 1998;11:726–733. [PubMed] [Google Scholar]
- 25.Chagnon F, Metz C, Bucala R, et al. MIF neutralizing can reverse myocardial dysfunction in endotoxinic shock. Circ Res. 2005;96:1095–1102. doi: 10.1161/01.RES.0000168327.22888.4d. [DOI] [PubMed] [Google Scholar]
- 26.Roxas B, Farjah M, Danziger RS. Aquaporin-2 transcript is differentially regulated by dietary salt in Sprague-Dawley and Dahl SS/Jr rats. Biochem Biophys Res Commun. 2002;296:755–758. doi: 10.1016/s0006-291x(02)00896-3. [DOI] [PubMed] [Google Scholar]
- 27.Bagshaw SM, Langenberg C, Wan L, et al. A systematic review of urinary findings in experimental septic acute renal failure. Crit Care Med. 2007;35:1592–1598. doi: 10.1097/01.CCM.0000266684.17500.2F. [DOI] [PubMed] [Google Scholar]
- 28.Johannes T, Mik EG, Ince C. Dual-wave-length phosphorimetry for determination of cortical and subcortical microvascular oxygenation in rat kidney. J Appl Physiol. 2006;100:1301–1310. doi: 10.1152/japplphysiol.01315.2005. [DOI] [PubMed] [Google Scholar]
- 29.Millar CGM, Thiermermann C. Intrarenal haemodynamics and renal dysfunction in endotoxaemia: Effects of nitric oxide synthase inhibition. Br J Pharmacol. 1997;121:1824–1830. doi: 10.1038/sj.bjp.0701335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boffa J-J, Arendshorst WJ. Maintenance of renal vascular reactivity contributes to acute renal failure during endotoxemic shock. J Am Soc Nephrol. 2005;16:117–124. doi: 10.1681/ASN.2004060441. [DOI] [PubMed] [Google Scholar]
- 31.Jonassen TEN, Graebe M, Promeneur D, et al. Lipopolysaccharide-induced acute renal failure in conscious rats: Effects of specific phosphodiesterase type 3 and 4 inhibition. J Pharmacol Exp Ther. 2002;303:364–374. doi: 10.1124/jpet.102.036194. [DOI] [PubMed] [Google Scholar]
- 32.Grinevich V, Knepper MA, Verbalis J, et al. Acute endotoxemia in rats induces down-regulation of V2 vasopressin receptors and aquaporin-2 content in the kidney medulla. Kidney Int. 2004;65:54–62. doi: 10.1111/j.1523-1755.2004.00378.x. [DOI] [PubMed] [Google Scholar]
- 33.Diamond JR, Yoburn DC. Nonoliguric acute renal failure. Arch Intern Med. 1982;142:1882–1884. [PubMed] [Google Scholar]
- 34.Versteilen AMG, Heemskerk AEJ, Groeneveld J, et al. Mechanisms of the urinary concentration defect and effect of desmopressin during endotoxemia in rats. Shock. 2008;29:217–222. doi: 10.1097/shk.0b013e3180ca9e53. [DOI] [PubMed] [Google Scholar]
- 35.Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Wen H, Frokiaer J, Kwon T-H, et al. Urinary excretion of Aquaporin-2 in rat is mediated by a vasopressin-dependent apical pathway. J Am Soc Nephrol. 1999;10:1416–1429. doi: 10.1681/ASN.V1071416. [DOI] [PubMed] [Google Scholar]
- 37.Albanese J, Leone M, Garnier F, et al. Renal effects of norepinephrine in septic and nonseptic patients. Chest. 2004;126:534–539. doi: 10.1378/chest.126.2.534. [DOI] [PubMed] [Google Scholar]
- 38.Patel B, Chittock D, Russel J, et al. Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology. 2002;96:576–582. doi: 10.1097/00000542-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Christensen BM, Marples D, Jensen UB, et al. Acute effects of vasopressin V2-receptor antagonist on kidney AQP-2 expression and subcellular distribution. Am J Physiol Renal Physiol. 1998;275:F285–F297. doi: 10.1152/ajprenal.1998.275.2.F285. [DOI] [PubMed] [Google Scholar]
- 40.Abid O, Sun Q, Sugimoto K, et al. Predictive value of microalbuminuria in medical ICU patients: Results of a pilot study. Chest. 2001;120:1984–1988. doi: 10.1378/chest.120.6.1984. [DOI] [PubMed] [Google Scholar]
- 41.Piepot HA, Boer C, Groeneveld AB, et al. Lipopolysaccharide impairs endothelial nitric oxide synthesis in rat renal arteries. Kidney Int. 2000;57:2502–2510. doi: 10.1046/j.1523-1755.2000.00109.x. [DOI] [PubMed] [Google Scholar]
- 42.Bouby N, Hassler C, Bankir L. Contribution of vasopressin to progression of chronic renal failure: Study in Brattleboro rats. Life Sci. 1999;65:991–1004. doi: 10.1016/s0024-3205(99)00330-6. [DOI] [PubMed] [Google Scholar]
- 43.Wu L, Tiwari MM, Messer KJ, et al. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol. 2007;292:F261–F268. doi: 10.1152/ajprenal.00263.2006. [DOI] [PubMed] [Google Scholar]
- 44.Han WK, Bonventre JV. Biologic markers for the early detection of acute kidney injury. Curr Opin Crit Care. 2004;10:476–482. doi: 10.1097/01.ccx.0000145095.90327.f2. [DOI] [PubMed] [Google Scholar]
- 45.Bernard AM, Thielemans NO, Lauwerys RR. Urinary protein 1 or Clara cell protein: A novel sensitive marker of proximal tubular dysfunction. Kidney Int. 1994;47:S34–S37. [PubMed] [Google Scholar]
- 46.Brown D. The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol. 2003;284:F893–F901. doi: 10.1152/ajprenal.00387.2002. [DOI] [PubMed] [Google Scholar]