Abstract
Objectives
Uterine serous carcinoma (USC) is an aggressive endometrial cancer associated with poor prognosis despite comprehensive surgical staging and adjuvant chemotherapy and radiation therapy. Biologic targets have yet to be fully explored in this disease and research on such targets could lead to clinical trials utilizing a new class of therapeutics. This study sought to evaluate primary USC tumors for molecular alterations in epidermal growth factor receptor (EGFR) and the recently characterized oncogene PIK3CA, which encodes the catalytic p110-alpha subunit of phosphatidylinositol 3-kinase (PI3K) and thus activates the AKT-mTOR oncogenic pathway.
Methods
Paraffin-embedded archival tissue of 45 primary USC tumors was utilized in this study. Immunohistochemical analysis of EGFR was performed and cases given a score of 0 to 12 calculated as the product of staining intensity (0 to 3+) and the percentage of positively stained cells (0-4), with 1=1-25%, 2=26-50%, 3=51-75%, and 4=76-100%. For mutational analysis, neoplastic tissue was microdissected and DNA was extracted with phenol-chloroform. Exons 18 through 21 of EGFR and exons 9 and 20 of PIK3CA, the most commonly mutated exons of these genes, were amplified and directly sequenced.
Results
When EGFR was evaluated, moderate or strong EGFR membranous staining was observed in 25/45 (56%) USC cases. Thus, a mutational analysis was performed on 35 cases, including all cases with moderate and strong EGFR staining. No mutations were identified in EGFR. In contrast, PIK3CA mutations were confirmed in 5/34 (15%) of USC cases. Four cases were mutated in exon 20 and one case was mutated in exon 9.
Conclusions
Since optimal treatment of uterine serous carcinoma remains unknown, novel therapeutic approaches need to be actively pursued. In the current study of primary USC tumors, oncogenic mutations of the PIK3CA gene were seen in 15% of USC cases. This represents the first report of this gene mutation in USC. In addition, EGFR stained positively in the majority of cases, suggesting a possible target protein. These findings warrant further investigation and suggest a potential role for therapeutic agents targeting the PI3K-AKT-mTOR pathway, such as rapamycin, as well as possible targets of EGFR in the treatment of uterine serous carcinoma.
Keywords: Uterine serous carcinoma, PIK3CA, EGFR
Introduction
Uterine serous carcinoma (USC) is a relatively rare histologic subtype of endometrial carcinoma that was first described by Lauchlan and Hendrickson in 1981[1, 2]. While USC comprises up to 10% of all primary endometrial cancers, it has an aggressive clinical course in comparison to the more common endometrioid adenocarcinoma with a pattern of spread that is similar to ovarian carcinoma. Uterine serous carcinomas thus account for a disproportionate number of deaths due to uterine cancer with overall 5-year survival rates between 35-81% for stages I and II, and 0-31% for stages III and IV[3, 4].
In contrast to uterine endometrioid adenocarcinoma, USC patients are less likely to be obese or in a hyperestrogenic state, are more often parous, and are diagnosed at an older age, typically in the postmenopausal period. It is thought that USC develops in an underlying atrophic endometrial epithelial background in association with early TP53 mutations through a different biologic mechanism from uterine endometrioid carcinoma [5]. In addition, the precursor lesion endometrial intraepithelial carcinoma has been well characterized and over 78% of these lesions have been shown to possess TP53 mutations [6]. This is in contrast to uterine endometrioid carcinomas, in which inactivation of the tumor suppressor gene PTEN is the most common molecular alteration.
There is currently no consensus on the optimal adjuvant therapy after a surgical staging procedure for USC; however, it is generally believed that aggressive adjuvant therapy is warranted even in early stage disease given the high recurrence rates with surgical resection alone. Both chemotherapy and radiation therapy, alone or in combination, have variable success rates; thus, investigators have begun to explore the potential of biologically targeted therapeutic agents. The purpose of the current study was to investigate the presence of potential targets in USC such as the epidermal growth factor receptor (EGFR or erbB1), a member of the erbB transmembrane receptor tyrosine kinases, as well as the novel oncogene PIK3CA, which is a downstream intracellular target of EGFR tyrosine kinase activation. EGFR has been found to be overexpressed in many solid tumors including colorectal, breast, and lung cancer. It has been implicated in malignant transformation and cellular proliferation via angiogenesis, anti-apoptosis and metastases. PIK3CA encodes the catalytic p110-α subunit of phosphatidylinositol 3-kinase (PI3K), a lipid kinase that generates phosphatidyl inositol-3,4,5-triphosphate (PIP3) by phosphorylating phosphatidyl inositol-3,4-diphosphate (PIP2). This in turn activates the AKT-mTOR oncogenic pathway. Recent literature suggests that PIK3CA mutations promote cell growth as well as invasion and are oncogenic in vitro and in vivo [7-9].
Methods
Specimen Collection
Forty-five cases of uterine serous carcinoma were retrospectively identified from an institutional database at Weill Medical College of Cornell University. A gynecologic pathologist reviewed the hematoxylin and eosin-stained slides of all cases in order to confirm the diagnoses. All cases were anonymized and Institutional Review Board approval was obtained.
Immunohistochemical Analysis
Immunohistochemical (IHC) analysis of EGFR was performed on paraffin-embedded archival tissue from 45 primary USC tumors. In order to avoid IHC methodology errors, we utilized the clinical core immunohistochemistry laboratory at our institution that performs EGFR staining uniformly for both clinical and research cases. Unstained four-micron sections of tumor tissue were deparaffinized in xylene and then pre-treated in a water bath of ph=6 for 40 minutes. Slides were pretreated with pepsin for 20 minutes and then treated with a 1:50 dilution of EGFR mouse monoclonal antibody (Zymed Laboratories, San Francisco, CA) and the EnVision + System (Dako, Carpinteria, CA). Cases were given a score of 0 to 12 calculated as the product of staining intensity (0 to 3+), with 0 =non-staining, 1+ =mild membranous staining, 2+ =moderate membranous staining, and 3+ = strong membranous staining, and the percentage of positively stained cells (0-4), with 1=1-25%, 2=26-50%, 3=51-75%, and 4=76-100%. A combined score was defined as having negative (score=0), weak (score=1-3), moderate (score=4-7), or strong (score=8-12) positivity. To obtain reliable expression analysis, two observers independently scored the tumor IHC slides and minor discrepancies that were encountered were settled by a third attending gynecologic pathologist.
Microdissection and DNA Extraction
Four-micron tissue sections were prepared from formalin-fixed, paraffin-embedded hysterectomy specimens of uterine serous carcinoma cases. Slides were stained with hematoxylin and areas of serous carcinoma were microdissected with a 26-gauge needle under direct light microscopic guidance to a concentration of >70% tumor cells. Tissue was then placed in TE-9 (500mM Tris, 20mM EDTA, 10mM NaCl, pH 9.0) with 1% SDS at 60°C overnight. DNA was extracted with phenol-chloroform and precipitated with ethanol as previously described [10].
EGFR and PIK3CA mutational analysis
Exons 18 through 21 of the EGFR gene and exons 9 and 20 of the PIK3CA gene are the most common sites of mutations in these genes, and PCR amplification of each exon was performed with subsequent DNA sequencing using the Applied Biosystems Automated 3730 DNA Analyzer (Foster City, CA). Two to 100 ng of genomic DNA were amplified using previously described exon-specific primers for EGFR [11] and PIK3CA [12]. For exon 20 of the PIK3CA gene, new primer sequences were constructed for optimal PCR conditions. A forward primer of 5’-CATTTGCTCCAAACTGACCA-3’ and a reverse primer of 5’-TGTGGAATCCAGAGTGAGCTT-3’ were used with the following PCR conditions to produce a 353 bp PCR product: five minutes (min) at 95°C for one cycle, 40 cycles at 95°C for one min, 60°C for one min, and 72°C for one min, followed by five minutes at 72°C for one cycle. Purification of PCR fragments was performed with the QIAquick PCR Purification Kit (Qiagen, Valencia, CA) and submitted for direct DNA sequence analysis. All potential mutations were verified by re-amplification from genomic DNA and direct sequencing of the mutated exon.
Results
Twenty-five of 45 (56%) uterine serous carcinomas stained moderately or strongly for EGFR by immunohistochemical analysis (Figure 1). The predominant pattern was diffuse membranous staining, consistent with the fact that EGFR is a transmembrane receptor tyrosine kinase. Given the high percentage of cases with EGFR positivity, mutational analysis was performed on exons 18 through 21, the four exons in the EGFR gene that encode for the kinase domain. Thirty-five cases were selected, including all 25 cases in which moderate or strong staining was displayed by IHC analysis. Direct DNA sequencing of all four exons revealed wild-type sequences for each case.
Figure 1.
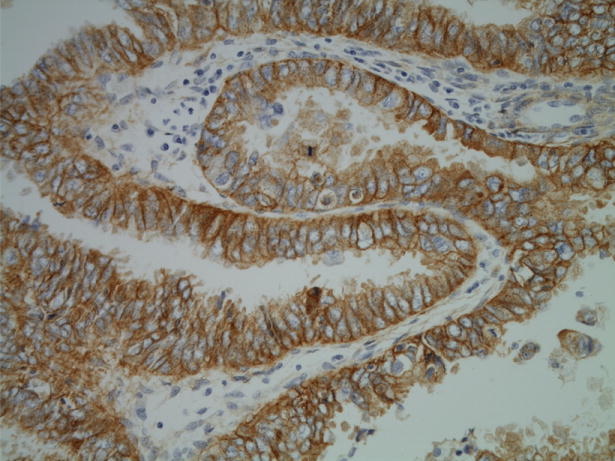
Uterine serous carcinoma with strongly positive membranous EGFR staining.
A downstream intracellular target of EGFR activation is the PI3K-AKT pathway. The oncogene PIK3CA was thus evaluated by mutational analysis with direct DNA sequencing of exons 9 and 20, which encode the helical and catalytic domains of the lipid kinase. Of the 34 USC cases in which DNA amplification and sequencing was performed, five (15%) contained confirmed PIK3CA mutations (Figure 2). Four cases had mutations in exon 20 and one case was mutated in exon 9. All mutations were missense mutations that have been described in the literature in other epithelial cancers (Table 1). Two of the five mutations were in codon 1047, which is known to be oncogenic [7, 9, 13, 14].
Figure 2.
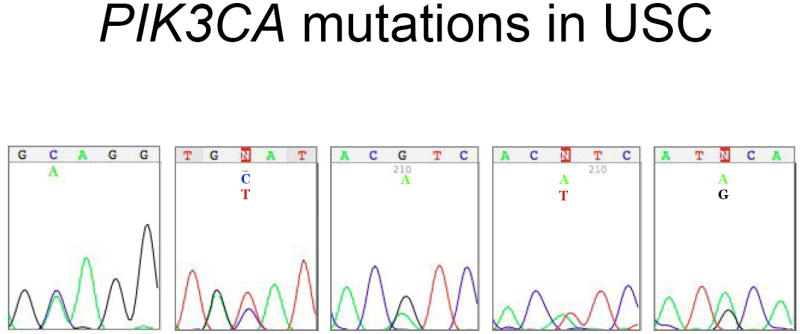
DNA sequences illustrating PIK3CA mutations in uterine serous carcinoma.
Table 1.
PIK3CA mutations discovered in uterine serous carcinoma.
| PIK3CA Mutation | Exon |
|---|---|
| Q546K | 9 |
| A1066V | 20 |
| H1047R | 20 |
| H1047L | 20 |
| Y1021C | 20 |
Discussion
The aggressive clinical course seen with uterine serous carcinoma has largely been attributed to the development of TP53 mutations early in its pathogenesis, with over 90% of uterine serous carcinomas containing a TP53 mutation [6]. Aside from mutations in TP53, however, much remains unknown regarding the molecular pathogenesis of USC. Given the paucity of treatment strategies for this disease, researchers are investigating the potential of utilizing biologically targeted agents for treatment. Potential targets that have been studied thus far have largely focused on HER-2/neu, but the results have been variable for overexpression by IHC and gene amplificiaton by FISH analysis. Protein overexpression ranges from 18%-26% [15, 16], and one study of a sample size of 10 cases found 80% HER-2/neu expression in USC [17]. FISH analysis is equally conflicting; with some studies reporting 47-71% of USC cases with HER-2/neu gene amplification [18, 19] and one study finding only 2/68 (2.9%) displayed gene amplification [15].
With the development of therapeutic agents targeted against EGFR, such as the tyrosine kinase inhibitors erlotinib and gefitinib and the chimeric monoclonal antibody cetuximab, we sought to evaluate whether the EGFR system is upregulated in USC. We observed a high expression rate of EGFR and thus propose that EGFR may be a potential target in these cancers. Multiple studies in non-small cell lung cancer have shown that the tyrosine kinase inhibitors appear to be effective against tumors that contain a mutation in the kinase domain encoded in exons 18 through 21 [11, 20, 21]. Since we did not find any activating mutations of EGFR in the cohort of USC tumors examined, it is unlikely that these inhibitors will be effective in USC as a single therapeutic agent; however, monoclonal antibodies such as cetuximab may warrant further study in the clinical setting. When we performed FISH on a subset of five USC samples that had strong IHC EGFR expression, we found no gene amplification (data not shown). However, we believe that given the current studies in the literature showing conflicting results regarding the utility of FISH in colorectal cancer, a lack of FISH gene amplification may not be predictive of response to cetuximab in USC. In addition, IHC of EGFR is currently used in metastatic colorectal cancer patients to determine eligibility for cetuximab therapy.
Studies in other solid tumors have shown that growth inhibition due to EGFR tyrosine kinase inhibitors is dependent on down regulation of the PI3K-AKT signaling pathway. In order to further investigate the AKT pathway, PIK3CA, the gene that encodes the catalytic p110-α subunit of PI3K, was examined. We report for the first time the presence of PIK3CA mutations in 15% of the USC cases examined. Oncogenic activation of PI3K promotes tumorigenesis via increased cell proliferation, cell survival, and motility [22]. Agents such as rapamycin and its derivative RAD001, both of which block mTOR, are able to inhibit PIK3CA-induced cellular transformation in vitro [7] and in vivo [8] and thus may be potentially useful in the treatment of tumors which harbor these mutations.
In addition, recent in vitro and in vivo data on multiple cell lines of non-small cell lung, pancreatic, breast and colon cancers, in which EGFR was either wildtype or not determined, suggest that erlotinib in combination with the mTOR inhibitor rapamycin has a synergistic effect on growth inhibition [23]. With AKT upregulation in the presence of PIK3CA mutations that is independent of AKT activation via EGFR, uterine serous carcinomas will likely need a multipoint interventional approach with a combination of biologically targeted agents such as an EGFR inhibitor and an AKT/mTOR inhibitor.
Footnotes
Conflict of Interest Statement The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lauchlan SC. Tubal (serous) carcinoma of the endometrium. Arch Pathol Lab Med. 1981;105(11):615–8. [PubMed] [Google Scholar]
- 2.Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6(2):93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kato DT, Ferry JA, Goodman A, Sullinger J, Scully RE, Goff BA, et al. Uterine papillary serous carcinoma (UPSC): a clinicopathologic study of 30 cases. Gynecol Oncol. 1995;59(3):384–9. doi: 10.1006/gyno.1995.9957. [DOI] [PubMed] [Google Scholar]
- 4.Bristow RE, Asrari F, Trimble EL, Montz FJ. Extended surgical staging for uterine papillary serous carcinoma: survival outcome of locoregional (Stage I-III) disease. Gynecol Oncol. 2001;81(2):279–86. doi: 10.1006/gyno.2001.6159. [DOI] [PubMed] [Google Scholar]
- 5.Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000;88(4):814–24. [PubMed] [Google Scholar]
- 6.Tashiro H, Isacson C, Levine R, Kurman RJ, Cho KR, Hedrick L. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol. 1997;150(1):177–85. [PMC free article] [PubMed] [Google Scholar]
- 7.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A. 2005;102(3):802–7. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bader AG, Kang S, Vogt PK. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci U S A. 2006;103(5):1475–9. doi: 10.1073/pnas.0510857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7(6):561–73. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, et al. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57(18):3935–40. [PubMed] [Google Scholar]
- 11.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 12.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 13.Isakoff SJ, Engelman JA, Irie HY, Luo J, Brachmann SM, Pearline RV, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65(23):10992–1000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 14.Zhao JJ, Liu Z, Wang L, Shin E, Loda MF, Roberts TM. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci U S A. 2005;102(51):18443–8. doi: 10.1073/pnas.0508988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slomovitz BM, Broaddus RR, Burke TW, Sneige N, Soliman PT, Wu W, et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J Clin Oncol. 2004;22(15):3126–32. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- 16.Villella JA, Cohen S, Smith DH, Hibshoosh H, Hershman D. HER-2/neu overexpression in uterine papillary serous cancers and its possible therapeutic implications. Int J Gynecol Cancer. 2006;16(5):1897–902. doi: 10.1111/j.1525-1438.2006.00664.x. [DOI] [PubMed] [Google Scholar]
- 17.Santin AD, Bellone S, Gokden M, Palmieri M, Dunn D, Agha J, et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clin Cancer Res. 2002;8(5):1271–9. [PubMed] [Google Scholar]
- 18.Rolitsky CD, Theil KS, McGaughy VR, Copeland LJ, Niemann TH. HER-2/neu amplification and overexpression in endometrial carcinoma. Int J Gynecol Pathol. 1999;18(2):138–43. doi: 10.1097/00004347-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Santin AD, Bellone S, Van Stedum S, Bushen W, Palmieri M, Siegel ER, et al. Amplification of c-erbB2 oncogene: a major prognostic indicator in uterine serous papillary carcinoma. Cancer. 2005;104(7):1391–7. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- 20.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 22.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 23.Buck E, Eyzaguirre A, Brown E, Petti F, McCormack S, Haley JD, et al. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol Cancer Ther. 2006;5(11):2676–84. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]


