Abstract
Purpose of review
Fibroblast growth factors (FGFs) are potent angiogenic inducers; however, their precise roles in angiogenesis have not been well understood. In this review, we will focus on specific roles played by FGFs in neovascularization.
Recent findings
Whereas FGFs promote a strong angiogenic response, it has been suggested that FGF-induced angiogenesis requires activation of the vascular endothelial growth factor (VEGF) system. Recent findings have endorsed this view: indirect contribution of FGF signaling to vascular development. A study using embryoid bodies demonstrated a non-immediate role played by FGFR1 in vasculogenesis since VEGF supplementation was sufficient to promote vascular development in Fgfr1-/- embryoid bodies. Moreover, another line of evidence indicated that myocardial FGF signaling is essential for mouse coronary development. The key role of FGF signaling is Hedgehog activation, which induces VEGF expression and formation of the coronary vasculature.
In addition to VEGF interaction, FGFs can control neovascularization by influencing other growth factors and chemokines such as PDGF, HGF and MCP-1, contributing to development of mature vessels and collateral arteries.
Summary
Although FGFs are potent angiogenic factors, they may indirectly control neovascularization in concert with other growth factors. Thus, the unique role played by FGFs might be organization of various angiogenic pathways and coordination of cell-cell interactions in this process.
Keywords: fibroblast growth factor, vascular endothelial growth factor, platelet-derived growth factor, angiogenesis, arteriogenesis
Introduction
In 1974, Gospodarowicz reported an activity in the bovine pituitary extract capable of stimulating the division of BALB/c 3T3 fibroblasts that later was determined to be basic FGF (FGF2) [1]. The FGF activity was subsequently demonstrated in many cell types including endothelial cells. Acidic FGF (FGF1), initially reported as endothelial cell growth factor (ECGF), was identified several years after the discovery of FGF2 [2-4]. The molecular characterization of FGF proteins was, however, not fully resolved until Shing, Folkman, and Klagsbrun discovered that heparin sepharose chomatography can be used as a very powerful method to purify heparin-binding growth factors including FGFs [5]. Since then, the FGF family, along with other angiogenic growth factor families, has been extensively studied with regard to its ability to promote angiogenesis, leading to accumulation of an enormous amount of knowledge. However, the precise role played by FGFs in this process remains elusive. Unlike VEGFs, FGFs are pleiotropic molecules capable of acting on a variety of cell types including cells of endodermal, mesenchymal and neuroectodermal origin. This raises a possibility of the FGF function to orchestrate angiogenesis by regulating various cell-cell interactions.
The FGF system comprises one of the most versatile growth factor signaling families in vertebrates, playing critical roles in a wide variety of biological processes [6]. In mice and humans, twenty-two FGF ligands and four tyrosine kinase receptors which are subjected to multiple splicing events have been identified [7]. Furthermore, there is a number of co-receptors and cell-surface and cytoplasmic proteins that modulate FGF signaling in a tissue and cell-type specific manner [8]. These signaling modulators may explain FGFs’ diverse functional properties which are capable of producing different effects even in the same cell type depending on the biological context.
One of the key barriers to deciphering the precise function of this complex family in angiogenesis is the paucity of information from knockout studies. Mouse embryos that are null for Fgfr1 or Fgfr2 die at very early stages of development, curtailing further studies to evaluate their contribution to vascular development [9-11]. Moreover, neither Fgfr3 nor Fgfr4 knockout mice show vascular defects [12-14]. At the same time, while FGF1 and FGF2 are known to induce angiogenesis in vivo, Fgf2-/- mice demonstrate a mild delay in wound repair, but no alteration in the vessel repair following mechanical injury [15, 16]. Essentially no abnormalities were found in mice lacking only Fgf1 [17]. Furthermore, the double knockout of Fgf1 and Fgf2 failed to show abnormalities in angiogenesis, implying extensive redundancy of the ligand system and leaving the distinct role of FGF1 or FGF2 in vascular development unclear [17].
Thus, key roles of FGFR1 and R2 in early development and the redundant functionality among various FGFs have combined to frustrate traditional knockout approaches to examine FGFs’ function in the vasculature. However, recent studies using advanced techniques have begun to illustrate distinct roles played by FGFs. In this paper, we will endeavor to elucidate specific roles played by the FGF system in neovascularization, especially with emphasis on interplay with other growth factors such as VEGF and platelet-derived growth factor (PDGF).
Lessons from clinical trials
Double-blind randomized clinical trials aiming at therapeutic application of angiogenic growth factors in patients with ischemic heart diseases have so far failed to demonstrate therapeutic efficacy of any single growth factor delivery [18, 19*]. This leads to the conclusion that administration of a single growth factor is not sufficient to support the formation of dependable blood vessels, suggesting that generating functionally reliable vasculature requires multiple growth factors and these growth factors play unique roles even though they can uniformly promote neovascularization in many experimental settings. It has been known that FGF2 and VEGF-A, two prototype angiogenic factors, stimulate distinct subsets of gene expression and generate different types of capillaries [20, 21]. Now, it is recognized that the understanding of the specific role of each growth factor is an important step to successfully promote therapeutic angiogenesis.
Angiogenic mechanism of FGF -controlling from behind the scenes
Several lines of evidence have indicated FGF regulation of the VEGF system in the angiogenic process [22]. FGF2 stimulates VEGF expression in endothelial cells and stromal cells, which is required for the FGF’s angiogenic response [23-25]. FGF signaling, furthermore, controls VEGFR2 signaling responsiveness (Murakami and Simons, unpublished observations). Ectopic expression of FGFR1 in hepatocytes, commonly observed in hepatocellular carcinoma, accelerates tumor growth by driving VEGF-induced angiogenesis [26]. One key player to explain this FGF-induced VEGF expression is the Shc protein, an adaptor molecule recruited to FGFRs upon activation, which plays a crucial role in receptor tyrosine kinase-dependent VEGF gene expression [7, 27].
Conversely, FGF signaling inhibition affects angiogenesis due to VEGF insufficiency. Glioma cell tumors expressing dominant-negative FGFR demonstrate a profound angiogenic defect paralleled by downregulation of VEGF expression [28]. Lack of FGF signaling in retinal pigmented epithelium during eye development strongly affects choroidal angiogenesis, including the absence of astrocytes which are responsible for VEGF production [29]. Similarly, FGF-induced angiogenesis is inhibited by a neutralizing antibody against VEGF-A, a blocking antibody against VEGFR or a VEGF-trap in many experimental settings [23, 25, 30, 31]. A genetic study also indicates similar dependency; identification of genetic loci that control the angiogenic response revealed that FGF2 responsiveness correlates generally with VEGF responsiveness and is partially explicable by VEGF responsiveness [32]. Thus, FGF signaling seems to require VEGF signaling for its angiogenic potential in vivo.
On the other hand, once VEGF is expressed, its ability to induce angiogenesis appears to be independent of FGF signaling. For example, while VEGF expression and vascular formation are impaired in Fgfr1 -/- embryoid bodies, VEGF supplementation corrects this defect. Furthermore, Vegfr2 -/- embryoid bodies fails to develop vessels in the presence of FGF2 [33]. Importantly, exogenous FGF2 is able to generate the vasculature in normal embryoid bodies, implying that the FGF system controls the angiogenic process upstream of VEGF.
Recent studies have further elucidated the distinct roles played by different growth factor systems in coronary artery development. FGF9, derived from epicardial and endocardial origin, activates FGF signaling to cardiomyoblast, resulting in Hedgehog signaling activation. This, in turn, induces VEGF-A, VEGF-B, VEGF-C and angiopoietin 2 expression, leading to the formation of the coronary vascular plexus [34**].
Caution is needed when determining direct dependency between growth factor systems since gene expression is subjected to complex regulation by feedback loops. Nonetheless, other examples also point to the indirect but central role of FGF signaling in angiogenesis. FGF2 directly stimulates in vitro mRNA synthesis of hepatocyte growth factor (HGF), another potent angiogenic growth factor, and in vivo HGF expression in ischemic muscle is influenced by FGF2 availability [35]. FGF2 further upregulates MCP-1 (monocyte chemoattractant protein-1) expression in cultured endothelial cells and controls its function in vivo [36, 37] (see below).
In contrast, the FGF effect on mural cells, i.e., vascular smooth muscle cells (VSMC) and pericytes, appear to be more complex. It is widely accepted that associations of mural cells with newly formed microvessels contributes to vessel maturation [38]. PDGF displays potent biological activities on VSMC which express abundant PDGFRs; therefore, it has been generally thought that PDGF, especially PDGF-BB, directly promotes mural cell recruitment [39]. Recent studies, however, indicates that PDGF-BB alone is not sufficient for vessel maturation and surprisingly, overexpression of PDGF-BB in tumor cells results in dissociation of smooth muscle cells from tumor vasculature [40, 41*]. These studies suggest that additional factors are required for vessel stability. Although FGF2 alone seems to induce modest vessel maturation, accumulating evidence indicates that a combination of FGF2 and PDGF-BB synergistically promote formation of stable vessels which remain stable for a long period of time even after depletion of angiogenic factors [42, 43]. In the absence of FGF2, endothelial cells lack responsiveness to PDGF-BB due to their restricted expression of PDGFR. FGF2 increases endothelial PDGFR levels, thereby enhancing sensitivity to PDGF-BB [41]. In line with this observation, FGF2’s priming effect on VSMC is also reported possibly through upregulation of PDGFR in VSMC [44]. PDGF-stimulated migratory function of VSMC is potentiated by FGF2 treatment [45]. Notably, this synergistic effect on long-term vessel stabilization is not observed with combinations of FGF2 and VEGF or PDGF-BB and VEGF [43].
FGF contribution to collateral development and muscle regeneration
Inflammatory responses that often occur in conjunction with neovascularization are known to accelerate vessel growth. Although a number of growth factors and cytokines are involved, FGFs potentially play a pivotal role in immune cell-mediated vessel growth. Monocyte/macrophage recruitment is an important step to promoting post-ischemic angiogenesis [46]. Monocyte chemoattractant protein-1 (MCP-1) is a cytokine belonging to CC chemokine family which regulates inflammation by recruiting immune cells, such as monocytes, to sites of tissue injury and infection. Recent studies have demonstrated that MCP-1 is one of the most potent drivers of arteriogenesis (collateral vessel formation) and the FGF system is involved in this process [47, 48]. Arteriogenesis is thought to occur by the growth and remodeling of preexisting arteries and by de novo arterialization of the capillary network [49]. The initiation of arteriogenesis does not seem to be triggered by either an increased expression of VEGF or hypoxia-inducible genes such as HIF-1α, and, also, the arteriogenic process is not accelerated by infusion of VEGF [50]. Both angiogenesis and arteriogenesis can occur in ischemic tissues as compensatory mechanisms for impaired arterial inflow and tissue perfusion. In the mouse critical limb ischemia model, FGF2 gene transfer induces MCP-1 and VEGF expression in non-endothelial mesenchymal cells such as VSMC and fibroblasts via distinct signaling pathways, thereby promoting arteriogenesis driven by MCP-1 and angiogenesis by VEGF. Unlike TNFα-induced MCP-1 expression which is observed in many cell types, FGF2-stumilated upregulation of MCP-1 is only found in non-endothelial mesenchymal cells. Blockade of MCP-1 activity significantly diminishes the FGF2-induced arteriogenic response [37].
In the post-ischemic tissue recovery, restoration of arterial blood supply normally leads to tissue regeneration coupled with concomitant angiogenesis. It has been reported that FGF2 and FGF6 strongly promote skeletal myocyte regeneration in conjunction with an increased angiogenic response [51]. Importantly, myogenesis and angiogenesis appear interdependent as it has been shown that myocyte-derived angiogenic factors are critical for angiogenesis [52]. Syndecans, a family of cell-surface transmembrane heparan sulfate proteoglycans (HSPGs) function as a non-tyrosine kinase receptor system for FGF signaling and positively regulate angiogenesis [53]. Syndecan-3 and syndecan-4 play important regulatory roles in skeletal muscle regeneration by promoting satellite cell maintenance, activation, proliferation, and differentiation [54]. Moreover, it is noteworthy that neural cell adhesion molecule (NCAM), which is abundantly expressed in regenerating myotubes, is an FGFR binding protein and implicated in modulation of FGF signaling [55].
FGF’s regulatory roles in neovascularization
Morphological differences between FGF- and VEGF-induced capillaries argues for differential roles of these growth factors in angiogenesis [21]. VEGF, being a potent inducer of vascular permeability, is known to cause edema and lead to formation of hemangiomas in high concentrations [56-58]. By contrast, these deleterious effects are not reported with FGF overexpression [59, 60]. Furthermore, in the experimental model of limb ischemia, comparison of FGF2 and VEGF-A gene delivery demonstrated that VEGF overexpression accelerates limb loss with a decreased blood perfusion recovery [31]. FGF2 gene delivery increases endogenous VEGF expression and FGF2’s therapeutic effect is abolished with an anti-VEGF neutrarizing antibody, strongly suggesting that although VEGF is critical for the neovascularization process, its expression needs to be tightly regulated [31]. In contrast, FGF-induced newly formed vessels are often characterized as functionally mature with increased mural cell investment.
In the process of mural cell recruitment in angiogenesis, N-cadherin appears to play a critical role with its ability to mediate heterophilic adhesion between endothelial cells and mural cells, thereby contributing to vessel maturation and stabilization [61, 62]. Underlying mechanisms to promote this endothelial-mural cell interaction are not well understood; however, a recent report strongly implicates involvement of sphingosine 1-phosphate (S1P), a sphingolipid metabolite found in high concentrations in platelets and blood. Inhibition of N-cadherin expression with small interfering RNA profoundly attenuates the process of vascular stabilization in vitro and in vivo [63]. Interestingly, N-cadherin is known to directly associate with FGFR and modify FGF signaling by inhibiting the ligand-induced internalization of cell-surface FGFR. It is, therefore, plausible that FGF-induced mural cell recruitment involves S1P signaling.
Related to its potential role in regulation of vessel stability, FGF may contribute to the maintenance of newly formed capillaries. This is suggested by a study using spontaneous β-cell pancreatic tumors of Rip1TaG2 mice. Whereas adenoviral expression of soluble VEGFR1 predominantly affected the initiation of tumor angiogenesis, soluble FGFR appeared to impair the maintenance of tumor angiogenesis [64].
The other layer of FGFs’ regulatory function may reside in the control of cell survival. FGF1 and FGF2 are widely accepted as cardioprotective agents. Although the underlying mechanism is not fully understood, there is a strong evidence supporting direct protective effect on cardiac myocytes by FGF2, independent of its angiogenic effect [65, 66*]. Similarly, FGF-2 has been shown to be a survival factor for vascular cells, i.e., VSMC and endothelial cells [67, 68]. FGF2 regulates endothelial apoptosis in the course of angiogenesis; however, its effect seems highly context dependent. FGF2 can activate anti-apoptotic factors phosphoinositide-3 kinase (PI3K)-Akt and Bcl-2 in the 2-dimentional (2D) endothelial culture system, thereby inhibiting apoptosis [69]. However, when endothelial cells are cultured in the 3D-collagen gel system, FGF2-induced p38 MAPK activation regulates angiogenesis by possibly increasing apoptosis [69, 70].
Conclusion
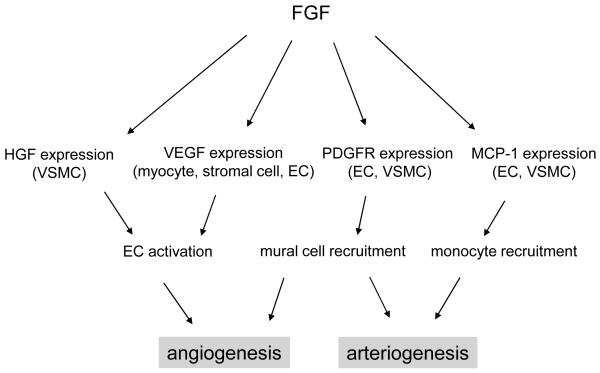
Angiogenesis, among other biological processes, is an intricate process that involves many cell types playing different roles. Given that the FGF system is capable of regulating other growth factor signaling, it is, therefore, reasonable to speculate that the FGF system is positioned upstream of more specialized growth factor systems such as VEGF for endothelial cells and PDGF for smooth muscle cells, thus orchestrating the entire angiogenic process in an indirect manner (summarized in figure 1).
Figure 1.
Indirect control of neovascularization by the FGF system FGFs promote angiogenesis and arteriogenesis by coordinating other growth factor systems. FGF-induced angiogenesis requires expression of VEGF in the subset of cells including cardiomyocyte, stromal cells and endothelial cells, resulting in the activation of endothelial VEGF signaling. In addition, FGF can induce HGF expression to drive angiogenesis. FGF potentiates PDGF-induced vascular maturation possibly through FGF-stimulated expression of PDGFR. Moreover, FGF stimulates MCP-1 synthesis in endothelial cells, which, in turn recruits monocytes to drive an arteriogenic response. EC, endothelial cells; VSMC, vascular smooth muscle cells
Growth factor functions are regulated in a complex fashion with multiple feedback systems influencing many cell types; hence, it is extremely difficult to elucidate unique roles of each growth factor unless it is specific to a single cell type. The limitation of current cell biology techniques which are largely dependent on the monoculture system basically precludes the investigation dealing with interplay between different cell types. In addition to largely uninformative knockout studies of individual FGF family members, the lack of suitable experimental technique is a major obstacle in the investigation of FGF functions in neovascular development. Therefore, one has to keep in mind that FGF’s true functions can be disguised because of oversimplified experimental settings and despite technical difficulties, it is critically important to design experiments to reflect more physiological situations.
Clarifying specific roles of angiogenic growth factors in the neovascularization process remains an arduous challenge; nonetheless, it will lead to an understanding of the precise mechanism of angiogenesis and, ultimately, the development of new therapeutic concepts.
Acknowledgements
This work is supported by National Institutes of Health grants (HL62289 and HL53793 to M.S, Funding Agency: National Heart, Lung and Blood Institute).
References
- [1].Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974;249:123–7. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- [2].Maciag T, Cerundolo J, Ilsley S, et al. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979;76:5674–8. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thomas KA, Riley MC, Lemmon SK, et al. Brain fibroblast growth factor: nonidentity with myelin basic protein fragments. J Biol Chem. 1980;255:5517–20. [PubMed] [Google Scholar]
- [4].Thomas KA, Rios-Candelore M, Fitzpatrick S. Purification and characterization of acidic fibroblast growth factor from bovine brain. Proc Natl Acad Sci U S A. 1984;81:357–61. doi: 10.1073/pnas.81.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shing Y, Folkman J, Sullivan R, et al. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984;223:1296–9. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- [6].Itoh N. The Fgf families in humans, mice, and zebrafish: their evolutional processes and roles in development, metabolism, and disease. Biol Pharm Bull. 2007;30:1819–25. doi: 10.1248/bpb.30.1819. [DOI] [PubMed] [Google Scholar]
- [7].Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–49. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- [8].Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- [9].Deng CX, Wynshaw-Boris A, Shen MM, et al. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–57. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- [10].Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–44. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- [11].Arman E, Haffner-Krausz R, Chen Y, et al. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci U S A. 1998;95:5082–7. doi: 10.1073/pnas.95.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Colvin JS, Bohne BA, Harding GW, et al. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–7. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- [13].Deng C, Wynshaw-Boris A, Zhou F, et al. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–21. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- [14].Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–23. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- [15].Ortega S, Ittmann M, Tsang SH, et al. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci U S A. 1998;95:5672–7. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhou M, Sutliff RL, Paul RJ, et al. Fibroblast growth factor 2 control of vascular tone. Nat Med. 1998;4:201–7. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Miller DL, Ortega S, Bashayan O, et al. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol. 2000;20:2260–8. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Simons M. Angiogenesis: where do we stand now? Circulation. 2005;111:1556–66. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- [19]*.Molin D, Post MJ. Therapeutic angiogenesis in the heart: protect and serve. Curr Opin Pharmacol. 2007;7:158–63. doi: 10.1016/j.coph.2006.10.006.This is a good review concisely describing current status of therapeutic angiogenesis
- [20].Jih YJ, Lien WH, Tsai WC, et al. Distinct regulation of genes by bFGF and VEGF-A in endothelial cells. Angiogenesis. 2001;4:313–21. doi: 10.1023/a:1016080321956. [DOI] [PubMed] [Google Scholar]
- [21].Cao R, Eriksson A, Kubo H, et al. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res. 2004;94:664–70. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- [22].Presta M, Dell’Era P, Mitola S, et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–78. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- [23].Seghezzi G, Patel S, Ren CJ, et al. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol. 1998;141:1659–73. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Claffey KP, Abrams K, Shih SC, et al. Fibroblast growth factor 2 activation of stromal cell vascular endothelial growth factor expression and angiogenesis. Lab Invest. 2001;81:61–75. doi: 10.1038/labinvest.3780212. [DOI] [PubMed] [Google Scholar]
- [25].Tsunoda S, Nakamura T, Sakurai H, Saiki I. Fibroblast growth factor-2-induced host stroma reaction during initial tumor growth promotes progression of mouse melanoma via vascular endothelial growth factor A-dependent neovascularization. Cancer Sci. 2007;98:541–8. doi: 10.1111/j.1349-7006.2007.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang X, Yu C, Jin C, et al. Ectopic activity of fibroblast growth factor receptor 1 in hepatocytes accelerates hepatocarcinogenesis by driving proliferation and vascular endothelial growth factor-induced angiogenesis. Cancer Res. 2006;66:1481–90. doi: 10.1158/0008-5472.CAN-05-2412. [DOI] [PubMed] [Google Scholar]
- [27].Saucier C, Khoury H, Lai KM, et al. The Shc adaptor protein is critical for VEGF induction by Met/HGF and ErbB2 receptors and for early onset of tumor angiogenesis. Proc Natl Acad Sci U S A. 2004;101:2345–50. doi: 10.1073/pnas.0308065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Auguste P, Gursel DB, Lemiere S, et al. Inhibition of fibroblast growth factor/fibroblast growth factor receptor activity in glioma cells impedes tumor growth by both angiogenesis-dependent and -independent mechanisms. Cancer Res. 2001;61:1717–26. [PubMed] [Google Scholar]
- [29].Rousseau B, Larrieu-Lahargue F, Bikfalvi A, Javerzat S. Involvement of fibroblast growth factors in choroidal angiogenesis and retinal vascularization. Exp Eye Res. 2003;77:147–56. doi: 10.1016/s0014-4835(03)00127-1. [DOI] [PubMed] [Google Scholar]
- [30].Kanda S, Miyata Y, Kanetake H. Fibroblast growth factor-2-mediated capillary morphogenesis of endothelial cells requires signals via Flt-1/vascular endothelial growth factor receptor-1: possible involvement of c-Akt. J Biol Chem. 2004;279:4007–16. doi: 10.1074/jbc.M307569200. [DOI] [PubMed] [Google Scholar]
- [31].Masaki I, Yonemitsu Y, Yamashita A, et al. Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ Res. 2002;90:966–73. doi: 10.1161/01.res.0000019540.41697.60. [DOI] [PubMed] [Google Scholar]
- [32].Rogers MS, Rohan RM, Birsner AE, D’Amato RJ. Genetic loci that control the angiogenic response to basic fibroblast growth factor. Faseb J. 2004;18:1050–9. doi: 10.1096/fj.03-1241com. [DOI] [PubMed] [Google Scholar]
- [33].Magnusson P, Rolny C, Jakobsson L, et al. Deregulation of Flk-1/vascular endothelial growth factor receptor-2 in fibroblast growth factor receptor-1-deficient vascular stem cell development. J Cell Sci. 2004;117:1513–23. doi: 10.1242/jcs.00999. [DOI] [PubMed] [Google Scholar]
- [34]**.Lavine KJ, White AC, Park C, et al. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006;20:1651–66. doi: 10.1101/gad.1411406.This study demonstrates a critical FGF’s regulatory role in coronary artery development in which many cell types are involved in a coordinated manner.
- [35].Onimaru M, Yonemitsu Y, Tanii M, et al. Fibroblast growth factor-2 gene transfer can stimulate hepatocyte growth factor expression irrespective of hypoxia-mediated downregulation in ischemic limbs. Circ Res. 2002;91:923–30. doi: 10.1161/01.res.0000043281.66969.32. [DOI] [PubMed] [Google Scholar]
- [36].Wempe F, Lindner V, Augustin HG. Basic fibroblast growth factor (bFGF) regulates the expression of the CC chemokine monocyte chemoattractant protein-1 (MCP-1) in autocrine-activated endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:2471–8. doi: 10.1161/01.atv.17.11.2471. [DOI] [PubMed] [Google Scholar]
- [37].Fujii T, Yonemitsu Y, Onimaru M, et al. Nonendothelial mesenchymal cell-derived MCP-1 is required for FGF-2-mediated therapeutic neovascularization: critical role of the inflammatory/arteriogenic pathway. Arterioscler Thromb Vasc Biol. 2006;26:2483–9. doi: 10.1161/01.ATV.0000244684.23499.bf. [DOI] [PubMed] [Google Scholar]
- [38].Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- [39].Ostman A. PDGF receptors-mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev. 2004;15:275–86. doi: 10.1016/j.cytogfr.2004.03.002. [DOI] [PubMed] [Google Scholar]
- [40].Lu H, Xu X, Zhang M, et al. Combinatorial protein therapy of angiogenic and arteriogenic factors remarkably improves collaterogenesis and cardiac function in pigs. Proc Natl Acad Sci U S A. 2007;104:12140–5. doi: 10.1073/pnas.0704966104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]*.Nissen LJ, Cao R, Hedlund EM, et al. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–77. doi: 10.1172/JCI32479.This study demonstrates synergistic effect played by FGF2 and PDGF-BB in tumor angiogenesis. The authors address the underlying mechanism in which these growth factors enhance EC and VSMC responsiveness.
- [42].Cao R, Brakenhielm E, Li X, et al. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. Faseb J. 2002;16:1575–83. doi: 10.1096/fj.02-0319com. [DOI] [PubMed] [Google Scholar]
- [43].Cao R, Brakenhielm E, Pawliuk R, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- [44].Kano MR, Morishita Y, Iwata C, et al. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci. 2005;118:3759–68. doi: 10.1242/jcs.02483. [DOI] [PubMed] [Google Scholar]
- [45].Pickering JG, Uniyal S, Ford CM, et al. Fibroblast growth factor-2 potentiates vascular smooth muscle cell migration to platelet-derived growth factor: upregulation of alpha2beta1 integrin and disassembly of actin filaments. Circ Res. 1997;80:627–37. doi: 10.1161/01.res.80.5.627. [DOI] [PubMed] [Google Scholar]
- [46].Li J, Brown LF, Laham RJ, et al. Macrophage-dependent regulation of syndecan gene expression. Circ Res. 1997;81:785–96. doi: 10.1161/01.res.81.5.785. [DOI] [PubMed] [Google Scholar]
- [47].Scholz D, Cai WJ, Schaper W. Arteriogenesis, a new concept of vascular adaptation in occlusive disease. Angiogenesis. 2001;4:247–57. doi: 10.1023/a:1016094004084. [DOI] [PubMed] [Google Scholar]
- [48].Deindl E, Hoefer IE, Fernandez B, et al. Involvement of the fibroblast growth factor system in adaptive and chemokine-induced arteriogenesis. Circ Res. 2003;92:561–8. doi: 10.1161/01.RES.0000061181.80065.7D. [DOI] [PubMed] [Google Scholar]
- [49].Annex BH, Simons M. Growth factor-induced therapeutic angiogenesis in the heart: protein therapy. Cardiovasc Res. 2005;65:649–55. doi: 10.1016/j.cardiores.2004.09.004. [DOI] [PubMed] [Google Scholar]
- [50].Deindl E, Buschmann I, Hoefer IE, et al. Role of ischemia and of hypoxia-inducible genes in arteriogenesis after femoral artery occlusion in the rabbit. Circ Res. 2001;89:779–86. doi: 10.1161/hh2101.098613. [DOI] [PubMed] [Google Scholar]
- [51].Doukas J, Blease K, Craig D, et al. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Mol Ther. 2002;5:517–27. doi: 10.1006/mthe.2002.0579. [DOI] [PubMed] [Google Scholar]
- [52].Tateno K, Minamino T, Toko H, et al. Critical roles of muscle-secreted angiogenic factors in therapeutic neovascularization. Circ Res. 2006;98:1194–202. doi: 10.1161/01.RES.0000219901.13974.15. [DOI] [PubMed] [Google Scholar]
- [53].Simons M, Horowitz A. Syndecan-4-mediated signalling. Cell Signal. 2001;13:855–62. doi: 10.1016/s0898-6568(01)00190-5. [DOI] [PubMed] [Google Scholar]
- [54].Cornelison DD, Filla MS, Stanley HM, et al. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- [55].Hinsby AM, Berezin V, Bock E. Molecular mechanisms of NCAM function. Front Biosci. 2004;9:2227–44. doi: 10.2741/1393. [DOI] [PubMed] [Google Scholar]
- [56].Baumgartner I, Rauh G, Pieczek A, et al. Lower-extremity edema associated with gene transfer of naked DNA encoding vascular endothelial growth factor. Ann Intern Med. 2000;132:880–4. doi: 10.7326/0003-4819-132-11-200006060-00005. [DOI] [PubMed] [Google Scholar]
- [57].Springer ML, Chen AS, Kraft PE, et al. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell. 1998;2:549–58. doi: 10.1016/s1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- [58].Lee RJ, Springer ML, Blanco-Bose WE, et al. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- [59].Giordano FJ, Ping P, McKirnan MD, et al. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med. 1996;2:534–9. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- [60].Tabata H, Silver M, Isner JM. Arterial gene transfer of acidic fibroblast growth factor for therapeutic angiogenesis in vivo: critical role of secretion signal in use of naked DNA. Cardiovasc Res. 1997;35:470–9. doi: 10.1016/s0008-6363(97)00152-1. [DOI] [PubMed] [Google Scholar]
- [61].Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn. 2000;218:472–9. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- [62].Tillet E, Vittet D, Feraud O, et al. N-cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell-derived angiogenesis. Exp Cell Res. 2005;310:392–400. doi: 10.1016/j.yexcr.2005.08.021. [DOI] [PubMed] [Google Scholar]
- [63].Paik JH, Skoura A, Chae SS, et al. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392–403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Compagni A, Wilgenbus P, Impagnatiello MA, et al. Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res. 2000;60:7163–9. [PubMed] [Google Scholar]
- [65].Hampton TG, Amende I, Fong J, et al. Basic FGF reduces stunning via a NOS2-dependent pathway in coronary- perfused mouse hearts. Am J Physiol Heart Circ Physiol. 2000;279:H260–8. doi: 10.1152/ajpheart.2000.279.1.H260. [DOI] [PubMed] [Google Scholar]
- [66]*.Kardami E, Detillieux K, Ma X, et al. Fibroblast growth factor-2 and cardioprotection. Heart Fail Rev. 2007;12:267–77. doi: 10.1007/s10741-007-9027-0.This is a comprehensive review of FGF-mediated cardioprotection.
- [67].Fox JC, Shanley JR. Antisense inhibition of basic fibroblast growth factor induces apoptosis in vascular smooth muscle cells. J Biol Chem. 1996;271:12578–84. doi: 10.1074/jbc.271.21.12578. [DOI] [PubMed] [Google Scholar]
- [68].Gospodarowicz D, Hirabayashi K, Giguere L, Tauber JP. Factors controlling the proliferative rate, final cell density, and life span of bovine vascular smooth muscle cells in culture. J Cell Biol. 1981;89:568–78. doi: 10.1083/jcb.89.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Karsan A, Yee E, Poirier GG, et al. Fibroblast growth factor-2 inhibits endothelial cell apoptosis by Bcl-2-dependent and independent mechanisms. Am J Pathol. 1997;151:1775–84. [PMC free article] [PubMed] [Google Scholar]
- [70].Matsumoto T, Turesson I, Book M, et al. p38 MAP kinase negatively regulates endothelial cell survival, proliferation, and differentiation in FGF-2-stimulated angiogenesis. J Cell Biol. 2002;156:149–60. doi: 10.1083/jcb.200103096. [DOI] [PMC free article] [PubMed] [Google Scholar]