Summary
Immunity to the intestinal parasite, Heligomosomoides polygyrus, is dependent on the successful generation of Th2 memory cells. We show that B cells contribute to immunity against H. polygyrus by producing antibody and by promoting expansion and differentiation of primary and memory Th2 cells. We also demonstrate that cytokine-producing “effector” B cells are essential for effective immunity to H. polygyrus. TNFα production by B cells is necessary for sustained Ab production, while IL-2 production by B cells is necessary for Th2 expansion and differentiation. These results show that B cells mediate protection to pathogens not only by presenting antigen and secreting antibody but also by producing cytokines that regulate the quality and magnitude of humoral and cellular immune responses.
Introduction
B lymphocytes produce antibodies (Abs) that facilitate clearance of pathogens and other antigens. However, emerging data examining mice and patients treated with a B cell depleting Ab (Rituximab, Rituxan, MabThera) suggest that B cells may regulate immune responses by additional, Ab-independent mechanisms. For example, auto-Ab titers do not drop significantly in B cell depleted SLE patients (Cambridge, 2004; Looney et al., 2004), yet these patients often experience complete clinical remission for many months or years. Likewise, autoimmune-prone mice that are B cell sufficient but unable to produce auto-Abs still exhibit symptoms of autoimmune pathology (Chan et al., 1999). Thus, B cells can be both protective and pathologic and appear to regulate immune responses by multiple mechanisms.
There are many ways, both Ab dependent and Ab dependent, by which B cells regulate immune responses to pathogens (Baumgarth et al., 2008; Dorner and Radbruch, 2007; Gray et al., 2007; Stephens and Langhorne, 2006). B cells express Toll-Like Receptors (Gray et al., 2007) and when activated also express co-stimulatory molecules (Linton et al., 2000; Linton et al., 2003). Activated B cells are also excellent APCs that can selectively internalize specific antigen through the BCR, facilitating antigen processing and presentation to CD4 T cells (Chen and Jensen, 2008; Lund et al., 2005). In fact, there is growing evidence to suggest that antigen-presenting B cells shape the magnitude and quality of the primary and memory CD4 T cell response, particularly when antigen is limiting (Cardillo et al., 2007; Crawford et al., 2006; Gillan et al., 2005; Iijima et al., 2008; Linton et al., 2000; Liu et al., 2007; Lund et al., 2006; McClellan et al., 2006; Ronet et al., 2008; Stephens and Langhorne, 2006). B cells also influence immune responses by producing cytokines (reviewed in (Lund, 2008; Lund et al., 2005). For example, production of lymphotoxin by B cells is necessary for the differentiation of follicular dendritic cells (Endres et al., 1999; Fu et al., 1998), the formation of B cell follicles (Gonzalez et al., 1998) and the development of specialized stromal cells in the spleen (Ngo et al., 2001). TNFα production by B cells can influence the generation of IFNγ-producing effector cells (Menard et al., 2007), while IL-10 production by B cells alters DC function and modulates T cell priming and effector T cell development (Moulin et al., 2000; Sun et al., 2005). In addition, IL-10-producing B cells (also known as Bregs) can act directly on effector T cells and repress T cell cytokine production thereby suppressing a number of T cell-mediated inflammatory diseases including SLE, EAE and CIA (reviewed in (Fillatreau et al., 2008; Mizoguchi and Bhan, 2006)).
B cells also secrete other cytokines including IL-2, IL-4, IL-13, IFNγ, IL-12 and TNFα (reviewed in (Lund et al., 2005; Pistoia, 1997)), however the functional role of these B cell-derived cytokines is largely unknown. We previously demonstrated that B cells can be subdivided into at least two different effector B cell subsets that produce distinct arrays of cytokines (Harris et al., 2000). B cells primed by cognate interactions with Th1 cells (Be-1 cells) produce IFNγ, IL-12p40, TNFα and IL-10, while B cells primed by cognate interactions with Th2 cells (Be-2 cells) produce IL-4, IL-2, IL-13, IL-6, IL-10 and TNFα. The signals that regulate the differentiation of these two subsets are distinct, with Be-1 cells developing in response to IFNγand IL-12 (Harris et al., 2005a) and Be-2 developing in response to IL-4 (Harris et al., 2005b). Interestingly, Be-1 and Be-2 cells can prime naïve CD4 T cells to differentiate into Th1 and Th2-like effectors in vitro (Harris et al., 2000), suggesting that these cytokine-producing effector B cells may function to amplify T cell dependent immune responses in vivo.
In this manuscript, we directly evaluated whether cytokine-producing effector B cells are required for protection to the intestinal nematode, Heligmosomoides polygyrus (Hp). We show that B cells are required for protection to Hp and that B cells mediate protection by producing Ab and by supporting the expansion and differentiation of primary and memory Th2 cells. We further demonstrate that cognate interactions between B and T cells are required for robust Th2 responses and that B cells regulate Th2 responses independently of Ab production. Importantly, we show that IL-4Rα expression by B cells is required for protection, suggesting that IL-4 primed Be-2 cells regulate immunity to H. polygyrus. In agreement with this finding, we demonstrate that B cell-derived IL-2 and TNFα regulate Ab and T cell responses to Hp and that the production of these cytokines by B cells is needed for protection. Thus, these data show that cytokine-producing effector B cells play important roles in the establishment and maintenance of humoral and cellular immune responses and are critically important for protection against some pathogens.
Results
Protective immunity to Hp is dependent on B cells
Oral inoculation of mice with Hp larvae (L3 Hp) results in the establishment of a chronic infection (Finkelman et al., 1997; Gause et al., 2003). The L3 Hp enter the submucosa of the small intestine, mature and emerge into the lumen as adult parasites after one week. Immunocompetent mice mount a robust primary Th2 response to the infection, however this immune response is unable to prevent the establishment of a chronic infection with the adult parasites. Parasitized animals can be cured of the chronic infection by treatment with pyrantel pamoate. Drug-cured mice respond rapidly to challenge infections with L3 Hp and are able to prevent the emergence of adult parasites. The protective recall response is dependent on IL-4-producing Th2 cells (Liu et al., 2004; Urban et al., 1991) as well as alternatively activated macrophages (Anthony et al., 2006), however the role for B cells in this response is unknown.
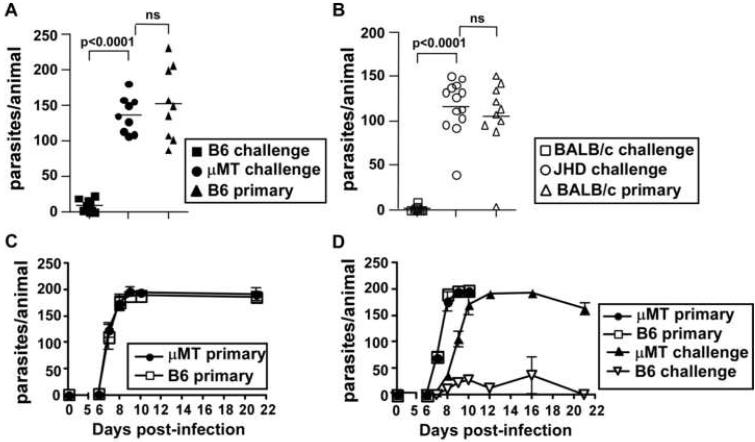
To determine whether B cells are required for the generation of a protective memory response to Hp, we orally infected C57BL/6 and BALB/c mice as well as B cell-deficient μMT and JHD mice with L3 Hp. The infected mice were treated 3 weeks later with pyrantel pamoate to eliminate adult parasites from the intestine. This infection/drug treatment cycle was repeated a second time to ensure the development of a robust memory immune response. Immune mice as well as naïve controls were then challenged with L3 Hp and parasites in the small intestine were enumerated 20 days later. As expected, C57BL/6 (Fig. 1A) or BALB/c (Fig. 1B) mice given a primary infection harbored substantial numbers of adult Hp parasites in the small intestine, whereas the memory C57BL/6 (Fig. 1A) and memory BALB/c (Fig. 1B) mice effectively prevented the emergence of adult parasites. In contrast, memory μMT (Fig. 1A) and memory JHD (Fig. 1B) mice harbored large numbers of adult parasites - equivalent to that seen in mice given a primary infection. Thus, B cells are required for the generation of a protective memory immune response to Hp.
Figure 1. B cells are required for protective immunity to Hp.
(A) C57BL/6 and μMT mice or (B) BALB/c and JHD mice were exposed to two rounds of L3 Hp infection and drug treatment with pyrantel pamoate. The memory mice and a group of naive mice (primary) were then challenged with L3 Hp and parasite burdens were determined on day 20. (C) C57BL/6 and μMT mice were infected with L3 Hp and the number of adult parasites in the intestine was determined on days 6, 7, 8, 9, 10 and 21 after infection. The data are shown as the mean ± SD (n=3-4 mice/group/timepoint). (D) C57BL/6 and μMT mice were infected with L3 Hp or were sham-infected. Three weeks later all mice were treated with pyrantel pamoate. Ten days after the last drug treatment, all mice were infected with L3 Hp. The number of adult parasites present in the intestinal lumen of the previously infected mice (memory) and previously naive mice (primary) was determined at the times indicated. The data are shown as the mean ± SD (n=3-4 mice/group/timepoint). The results shown are representative of two or more independent experiments.
To determine whether the failure of B cell deficient mice to mount a protective memory immune response to Hp was due to alterations in the establishment of the initial infection, we infected naïve C57BL/6 and μMT mice with L3 Hp and measured the emergence of adult parasites into the intestinal lumen over the next three weeks. As shown in Fig. 1C, the emergence of adult parasites into the lumen was identical in C57BL/6 and μMT mice both in terms of magnitude and kinetics. Next, to test whether a memory immune response to Hp was initiated in the absence of B cells, we infected naïve C57BL/6 and μMT mice with L3 Hp, waited 21 days for the establishment of a chronic infection and then treated the infected mice and a cohort of naïve mice with pyrantal pamoate to eliminate the adult parasites in the infected mice. Two weeks later, we infected the naïve and memory mice with L3 Hp and measured the emergence of adult parasites into the lumen of the intestine. In the C57BL/6 and μMT animals receiving a primary infection, adult parasites began emerging on day 7, reaching maximal levels between days 8 and 9 (Fig. 1D). In contrast, adult parasites began emerging from memory C57BL/6 and μMT on day 8 (Fig. 1D). Only a small number of adult parasites were found in the memory B6 mice (Fig. 1D). In contrast, the memory μMT mice had significantly greater numbers (p<0.003) of healthy adult parasites present in the lumen on day 8 and by days 10-12 post-challenge the number of adult parasites present in the intestine was equivalent to that seen in primary infected mice (Fig. 1D). Thus, while an immune response to Hp was initiated in B cell deficient mice it was neither sustained nor protective and only modestly delayed the entry of adult worms into the intestinal lumen.
Protective immunity to Hp is not regulated by B cell dependent lymphoid tissue organogenesis
The prior experiments were performed with B cell deficient mice and it is reported that B cells organize the cellular composition and architecture of the spleen (Ngo et al., 2001) and are necessary for the organogenesis of Peyer’s patches (PP) (Golovkina et al., 1999). It is reported that bone marrow (BM) reconstitution does not rescue the lymphoid tissue developmental defects in μMT mice (Golovkina et al., 1999; Ngo et al., 2001), indicating that B cells must be present during fetal ontogeny to promote proper development of the PP and spleen. These B cell dependent defects in lymphoid tissue organogenesis are known to alter the development of some DC and T cell subsets (Ngo et al., 2001) which has the potential to affect immune responses. Thus, it was possible that the inability of B cell deficient mice to clear Hp could simply be due to a requirement for B cells during fetal lymphoid tissue development. To determine whether the loss of B cells during organogenesis was responsible for the increased susceptibility of these mice to Hp, we transferred bone marrow (BM) from C57BL/6 or μMT mice into lethally irradiated μMT hosts to generate animals that lacked B cells during embryonic development but were either B cell sufficient or deficient at the time of infection (Suppl. Fig. 1A). After exposing the BM chimeric mice to two rounds of L3 Hp infection and pyrantal pamoate treatment, we challenged the mice with larvae and determined the number of adult parasites on day 15. As expected, μMT mice reconstituted with μMT BM lacked B cells (Suppl. Fig 1B) and were unable to mount a protective immune response to Hp (Suppl. Fig. 1C). However, μMT mice reconstituted with C57BL/6 BM were competent to express B cells (Suppl. Fig. 1B) and were fully protected from reinfection (Suppl. Fig. 1C), despite having a disorganized spleen and lacking PPs. Together, these data demonstrate that immunity to Hp is not dependent on the presence of B cells during fetal development. Instead the data suggest that B cells mediate their protective effect during the course of the immune response to Hp.
Hp-specific Ab is necessary for protection
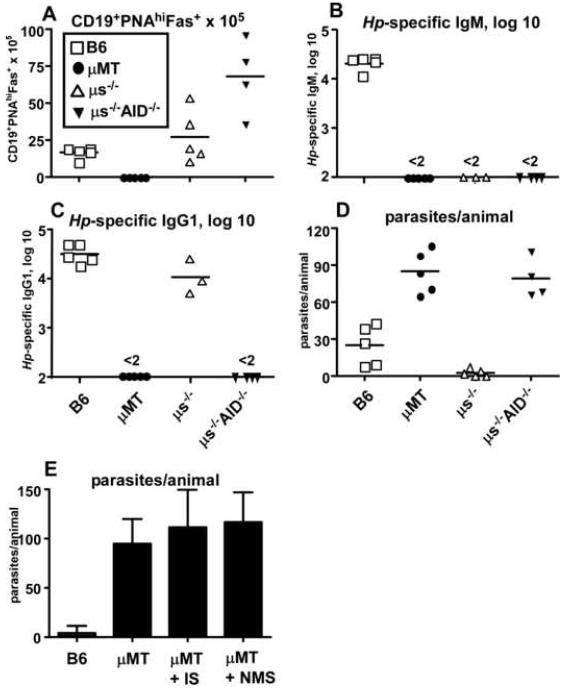
Although we showed a role for B cells during the establishment of a protective immune response to Hp, it was unclear how B cells might mediate protection. The role for Ab in immunity to most nematodes is controversial, with the majority of groups reporting minimal impact of Ab on pathogen clearance (Anthony et al., 2007). IgE is the only Ab isotype previously tested for efficacy in immunity to Hp and, despite the robust IgE response that is generated after Hp infection, IgE is dispensable for protection to Hp (Anthony et al., 2007; Finkelman et al., 1997). To determine whether other isotypes of Ab were necessary for protection, we performed a Hp challenge infection in mice that are unable to undergo affinity maturation or secrete Ab of any isotype (μs-/-AID-/-) (Kumazaki et al., 2007) and compared protection in these mice with B cell sufficient (C57BL/6), B cell-deficient (μMT) and secretory IgM-deficient (μs-/-) (Boes et al., 1998) animals. Mice were exposed to two rounds of infection and drug treatment prior to the final challenge infection. We enumerated B cells, Ab titers and parasite burdens on day 13 after the final challenge infection. Activated B cells with a germinal center phenotype (CD19+PNAhiFAS+) were detected in the mesenteric lymph nodes (mLNs) of infected C57BL/6, μs-/- and μs-/-AID-/- mice, but were not found in any tissues of μMT mice (Fig. 2A). Similar to a previous report (Hase et al., 2008), larger numbers of germinal center B cells were present in mice lacking AID (μs-/-AID-/-). As expected, no Ab of any isotype was detected in the infected μMT mice (Fig. 2B-C) and these animals did not clear the challenge infection (Fig. 2D). Hp-specific IgM was not detected in serum from the Hp-infected μs-/- mice (Fig. 2B), but these mice were competent to secrete Hp-specific class-switched IgG1 (Fig. 2C) and IgE (data not shown). In contrast, the μs-/-AID-/- mice could not secrete any Ab, regardless of isotype (Fig. 2B-C). Interestingly, the μs-/- mice efficiently cleared the challenge Hp infection, in spite of lacking Hp-specific IgM (Fig. 2D). In contrast, the μs-/-AID-/- mice were not protected from reinfection (Fig. 2D). Together, the data suggest that a class-switched (non-IgE) affinity matured Ab response is needed for protection to Hp.
Figure 2. Isotype-switched Ab is necessary but not sufficient for protection from Hp challenge.
C57BL/6, μMT, μs-/- or μs-/-AID-/- mice were exposed to two rounds of infection with L3 Hp and drug treatment with pyrantel pamoate. Mice were challenged with L3 Hp and the (A) number of CD19+PNAhiFAS+ germinal center B cells in the mLNs was determined 13 days later by flow cytometry. (B) Titers of Hp-specific IgM and (C) Hp-specific IgG1 were determined by ELISA. (D) The number of adult parasites present in the intestinal lumen was determined. (E) C57BL/6 and μMT mice were exposed to two rounds of Hp infection and drug treatment. 200 μl of Hp immune serum (IS) or normal mouse serum (NMS) was injected (i.p.) into the memory μMT and B6 recipients 2 days before, the day of, and two days following a challenge infection with Hp. Parasite burdens were determined 19 days later. Data are represented as the mean ± SD (n=10 mice/group). Data are representative of two individual experiments.
Next, to address whether Ab production was the only effector function of B cells required for immunity to Hp, we performed serum transfer experiments. We exposed C57BL/6 and μMT mice to two rounds of Hp infection and pyrantal pamoate treatment. We then injected the μMT memory mice with normal mouse serum or serum from Hp-immune C57BL/6 mice three times - two days before a final challenge with Hp larvae, the day of challenge and two days after the challenge infection. We measured parasite burden in the intestine 19 days after the final challenge. Despite being able to detect the transferred Hp-specific IgG1 in μMT recipients for at least two weeks after transfer (data not shown), no protection was observed in the memory μMT mice that received immune serum (Fig. 2E). Taken together, these data suggest that while Hp-specific Ab is necessary for protection, it may not be sufficient to mediate protection in μMT mice.
B cells are required for the development of Hp-specific primary effector Th2 cells
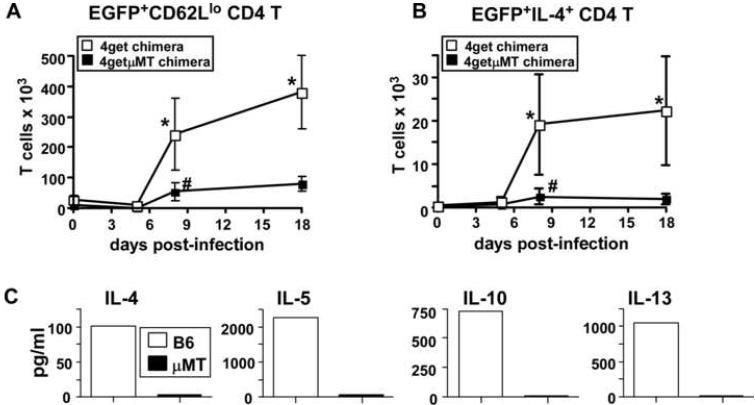
Collectively, our data suggested that B cells might play both an Ab dependent and Ab independent role in protection to Hp. Given the critical role for Th2 cells in protection to Hp (Liu et al., 2004), we next asked whether B cells are necessary for the initial priming or expansion of Th2 effectors. To test this hypothesis, we intercrossed 4get IL-4 reporter mice (EGFP expression reports IL-4 transcription (Mohrs et al., 2001)) with μMT mice to generate B cell-deficient IL-4 reporter mice (4getμMT). To eliminate the confounding variable of B cell deficiency during lymphoid tissue development and its potential impact on T cell responses (Ngo et al., 2001), we again made BM chimeras using lethally irradiated μMT hosts reconstituted with either 4getμMT BM to generate the B cell deficient 4getμMT chimeras or with 4get BM to generate the B cell sufficient 4get chimeras (see Suppl. Fig. 2A). We infected both groups of chimeric mice with Hp larvae and then used FACS analysis to determine the percentage and number of IL-4 transcript (EGFP+) expressing CD4 T cells present in the MLNs of the infected 4get and 4getμMT mice (Suppl. Fig. 2B). As shown in Fig. 3A, the numbers of EGFP+CD62Llo CD4 T cells began to increase in the mLNs between days 5 and 8 after infection. At this time, the number of EGFP+CD62Llo CD4 T cells was significantly higher in the 4get chimeras than in the B cell-deficient 4getμMT chimeras - a trend that continued through day 18 (Fig 3A).
Figure 3. B cells regulate Hp-specific Th2 cell commitment, expansion and differentiation.
B cell deficient μMT mice were lethally irradiated and reconstituted with 4get BM (4get chimeras) or with B cell deficient 4get BM (4getμMT chimeras). (A) The chimeric mice were infected with Hp and the number of EGFP+CD62Llo CD4 cells in the mLNs was determined by flow cytometry at various times. (B) Cells from the mLNs were stimulated with platebound anti-CD3 for 4 hours in the presence of Brefeldin A and the numbers of IL-4+EGFP+ CD4 T cells were determined using flow cytometry. Data are shown as the mean ± SD of 4-5 mice/group/timepoint. *Statistically significant differences (p≤0.02) between the 4get and 4getμMT groups were found at each of the indicated timepoints. #Statistically significant differences (p≤0.05) between day 0 4getμMT mice and day 8 4getμMT mice are indicated. (C) C57BL/6 and μMT mice were infected with L3 Hp and CD4 cells were purified from pooled mLNs 21 days later. Purified CD4 T cells were restimulated with Hp antigen-pulsed APCs and cytokine levels in the supernatants were determined by cytometric bead array. The results shown are representative of 3 independent experiments.
Next, to assess the functionality of the few IL-4 transcript expressing CD4 T cells present in the 4getμMT mLNs, we isolated cells from the infected mice at various times post-infection, stimulated the cells with platebound anti-CD3 and then assessed IL-4 protein production by the EGFP+ CD4 T cells (Suppl. Fig. 2C). Interestingly, while a large proportion of the EGFP+CD4+ T cells from the 4get chimeras produced IL-4 upon anti-CD3 restimulation, almost no IL-4 producing T cells were identified in 4getμMT chimeras at any timepoint examined (Fig. 3B). To confirm that these data from the reporter mice accurately reflected functional differences in Hp-specific Th2 cells, we purified CD4 T cells from the mLNs of day 21 Hp-infected C57BL/6 and μMT mice, restimulated them with Hp antigen-pulsed APCs and measured cytokine production in the supernatant. As shown in Fig. 3C, CD4 T cells from C57BL/6 mice produced IL-4, IL-5, IL-10 and IL-13 upon restimulation with Hp antigen, whereas T cells from μMT mice produced low to undetectable amounts of these cytokines (Fig. 3C). These data demonstrate that B cells, while not necessarily obligate for T cell priming, are essential for the optimal expansion and differentiation of functional antigen-specific Th2 effector cells during the primary response to Hp.
Th2 memory responses to Hp are dependent on the presence of B cells
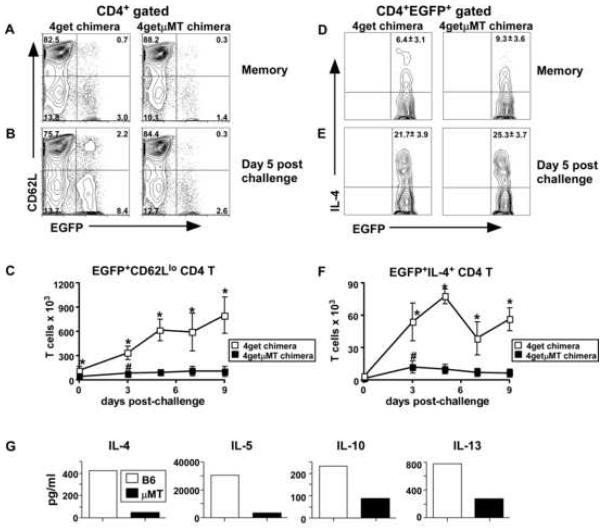
Given the importance of B cells during the initial expansion of Hp specific Th2 effectors, we next addressed whether B cells were needed to generate an optimal Th2 memory response. We again reconstituted irradiated μMT hosts with 4getμMT or 4get BM to generate the same two groups of chimeric mice used in the previous experiment (see Suppl. Fig 2A). We infected both groups of chimeric mice with L3 Hp, cured them of infection with pyrantal pamoate on day 21 and rested the mice until day 60. Using FACS analysis, we found that the frequency of CD4 T cells that were EGFP+CD62Llo was two-fold reduced in the mLNs of the memory B cell deficient 4getμMT BM chimeras (Fig. 4A). To address whether these memory cells were competent to expand after a challenge infection, we inoculated a cohort of the day 60 memory mice with L3 Hp and compared the percentage and number of EGFP+ CD4 T cells in the mLNs of the mice at various timepoints post-challenge. As shown in Figure 4B, the frequency of EGFP+CD62Llo CD4 T cells was greatly reduced in the day 5 post-challenge 4getμMT chimeras. Furthermore, the number of EGFP+CD62Llo cells in the mLNs 4getμMT mice was significantly and dramatically decreased relative to those in the 4get chimeras at all timepoints post-challenge (Fig. 4C).
Figure 4. Th2 memory responses to Hp are dependent on B cells.
B cell deficient μMT mice were irradiated and reconstituted with BM from either 4get mice (4get chimeras) or B cell deficient 4get mice (4getμMT chimeras). Chimeric mice were infected with Hp and drug treated on day 21. On day 60 a cohort of mice was analyzed (memory timepoint) and the remainder of the mice were challenged with L3 Hp and mLN cells were analyzed for expression of CD4, EGFP and CD62L by flow cytometry. (A-B) Plots gated on live CD4+ cells and are representative of mice at the (A) memory timepoint and (B) 5 days post-challenge. The numbers in each quadrant indicate the percentage of CD4+ cells that express EGFP or CD62L. (C) The number of EGFP+CD62Llo CD4 T cells present in the mLNs at the memory timepoint and various times post-challenge was determined. (D-E) Cells from mLNs of (D) memory and (E) day 5 post-challenged mice were restimulated with platebound anti-CD3 in the presence of Brefeldin A for 4 hours and the expression of IL-4 and EGFP on the CD4 T cells was determined by flow cytometry. The percent of EGFP+CD4+ cells co-expressing IL-4 is indicated in the plot (mean ±SD of 5 mice/group). (F) The number of EGFP+IL-4+ CD4 T cells present in the mLNs at the memory timepoint and various times post-challenge was determined. Data in panels C and F are shown as the mean ± SD of 4-5 mice/group. *Statistically significant differences (p≤0.02) between the 4get and 4getμMT groups were found at each of the indicated timepoints. #Statistically significant differences (p≤0.03) between day 0 4getμMT mice and day 3 4getμMT mice are indicated. (G) C57BL/6 and μMT mice were infected with Hp, drug treated and then challenged with 200 L3 Hp. Ten days later, CD4 cells were purified from pooled mLNs and restimulated with Hp antigen-pulsed APCs overnight. Cytokines in the supernatants were quantified by cytometric bead array. The results shown are representative of 3 independent experiments.
To assess the functionality of the memory CD4 T cells in the 4getμMT mice, we isolated mLN cells from the memory and re-challenged mice, stimulated them with platebound anti-CD3 and assessed IL-4 production by the EGFP+ CD4 T cells. The frequency of IL-4 expressing EGFP+ CD4 T cells was essentially identical between the 4get and 4getμMT chimeras regardless of whether the T cells were resting memory (Fig. 4D) or reactivated memory (Fig. 4E). However, when we calculated the number of IL-4 producing EGFP+ CD4 T cells present in the mLNs of 4get and 4getμMT chimeras, we observed a dramatic reduction in the number of cytokine-producing effector memory T cells in the B cell deficient animals (Fig. 4F). Finally, to confirm that the reduced numbers of IL-4 producing CD4 T cells in the memory 4getμMT mice reflected a specific impairment in the generation and/or expansion of Hp-specific Th2 cells, we exposed C57BL/6 and μMT mice to two rounds of infection and drug treatment, challenged them with L3 Hp and then restimulated purified CD4 T cells from day 10 mLNs overnight with Hp antigen-pulsed APCs. We found that CD4 T cells from infected memory C57BL/6 mice produced elevated amounts of IL-4, IL-5, IL-10 and IL-13, whereas CD4 T cells from infected memory μMT mice produced much lower levels of these cytokines (Fig. 4G). Taken altogether, these data demonstrate that B cells, while not obligate for the generation or maintenance of memory Th2 cells, are required to support the rapid expansion of functional antigen-specific Th2 memory cells after challenge infection.
B cells regulate Th2 memory responses independently of Ab production
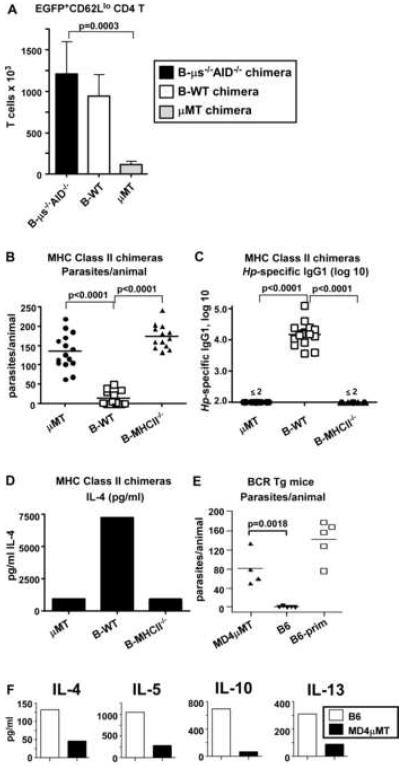
Our data indicated that B cells are required for the development of an optimal Th2 memory response and are also necessary to make class-switched Abs. To address whether B cells control Th2 responses to Hp indirectly by making Ab that regulates Th2 memory cell function, we measured Th2 responses to Hp in chimeric mice that are B cell sufficient, but are unable to secrete Ab. To do this, we took advantage of the mixed BM chimera method (Lee et al., 2003) as this method permitted us to selectively express or delete particular genes in a specific hematopoietic lineage cell. In this experiment (Suppl. Fig. 3), we used lethally irradiated μMT mice as hosts for all groups. We reconstituted one group of μMT hosts with a mixture of 75% 4getμMT BM and 25% μs-/-AID-/- BM (B-μs-/-AID-/- chimeras). Since all of the B cells in these chimeras must develop from the μs-/-AID-/- BM, the B cells in these mice are unable to secrete Ab of any isotype. As controls, we reconstituted μMT hosts with a mixture of 75% 4getμMT BM and 25% C57BL/6 BM (B-WT chimeras). Since all of the B cells in these chimeras must develop from the C57BL/6 BM, the B cells are normal and competent to produce Ab. Finally, we reconstituted μMT hosts with 75% 4getμMT BM and 25% μMT BM (μMT chimeras). These chimeras are B cell deficient as none of the transferred BM can give rise to B cells. Finally, 75% of all T cells in all three groups of chimeras will be derived from 4getμMT BM and therefore are competent to express EGFP when the IL-4 locus is transcribed.
We infected the chimeric mice with L3 Hp, treated them with pyrantal pamoate and then challenged them with Hp larvae. Six days later, we isolated cells from the mLNs and determined the number of CD4+EGFP+CD62Llo cells. As expected, the number of EGFP+CD62Llo CD4 T cells was greatly diminished in the B cell deficient μMT chimeras compared to the B-WT controls (Fig. 5A). In contrast, the number of EGFP+CD62Llo CD4 T cells was similar in B-WT chimeras and B-μs-/-AID-/- chimeras (Fig. 5A). Thus, the presence of B cells, even in the absence of Ab, is sufficient to generate a robust Th2 memory response. These data therefore indicate that B cells regulate memory CD4 T cell responses to Hp by an Ab-independent mechanism.
Figure 5. Th2-mediated immunity to Hp is dependent on antigen-presenting, antigen-specific B cells, but does not require Ab-producing B cells.
(A) Irradiated μMT mice were reconstituted with 75% 4getμMT BM and 25% μMT BM (μMT chimeras), with 75% 4getμMT BM and 25% C57BL/6 BM (B-WT chimeras) or with 75% 4getμMT BM and 25% μs-/-AID-/- BM (B-μs-/-AID-/- chimeras). The chimeric mice were infected with Hp, drug treated and then challenged with 200 L3 Hp. EGFP+CD62Llo CD4 T cells in the mLNs were enumerated by flow cytometry 6 days after challenge. (B-D) Irradiated μMT mice were reconstituted with 75% μMT BM and 25% μMT BM (μMT chimeras), with 75% μMT BM and 25% C57BL/6 BM (B-WT chimeras) or with 75% μMT BM and 25% MHCII-/- BM (B-MHCII-/- chimeras). The reconstituted mice were exposed to two rounds of Hp infection and drug treatment and were then challenged with 200 L3 Hp. (B) Parasite burdens were determined for individual animals on day 21 and (C) serum titers of Hp-specific IgG1 were determined by ELISA. (D) CD4 T cells were purified from pooled mLNs and stimulated with platebound anti-CD3 overnight. IL-4 levels in the T cell supernatants were determined by ELISA. (E-F) B6 mice and MD4μMT mice were exposed to two rounds of Hp infection and drug treatment and were then challenged with L3 Hp. (E) Parasite burdens were determined for individual animals. (F) Cells from the mLNs of each group were pooled and the CD4 T cells were purified and stimulated on anti-CD3 coated plates. Cytokine levels in the culture supernatants were determined by cytokine bead array at 24 hr post-restimulation. The data are shown as the mean ± SD of 4-5 mice/group. The results shown are representative of 2 or more independent experiments.
Antigen-presenting Hp-specific B cells are necessary for the development of functional Th2 memory cells and for protection
To address how B cells regulate Th2 memory responses to Hp, we asked whether B cells promote the differentiation of T cells into Th2 effectors and memory cells by presenting antigen. To address this question, we generated mixed BM chimeras (Suppl. Fig. 4A) using irradiated μMT hosts which were reconstituted with 100% μMT BM (μMT chimeras), 75% μMT BM + 25% MHCII-/- BM (B-MHCII-/- chimeras) or with 75% μMT BM + 25% C57BL/6J BM (B-WT chimeras). As expected, by 8 weeks post-reconstitution the percentage of B cells present in peripheral blood was equivalent between the B-WT and B-MHCII-/- chimeras (Suppl. Fig. 4B). Likewise, the number of B cells present in the spleens of these two types of chimeras was indistinguishable (Suppl. Fig. 4C). The B cells from the B-MHCII-/- chimeras were Class II deficient but other hematopoietic cells were competent to express class II (Suppl. Fig. 4D). In contrast, B cells as well as other APCs from the B-WT chimeras were able to express class II and the percentage of class II-expressing non-B cells was very similar between all the groups (Suppl. Fig. 4D). These chimeric mice were exposed to two rounds of L3 Hp infection and drug treatment and were then challenged with L3 Hp. As expected, the B-WT chimeras cleared the infection, whereas the μMT chimeras did not (Fig. 5B). Interestingly, the B-MHCII-/- chimeras were also unable to clear the infection (Fig. 5B), demonstrating that cognate interactions specifically between B cells and CD4 T cells are important for immunity to Hp. Next, we measured the titers of Hp-specific Ab in the serum of chimeric mice to determine whether antigen presentation by B cells was necessary to produce the protective isotype-switched Ab. As shown in Fig. 5C, Hp-specific IgG1 was easily detected in the serum of B-WT, but not μMT chimeras or B-MHCII-/- chimeras. To evaluate the CD4 T cell response in the Hp challenged mice we purified CD4 T cells, restimulated them with anti-CD3 overnight and measured IL-4 accumulation in the supernatant. T cells from B-WT mice produced large quantities of IL-4, while T cells from μMT and B-MHCII-/- chimeras produced much smaller quantities (Fig. 5D).
Finally, to address whether the protective B cells had to be antigen-specific to promote protection to Hp, we crossed BCR transgenic MD4 mice (Goodnow et al., 1988) onto the B cell deficient μMT background (MD4μMT) to generate mice with a monoclonal repertoire of B cells specific for an irrelevant antigen (lysozyme). These mice were exposed to two rounds of infection and drug treatment and then challenged with L3 Hp. Interestingly, the MD4μMT mice were not protected against Hp challenge infection (Fig. 5E). Furthermore, Th2 cytokine production by the anti-CD3 restimulated CD4 T cells isolated from the mLNs of the MD4μMT mice was greatly diminished relative to the response of CD4 T cells from C57BL/6J mice (Fig. 5F). Together, these data show that cognate T/B cell interactions are required for the activation and differentation of Hp-specific B and T cells and that this cognate interaction is necessary for the development of a protective memory response to Hp.
IL-4 primed B cells are required for immune protection to Hp
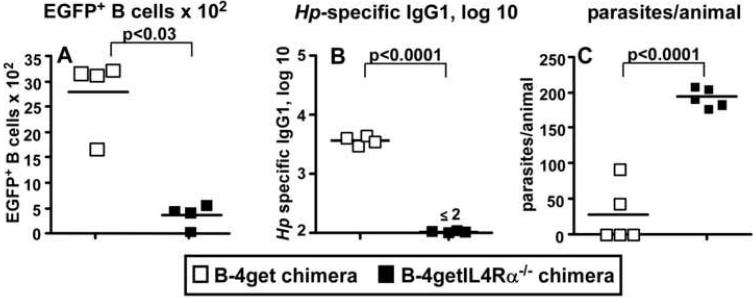
Protective immunity to Hp is dependent on IL-4-producing Th2 cells (Liu et al., 2004). We previously showed that B cells primed in the presence of IL-4 (Be-2 cells) are excellent antigen presenting cells and promote the in vitro differentiation of naïve T cells into IL-4 producing Th2 cells (Harris et al., 2000). To test whether IL-4 primed B cells are necessary for protection to Hp, we generated mixed BM chimeras by lethally irradiating B cell deficient JHD mice and reconstituting them with a mixture of 80% JHD BM and 20% 4get IL-4-reporter BM (B-4get chimeras). Since all the B cells in these chimeras develop from the 4get BM, they will express EGFP in any cell in which IL-4 transcripts are also expressed (Suppl. Fig. 5A). We also irradiated a second group of JHD mice and reconstituted these animals with 80% JHD BM and 20% 4getIL4Rα-/- BM. The B cells in these mice (B-4getIL4Rα-/- chimeras) develop from the 4getIL4Rα-/- BM and will express EGFP when the IL-4 locus is transcribed. However, these reporter B cells are unable to express the IL-4Rα subunit, preventing them from responding to IL-4 or IL-13 and blocking their ability to differentiate into Be-2 cells (Harris et al., 2005b). We exposed both groups of chimeric mice to two rounds of Hp and drug treatment and then challenged them with Hp larvae. Similar to our previous observations (Harris et al., 2005b), a small number of B cells from the mLNs of the B-4get chimeras expressed EGFP (Fig. 6A and Suppl. Fig. 5B), indicating that these B cells are transcribing the IL-4 gene. In contrast, the number of IL-4Rα deficient 4getB cells expressing EGFP (Fig. 6A and Suppl. Fig. 5B) was significantly lower than that seen in the B-WT chimeras, demonstrating that IL-4 producing Be-2 cells do not develop efficiently in vivo when B cells cannot receive an IL-4/IL-13 signal.
Figure 6. IL-4Rα expression on B cells is required for the in vivo development of Be-2 cells and for immunity to Hp.
B cell-deficient JHD mice were irradiated and reconstituted with 80% JHD BM and 20% 4get BM (B-4get) or with 80% JHD BM and 20% 4getIL-4Rα-/- BM (B-4getIL-4Rα-/-). Reconstituted mice were exposed to two rounds of L3 Hp infection and drug treatment and then challenged with 200 L3 Hp. (A) The number of live (PIneg), EGFP-expressing CD19+CD4neg B cells in the mLNs was determined using flow cytometry. EGFP+ B cells present in the MLN of uninfected 4get or 4getIL-4Rα-/- mice represent ≤0.01% of the total B cell pool or 100-300 B cells/MLN (Harris et al., 2005b). (B) Titers of Hp-specific IgG1 in serum were determined by ELISA. (C) Adult parasites were enumerated by counting. The results shown are representative of 2 independent experiments.
To test whether IL-4Rα-expressing B cells are needed for protection to Hp, we measured Hp-specific Ab responses and parasite burden in the chimeric mice. The B-4get chimeras made a robust Hp-specific IgG1 response (Fig. 6B) and efficiently prevented parasite emergence (Fig. 6C). In contrast, the B-4getIL4Rα-/- chimeras did not produce Hp-specific IgG1 (Fig. 6B) and were not protected from the challenge infection (Fig. 6C). Together, these data demonstrate that B cells primed in the presence of IL-4 (Be-2 cells) are required for protection from Hp challenge infections.
B cell-derived cytokines are necessary for effective humoral and cellular immunity to Hp
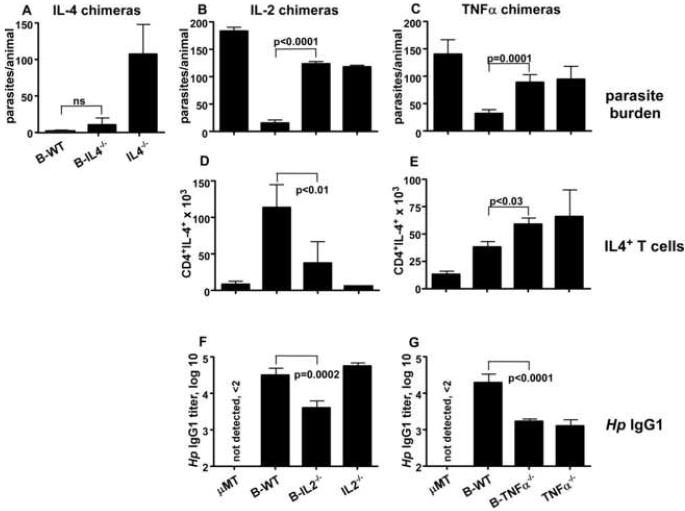
Prior data from our laboratory showed that Be-2 cells can regulate Th2 differentiation in vitro by producing IL-4 (Harris et al., 2000). Given that IL-4 is a critical cytokine for protection to Hp (Liu et al., 2004) and that B cells make IL-4 in response to Hp infection (Harris et al., 2000), we assessed whether IL-4 expressing B cells are necessary for immune protection. We therefore produced mixed BM chimeras in irradiated μMT hosts making chimeras with normal B cells (B-WT), chimeras with IL-4 deficient B cells (B-IL-4-/- chimeras), chimeras with IL-4 deficiency in all hematopoietic lineages (IL-4-/- chimeras) and chimeras that are B deficient (μMT chimeras) (Suppl. Fig. 6). We exposed the chimeras to two rounds of Hp infection and drug treatment then challenged the mice again with L3 Hp. As expected, the IL-4-/- chimeras did not clear the challenge infection, however B-IL-4-/- mice cleared the infection (Fig. 7A) and made a normal Hp-specific IgG1 response (not shown). Thus, while IL-4 is critical for immune control of Hp, IL-4-producing B cells are not necessary for protection.
Figure 7. Protective humoral and cellular immune responses to Hp are controlled by cytokine producing effector B cells.
(A) Irradiated μMT mice were reconstituted with 100% μMT BM (μMT chimeras), with 75% μMT BM and 25% C57BL/6 BM (B-WT chimeras) or with 75% μMT BM and 25% IL-4-/- BM (B-IL-4-/- chimeras). The reconstituted mice were exposed to 2 rounds of L3 Hp infection and drug treatment and were then challenged with L3 Hp. Parasite burden was determined on day 21. (Panel B, D, F) Irradiated μMT mice were reconstituted with 75% μMT BM and 25% IL-2-/- BM (B-IL-2-/- chimeras), with 100% IL-2-/- BM (IL-2-/- chimeras), with 100% μMT BM (μMT chimeras) or with 75% μMT BM and 25% C57BL/6 BM (B-WT chimeras). (Panel C, E, G) Irradiated μMT mice were reconstituted with 75% μMT BM and 25% TNFα-/- BM (B-TNFα-/- chimeras), with 100% TNFα-/- BM (TNFα-/- chimeras), with 100% μMT BM (μMT chimeras) or with 75% μMT BM and 25% C57BL/6 BM (B-WT chimeras). In separate experiments, the cytokine chimeras along with control B-WT and μMT chimeras were infected and drug-treated twice and then challenged with L3 Hp. Parasite burden in the lumen was determined on (B) day 12, or (C) day 10 post-infection. (D-E) Cells from mLNs of cytokine chimeras and controls were stimulated in the presence of Brefeldin A for 5 hours on anti-CD3 coated plates and the number of IL-4-producing CD4 T cells was determined by flow cytometry. (F-G) Serum titers of Hp-specific IgG1 were determined by ELISA. All data is shown as the mean ± SD of ≥ 5 mice/group. The results shown are representative of 2 or more independent experiments.
Since Be-2 cells make cytokines other than IL-4, including IL-10, LTα, IL-2 and TNFα (Harris et al., 2000), we generated additional mixed BM chimeras (see Suppl. Fig. 6) to test whether the production of these cytokines by B cells was required for effective protection against Hp. We found that neither IL-10 nor LTα production by B cells is necessary for protection from Hp challenge infections (not shown). However, mice with IL-2-deficient B cells exhibited only partial protection to Hp challenge infection and presented with a parasite burden that, while lower than that observed in the B deficient μMT chimeras, was significantly higher than the B-WT chimeras (Fig. 7B). Likewise, mice with TNFα-deficient B cells were only partially protected after challenge with Hp and exhibited a parasite burden that was intermediate between the protected B-WT and the susceptible μMT chimeras (Fig. 7C). Interestingly, the parasite burdens in the animals that lacked expression of IL-2 or TNFα specifically within B lineage cells were nearly identical to the parasite burdens found in mice that were deficient in TNFα or IL-2 in all hematopoietic lineages (Fig. 7B-C). Importantly, the chimeras with cytokine deficient B cells did not clear the adult parasites even out to day 21 (data not shown), indicating that these B cells were not able to prevent the establishment of a chronic infection. Thus, B cell-derived IL-2 and TNFα are necessary for the establishment of protective immunity to Hp.
To address how IL-2 and TNFα production by B cells regulates immunity to Hp, we analyzed cellular and humoral immune responses after challenge in the chimeric mice. We found that T cells from B-IL-2-/- and IL-2-/- chimeras produced very little IL-4 after restimulation with anti-CD3, relative to T cells from B-WT chimeras (Fig. 7D). In contrast, the numbers of IL-4 producing T cells in B-TNFα-/- or TNFα-/- mice were actually greater than the number of IL-4 producing T cells present in the mLN of B-WT chimeras (Fig. 7E). Next, to address whether IL-2 or TNFα-producing B cells modulate humoral immunity to Hp, we measured Hp-specific IgG1 titers in the serum of the chimeric mice. Hp-specific IgG1 titers were reduced in the B-IL-2-/- chimeras, compared to the IgG1 titers observed in B-WT mice (Fig. 7F). The titers of Hp-specific IgG1 were also reduced in the B-TNFα-/- and TNFα-/- chimeras (Fig. 7G) compared to those in B-WT mice. Thus, these data show that B cell-derived TNFa regulates humoral, but not cellular, immunity to Hp while IL-2 made by B cells modulates both cellular and humoral immunity to this pathogen. Taken altogether, the data show that B cells are necessary for both humoral and cellular immune responses to Hp and that cytokine production by B cells is one critical mechanism by which they mediate their protective effects. The implications of these findings are discussed.
Discussion
The data presented here show that B cells are an essential component of immunity to Hp and that B cells mediate their protective effects by regulating both the humoral and cellular immune response to this parasite. B cells utilize multiple effector mechanisms to regulate immunity to Hp, which include producing Ab, presenting antigen and secreting cytokines. Our data show that μs-/-AID-/- mice, which lack the ability to secrete Ab and cannot undergo affinity maturation, are not protected in a rechallenge infection with Hp. Although Ab is generated in response to infection with a variety of helminths, the protective role for Ab in these infections is variable and no obvious role for Hp-specific Ab has been reported. IgE deficient ((Anthony et al., 2007; Finkelman et al., 1997), WW and FL unpublished) and FcεR1a deficient mice (WW and FL unpublished) are immune to rechallenge infection with L3 Hp. Furthermore, transfer of Hp-immune serum to naïve mice did not confer protection against Hp challenge (Penttila et al., 1984), minimally suggesting that Ab by itself is not sufficient to mediate protection. Our new data clearly show a role for class-switched Ab and affinity matured Ab in protection to Hp (Fig. 2). While we do not yet know how Ab facilitates clearance of the larvae, it may be mediated by FcR-expressing cells as mice deficient in the Fc common γ chain subunit (FcER1γ) are susceptible to challenge infection (WW and FL unpublished).
Interestingly, an older report showed that naïve animals receiving both Hp-specific immune serum and immune lymphocytes are protected from Hp infection (Behnke and Parish, 1981), suggesting that the combination of Hp-specific Ab and memory lymphocytes is essential for immunity. However, the transfer of Hp immune serum to μMT mice that were previously infected (to elicit memory T cells) did not provide protection from Hp infection. This surprising result suggested to us that the cellular memory immune response may also be compromised in B cell deficient mice. In fact, we found that B cells play a critical role in the primary expansion and differentiation of IL-4-producing Th2 effector cells in response to Hp infection (Fig. 3). These data are consistent with previous observations showing that primary Th2 responses to protein antigens delivered with live N. brasiliensis larvae (Liu et al., 2007) as well as protein antigens delivered in alum (Crawford et al., 2006) are dependent on B cells. Although B cells were not required for the initial activation of Ag-specific T cells in these experiments, the B cells appear to play an important role in primary Th2 expansion (Crawford et al., 2006; Liu et al., 2007; Ronet et al., 2008). B cells also appear to be important for memory Th2 responses to protein antigens delivered in alum (Linton et al., 2000) and consistent with this, we find that B cells play a key role in regulating Th2 memory responses to Hp (Fig. 4). Interestingly, we find that while the number of memory Th2 T cells is lower in B cell deficient mice than in WT mice, the difference is less than two-fold and these cells are equally competent to produce IL-4 upon restimulation. However, we find that the expansion of Th2 memory cells and their subsequent differentiation into secondary cytokine-secreting effector cells is highly dependent on B cells. While we do not yet know whether B cells directly alter the quality of memory T cells that are generated during the primary response or whether B cells are necessary for memory T cell re-activation during the secondary immune response, our data show that B cells are important for the expansion of cytokine-producing Th2 effectors in both primary and secondary responses to Hp.
Given the requirement for Th2 cells in immunity to Hp, it is reasonable to postulate that the poor Th2 response induced in the B cell deficient animals contributes to the lack of immunity seen in these mice. Importantly, by using μMT hosts for all our BM chimeras we were able to demonstrate that the changes in T cell immunity we observed were not simply due to the lymphoid tissue defects associated with the loss of B cells during fetal development (Golovkina et al., 1999; Ngo et al., 2001). However, it is certainly possible that the loss of B cells during infection led to changes in other T cell subsets. We did not observe an increase in the number of IFNγ-expressing Hp-specific T cells, indicating that the loss of B cells did not shift the response from a type 2 to type 1 response (data not shown). We did, however, find that the number of antigen-specific IL-10, IL-5 or IL-13 producing T cells was greatly reduced in the B cell deficient mice (Fig. 3-4) and we also observed reductions in the numbers of Foxp3+CD25+ T cells (data not shown). It is important to note that we found no evidence that IL-10 expressed by any cell type is necessary for protection to Hp (data not shown), suggesting that IL-10 producing Tregs are not critically important for Hp clearance. Thus, while we cannot exclude the possibility that B cells regulate immunity to Hp by affecting other T cell subsets, we can conclude that one important way in which B cells regulate cellular immune responses to Hp is by controlling primary and memory Th2 expansion and differentiation.
How might B cells affect Th2 expansion and differentiation? We show that MHC class II-dependent interactions between B and T cells are necessary for the development of effective Th2 responses to Hp (Fig. 5), suggesting a potential role for B cell mediated co-stimulation. One likely candidate that we considered was OX40L as expression of OX40L on B cells is necessary for the development of optimal Th2 memory responses to OVA and alum (Linton et al., 2003). and OX40L is required for protection to Hp challenge infection (Ekkens et al., 2003). However, we found that OX40L expression by B cells is not required for the generation of a protective humoral or cellular immune response to Hp (data not shown). Instead, we found that mice lacking IL-4Rα specifically on B cells are unable to make an effective memory response to Hp (Fig. 6), leading us to conclude that B cells primed in the presence of IL-4 (or Be-2 like B cells), are required for immunity to Hp. However, despite the ability of Be-2 cells to make IL-4, IL-4 producing B cells are not required to generate a protective immune response to Hp (Fig. 7).
Be-2 cells also make large quantities of IL-2 (Harris et al., 2000); a cytokine that is known to play a central role in Th2 differentiation (Cote-Sierra et al., 2004). In fact, we show that IL-2 made specifically by B cells is required for protection to Hp (Fig. 7). Furthermore, we found that the loss of IL-2 producing B cells correlates with a significant decrease in the number of IL-4 producing Th2 cells in mice rechallenged with Hp. In agreement with these findings, anti-IL-2 treatment of BALB/c mice susceptible to L. major results in the suppression of the non-protective Th2 response and the induction of a protective IFNγ response (Heinzel et al., 1993). Interestingly, B cells were shown to be the major cellular source of IL-2 in the non-protected L. major-infected BALB/c mice (Heinzel et al., 1993) and B cells are obligate for the non-curing phenotype of BALB/c mice (Ronet et al., 2008). Thus, it is tempting to speculate that IL-2 producing Be-2 cells may prevent clearance of L. major in BALB/c mice. Although IL-2 is not considered a Th2 polarizing cytokine and does not promote Th2 survival or cell growth (Cote-Sierra et al., 2004), it does improve accessibility of transcription factors to the IL-4 locus - thereby controlling Th2 differentiation. Given the reduction in the Th2 response in the absence of IL-2 producing B cells, it was not surprising to find that the class-switched Ab response to Hp is also reduced. It was, however, surprising to find that mice that completely lack the ability to produce IL-2 in hematopoietic lineage cells produced nearly normal levels of Hp-specific IgG1 (Fig. 7). While we do not know the reason for this, we speculate that the loss of Tregs in these mice may enhance the humoral immune response to the point that it overcomes the reduction seen in the mice that are IL-2 deficient selectively in B lineage cells. Regardless, the impairment in protection against Hp observed in the in the absence of IL-2-producing B cells is likely due to the attenuation of both the cellular and humoral immune responses to Hp.
IL-2 was not the only cytokine made by Be-2 cells that regulates immunity to Hp as we found that chimeric mice in which the B cells are selectively unable to produce TNFα do not develop effective protection to Hp. These results were somewhat surprising as Hp infection induces a dominant Th2 response, and TNFα is not a cytokine traditionally associated with Th2 responses. Indeed, TNFα production by B cells has been shown to enhance IFNγ production by T cells in T. gondii infected mice (Menard et al., 2007). However, there are several examples in which TNFα regulates Th2 responses (Cazzola and Polosa, 2006) - most notably in mice infected with the nematode T. muris (Artis et al., 1999). Although TNFα is not required for the development of Th2 cells after infection with T. muris (Artis et al., 1999) or, as we show, after infection with Hp, TNFα is important for modulating the activity of IL-13 during T. muris expulsion (Artis et al., 1999). At this point, we do not know whether B cell-derived TNFα regulates the IL-13 response to Hp - or even whether IL-13 is necessary for protection to Hp. However, our data do show that the loss of TNFα-producing B cells leads to a significant decline in the class-switched Ab response. This result was unexpected, as TNFα production by B cells is not required to generate Ab responses to sheep red blood cells (Endres et al., 1999). However, our preliminary results indicate that long-lived Ab responses to mucosal antigens, but not systemic antigens, are dependent on TNFα-producing B cells (WW and FEL unpublished observation). In any event, given the importance of Ab in protection to Hp, it is reasonable to propose that one, of perhaps multiple, ways in which TNFα-producing B cells contribute to Hp immunity is by promoting enduring Ab responses.
In summary, our data demonstrate that B cells use a variety of effector mechanisms, including secretion of Ab, presentation of antigen, enhancement of Th2 primary and memory responses, and production of cytokines to promote immunity to Hp. While it has been known for many years that B cells have the ability to carry out each of these functions, this is the first demonstration that B cells must successfully carry out every one of these functions in order to protect the host against challenge infection with a single pathogen. Importantly, we also showed that cytokines made by B cells appear to control both the activity of the B cells themselves as well as the expansion and differentiation of effector and memory Th2 cells. Thus, our experiments fill an important gap as they show for the first time that multiple cytokines made by B cells regulate both humoral and cellular immune responses to infectious organisms. In the context of immunity to particular pathogens, cytokine production by B cells can clearly be beneficial. However, in the context of immune responses to “inappropriate” stimuli such as autoantigens and allergens, these same cytokine-producing effector B cells may facilitate a positive feedback loop between B and T cells, leading to amplification and immunopathology. Thus, cytokine-producing effector B cell subsets have the potential to influence both protective and damaging immune responses in vivo and therefore may represent future targets for vaccination, inhibition and depletion based therapeutics.
Experimental Procedures
Mice and BM chimeras
All animals used in these experiments were bred and maintained in the Trudeau Institute Animal Breeding Facility. All procedures involving animals were approved by the Trudeau Institute Institutional Animal Care and Use Committee and were conducted according to the principles outlined by the National Research Council. Mouse strains and their origins are described in Supplemental Table. 1. BM chimeric mice were generated by lethally irradiating recipients with 950 Rads from a 137Cs source and reconstituted them with 107 total BM cells (Lund et al., 2006). BM chimeras mice were allowed to reconstitute for 8-12 weeks before infection. See Suppl. Fig. 1 for description of conventional reciprocal BM chimeras and representative reconstitution profile. See Suppl. Figs. 3-6 for description of mixed BM chimeras used in the individual experiments and see Suppl. Fig. 4 for representative reconstitution profiles.
Hp stocks, antigen and infection
Hp stocks were routinely passaged through BALB/c mice. Soluble antigen was prepared from adult parasites as previously described (Harris et al., 2000). Mice were infected by gavage with 100-200 L3 Hp larvae. Adult Hp parasites were eliminated by 2 oral administrations of 100 mg/kg Pyrantel pamoate (Effcon Laboratories) delivered 5 days apart. Mice were challenged 10 days after the last drug treatment with 200 L3 Hp. Adult Hp parasites in the intestinal lumen were enumerated by visual inspection under a dissecting microscope at any time between days 10-21 post-challenge infection as parasite burden in the intestine peaked ∼10 days post infection and remained at plateau levels for at least 21 days (Fig. 1).
Intracellular staining and flow cytometry
Single cell suspensions from mLNs were stimulated on anti-CD3 coated plates (2 μg/ml) in the presence of Brefeldin-A (10 μg/ml) for 4-5 hours. The restimulated cells were stained with anti-CD4, fixed in 4% paraformaldehyde, permeabilized with 0.1% NP40 and stained with Abs against EGFP (Invitrogen) and IL-4 (BD Biosciences). Conjugated Abs to CD19, FAS, CD62L and CD4 were purchased from BD Biosciences. Peanut agglutinin (PNA) was obtained from Vector Laboratories. Samples were analyzed using a dual laser FACSCalibur™ or a FACScan (BD Biosciences) with a multicolor upgrade (Cytek Development). Data were analyzed with FlowJo software (TreeStar).
T cell purification and restimulation
CD4 T cells were purified (∼95% purity) from mLNs by positive selection over LS columns using anti-CD4 MACS beads (Miltenyi Biotec). Purified CD4 T cells (5 × 105/ml) were stimulated with plate-bound anti-CD3 (10 μg/ml) or with mytomycin C-treated (10 μg/ml), Hp antigen-pulsed (20 μg/ml), T cell-depleted splenocytes at a 1:1 ratio at 1 × 106/ml. Supernatants were removed for analysis 24 hours later.
ELISAs and cytometric bead array
Cytokines were measured by ELISA using Ab pairs from BD Biosciences or by Luminex™-based multiplex cytometric bead array using Beadlyte™ multi-cytokine beads (Upstate Biotech). Hp-specific Abs were assayed by ELISA using plates coated with 2 μg/ml Hp antigen. Serum samples were serially diluted and bound Abs were detected using anti-mouse IgM or anti-mouse IgG1 (Southern Biotech). Total IgE was measured by using anti-mouse IgE Ab pairs from BD in ELISAS.
Serum transfers
B6 donor mice were infected with 200 L3 Hp, treated with pyrantal pamoate on day 30 and then rechallenged with 200 L3 Hp 2 weeks later. Hp immune serum was collected from the euthanized donor mice on day 21. 200 μl of the Hp immune serum or normal mouse serum was injected i.p. into memory μMT and B6 recipients on day -2, 0 and 2 days following a challenge infection with Hp.
Statistical analyses
Data were analyzed using the unpaired Student’s T test using Prism 4.0 (GraphPad Software, Inc). Values of p≤0.05 were considered significant.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. J. Kearney (Univ. Alabama- Birmingham) for providing μMTMD4 mice, Dr. R. Corley (Boston Univ.) for providing μs-/- mice, and Dr. Rachael Gerstein (Univ. Mass.) for providing the AID-/- mice. This manuscript is dedicated to the memory of Frank Sprague, who played an instrumental role in the completion of this project. This work was funded by NIH AI-50844, NIH AI-68056 and Trudeau Institute.
Abbreviations
- Ag
antigen
- BCR
B cell receptor
- HPSE
H. polygyrus soluble extract
- BM
bone marrow
- WT
wildtype
- ICCS
intracellular cytokine staining
- IS
immune serum
- NMS
normal mouse serum
- Hp
Heligomosomoides polygyrus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Urban JF, Jr., Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D, Humphreys NE, Bancroft AJ, Rothwell NJ, Potten CS, Grencis RK. Tumor necrosis factor alpha is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during helminth infection. J. Exp. Med. 1999;190:953–962. doi: 10.1084/jem.190.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N, Choi YS, Rothaeusler K, Yang Y, Herzenberg LA. B cell lineage contributions to antiviral host responses. Curr. Top. Microbiol. Immunol. 2008;319:41–61. doi: 10.1007/978-3-540-73900-5_3. [DOI] [PubMed] [Google Scholar]
- Behnke JM, Parish HA. Transfer of immunity to Nematospiroides dubius: cooperation between lymphoid cells and antibodies in mediating worm expulsion. Parasite Immunol. 1981;3:249–259. doi: 10.1111/j.1365-3024.1981.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- Cambridge G. Serological changes following B cell depletion therapy. Arthritis Rheum. 2004;50:S645. [Google Scholar]
- Cardillo F, Postol E, Nihei J, Aroeira LS, Nomizo A, Mengel J. B cells modulate T cells so as to favour T helper type 1 and CD8+ T-cell responses in the acute phase of Trypanosoma cruzi infection. Immunology. 2007;122:584–595. doi: 10.1111/j.1365-2567.2007.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola M, Polosa R. Anti-TNF-alpha and Th1 cytokine-directed therapies for the treatment of asthma. Curr Opin Allergy Clin Immunol. 2006;6:43–50. doi: 10.1097/01.all.0000199798.10047.74. [DOI] [PubMed] [Google Scholar]
- Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Jensen PE. The role of B lymphocytes as antigen-presenting cells. Arch Immunol Ther Exp (Warsz) 2008;56:77–83. doi: 10.1007/s00005-008-0014-5. [DOI] [PubMed] [Google Scholar]
- Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc. Natl. Acad. Sci. USA. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J. Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- Dorner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. doi: 10.1016/j.immuni.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Ekkens MJ, Liu Z, Liu Q, Whitmire J, Xiao S, Foster A, Pesce J, VanNoy J, Sharpe AH, Urban JF, Gause WC. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J. Immunol. 2003;170:384–393. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- Endres R, Alimzhanov MB, Plitz T, Futterer A, Kosco-Vilbois MH, Nedospasov SA, Rajewski K, Pfeffer K. Mature follicular dendritic cell networks depend on expression of lymphotoxin b receptor by radioresistant stromal cells and of lymphotoxin b and tumor necrosis factor by B cells. J. Exp. Med. 1999;189:159–167. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol. 2008;8:391–397. doi: 10.1038/nri2315. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JFJ. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: Lessons from studies with rodent models. Annu. Rev. Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- Fu Y-X, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin a-dependent fashion. J. Exp. Med. 1998;187:1009–1018. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause WC, Urban JF, Jr., Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–277. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Gillan V, Lawrence RA, Devaney E. B cells play a regulatory role in mice infected with the L3 of Brugia pahangi. Int. Immunol. 2005;17:373–382. doi: 10.1093/intimm/dxh217. [DOI] [PubMed] [Google Scholar]
- Golovkina TV, Shlomchik M, Hannum L, Chervonsky A. Organogenic role of B lymphocytes in mucosal immunity. Science. 1999;286:1965–1968. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Mackay F, Browning JL, Kosco-Vilbois MH, Noelle RJ. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J. Exp. Med. 1998;187:997–1007. doi: 10.1084/jem.187.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Gray D, Gray M, Barr T. Innate responses of B cells. Eur. J. Immunol. 2007;37:3304–3310. doi: 10.1002/eji.200737728. [DOI] [PubMed] [Google Scholar]
- Harris DP, Goodrich S, Gerth AJ, Peng SL, Lund FE. Regulation of IFN-gamma production by B effector 1 cells: essential roles for T-bet and the IFN-gamma receptor. J. Immunol. 2005a;174:6781–6790. doi: 10.4049/jimmunol.174.11.6781. [DOI] [PubMed] [Google Scholar]
- Harris DP, Goodrich S, Mohrs K, Mohrs M, Lund FE. Cutting edge: the development of IL-4-producing B cells (B effector 2 cells) is controlled by IL-4, IL-4 receptor alpha, and Th2 cells. J. Immunol. 2005b;175:7103–7107. doi: 10.4049/jimmunol.175.11.7103. [DOI] [PubMed] [Google Scholar]
- Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- Hase K, Takahashi D, Ebisawa M, Kawano S, Itoh K, Ohno H. Activation-induced cytidine deaminase deficiency causes organ-specific autoimmune disease. PLoS ONE. 2008;3:e3033. doi: 10.1371/journal.pone.0003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel FP, Rerko RM, Hatam F, Locksley RM. IL-2 is necessary for the progression of Leishmaniasis in susceptible murine hosts. J. Immunol. 1993;150:3924–3933. [PubMed] [Google Scholar]
- Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J. Exp. Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki K, Tirosh B, Maehr R, Boes M, Honjo T, Ploegh HL. AID-/-mus-/- mice are agammaglobulinemic and fail to maintain B220-CD138+ plasma cells. J. Immunol. 2007;178:2192–2203. doi: 10.4049/jimmunol.178.4.2192. [DOI] [PubMed] [Google Scholar]
- Lee BO, Moyron-Quiroz J, Rangel-Moreno J, Kusser KL, Hartson L, Sprague F, Lund FE, Randall TD. CD40, but not CD154, expression on B cells is necessary for optimal primary B cell responses. J. Immunol. 2003;171:5707–5717. doi: 10.4049/jimmunol.171.11.5707. [DOI] [PubMed] [Google Scholar]
- Linton P-J, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J. Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick RC, Bradley LM. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J. Exp. Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liu Z, Rozo CT, Hamed HA, Alem F, Urban JF, Jr., Gause WC. The Role of B Cells in the Development of CD4 Effector T Cells during a Polarized Th2 Immune Response. J. Immunol. 2007;179:3821–3830. doi: 10.4049/jimmunol.179.6.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu Q, Pesce J, Anthony RM, Lamb E, Whitmire J, Hamed H, Morimoto M, Urban JF, Jr., Gause WC. Requirements for the development of IL-4-producing T cells during intestinal nematode infections: what it takes to make a Th2 cell in vivo. Immunol. Rev. 2004;201:57–74. doi: 10.1111/j.0105-2896.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- Lund FE. Cytokine-producing B lymphocytes - key regulators of immunity. Curr. Opin. Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr. Dir. Autoimmunity. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J. Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- McClellan KB, Gangappa S, Speck SH, Virgin H.W.t. Antibody-independent control of gamma-herpesvirus latency via B cell induction of anti-viral T cell responses. PLoS Pathog. 2006;2:e58. doi: 10.1371/journal.ppat.0020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard LC, Minns LA, Darche S, Mielcarz DW, Foureau DM, Roos D, Dzierszinski F, Kasper LH, Buzoni-Gatel D. B cells amplify IFN-gamma production by T cells via a TNF-alpha-mediated mechanism. J. Immunol. 2007;179:4857–4866. doi: 10.4049/jimmunol.179.7.4857. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Bhan AK. A case for regulatory B cells. J. Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Moulin V, Andris F, Thielmans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: Increased Interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J. Exp. Med. 2000;192:475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J. Exp. Med. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttila IA, Ey PL, Jenkin CR. Infection of mice with Nematospiroides dubius: demonstration of neutrophil-mediated immunity in vivo in the presence of antibodies. Immunology. 1984;53:147–154. [PMC free article] [PubMed] [Google Scholar]
- Pistoia V. Production of cytokines by human B cells in health and disease. Immunol. Today. 1997;18:343–350. doi: 10.1016/s0167-5699(97)01080-3. [DOI] [PubMed] [Google Scholar]
- Ronet C, Voigt H, Himmelrich H, Doucey MA, Hauyon-La Torre Y, Revaz-Breton M, Tacchini-Cottier F, Bron C, Louis J, Launois P. Leishmania major-specific B cells are necessary for Th2 cell development and susceptibility to L. major LV39 in BALB/c mice. J. Immunol. 2008;180:4825–4835. doi: 10.4049/jimmunol.180.7.4825. [DOI] [PubMed] [Google Scholar]
- Stephens R, Langhorne J. Priming of CD4+ T cells and development of CD4+ T cell memory; lessons for malaria. Parasite Immunol. 2006;28:25–30. doi: 10.1111/j.1365-3024.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- Sun CM, Deriaud E, Leclerc C, Lo-Man R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity. 2005;22:467–477. doi: 10.1016/j.immuni.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Urban JFJ, Katona IM, Paul WE, Finkelman FD. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc. Natl. Acad. Sci. 1991;88:5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.