Abstract
Background
Chronic renal failure (CRF) has been shown to significantly reduce the nonrenal clearance and alter bioavailability of drugs predominantly metabolized by the liver and intestine.
Objectives
The purpose of this article is to review all significant animal and clinical studies dealing with the effect of CRF on drug metabolism and transport.
Methods
The National Library of Medicine PubMed was utilized with the search terms ‘chronic renal failure, cytochrome P450, liver metabolism, efflux drug transport and uptake transport’ including relevant articles back to 1969.
Results
Animal studies in CRF have shown a major downregulation (40-85%) of hepatic and intestinal cytochrome P450 (CYP) metabolism. High levels of parathyroid hormone, cytokines, and uremic toxins have been shown to reduce CYP activity. Phase II reactions and drug transporters such as P-glycoprotein (Pgp) and organic anion transporting polypeptide (OATP) are also affected.
Conclusion
CRF alters intestinal, renal, and hepatic drug metabolism and transport producing a clinically significant impact on drug disposition and increasing the risk for adverse drug reactions.
Keywords: chronic renal failure, cytochrome P450, CYP, drug transport, efflux transporters, hepatic clearance, P-glycoprotein, uremic toxins
1. Introduction
It is a basic principle of clinical pharmacokinetics that drugs predominantly cleared by the kidney require dose adjustment in acute and chronic renal failure (ARF and CRF). It is widely assumed that it is unnecessary to dose adjust drugs cleared exclusively by hepatic metabolism and transport in the CRF patient population. However, accumulated data from animal and human studies do not support this assumption [1-6]. In the past 5 years, a number of reviews have appeared in the literature, which detail the effects of CRF on drug metabolism and transport including hepatic, renal, and intestinal processes [7-11]. This review was undertaken to illustrate various mechanisms by which CRF can alter drug metabolism and transport and to provide examples of actual or potential clinical relevance. Citations were derived from PubMed in years 1990-2008 using the search terms, ‘liver metabolism and transport, chronic renal failure, intestinal transport and cytochrome P450 metabolism, ‘including other key articles dating back to 1969. Recently, exciting new studies including intestinal, hepatic, and renal drug transporters have begun to elucidate the mechanisms of this phenomenon. The major focus of this review is to summarize these recent studies.
2. Background Pharmacokinetics
The following is a short review of basic pharmacokinetic principles focusing on hepatic clearance[12]. Drug absorption, nonrenal clearance, and volume of distribution of drugs are in fact altered by CRF via changes in hepatic clearance, intestinal absorption and first pass metabolism, hepatic, renal, and intestinal transport, plasma protein binding, and tissue binding. Perturbations of plasma protein binding of drugs occur with CRF which usually leads to an increase in the free unbound fraction (fu). Conformational changes in albumin resulting in altered albumin binding affinity or competition with uremic toxins are thought to be the mechanism. These effects may be partially reversed by dialysis.[13-28]. CYP activity in the kidney is estimated to be 20% of liver activity per gram of tissue. Conjugation reactions such as glucuronidation and acetylation are also impaired by CRF [28].
Hepatic clearance (CLH) is a function of hepatic blood flow (QH) and hepatic extraction ratio (EH) (Eq 1) [1,29].
| Eq(1) |
The relationship between hepatic blood flow, intrinsic hepatic clearance, and protein binding are expressed in the well stirred model of total hepatic clearance (CLHTOT) developed by Wilkinson and Shand (Eq 2) [29].
| Eq(2) |
where: fu = unbound fraction; CLINT = intrinsic hepatic clearance
Thus, intrinsic hepatic clearance and free unbound fraction of the drug are the determinants of total hepatic clearance. Intrinsic hepatic clearance relates the rate of metabolism at steady-state to the free unbound fraction in blood. If the drug is highly extracted we assume CLINT fu > > QH, then CLHTOT = QH and total hepatic clearance is blood flow limited. If the drug is a low extraction agent then we assume QH > > CLINT fu then CLHTOT = CLINT fu and would be highly influenced by changes in plasma protein binding and intrinsic hepatic clearance as simulated in empirically derived models [30]. Hepatic blood plasma flow has been shown to be unaffected by CRF in the absence of congestive heart failure and chronic liver disease [31].
Bioavailability is determined by the extent of intestinal absorption and intestinal and hepatic metabolism and transport. For a highly extracted drug with low systemic bioavailability (F), the bioavailability can be estimated by the following equation (Eq. 3) [1,29].
| Eq(3) |
For highly extracted drugs, CLINT fu > > QH and F = QH/ClINT fu. Since CRF reduces CLINT, bioavailability can be significantly increased. Reduced plasma protein binding due to CRF would be expected to increase the fu reducing bioavailability. The relative magnitude of changes in fu and CLINT will determine the net effect on F. Oral clearance and apparent oral clearance is influenced by F and defined by the following equation (Eqs 4 and 5) [29].
| Eq(4) |
| Eq(5) |
The disposition of drugs by the liver can be altered by CRF in both high and low extraction states. For low extraction drugs, reduced CLINT leads to an increase in both free and total steady-state drug concentrations. Although the total hepatic clearance of high extraction drugs is blood flow limited, reductions in CLINT and/or increases in fu can alter F.
Sun has proposed that the relative contribution of drug transport and metabolism to drug disposition can be predicted based on the physiochemical properties such as the drug solubility and permeability criteria of the Biopharmaceuticals Classification System (BCS) [10,32]. In drugs with high solubility and high permeability, Class I, enzymes effects predominate. In Class II, high permeability low solubility, both enzymes and transporters are important. Class III, high solubility and low permeability, transporter effects predominate.
3. Animal Studies: Effect of Renal Failure on CYP Metabolism
Animal models of acute and chronic renal failure since the late 1960s have shown reduced CYP activity with retained susceptibility to induction [33-36]. An early ARF study in rats showed increased bioavailability of propranolol, a highly extracted drug [37]. The same investigators then showed reduced hepatic extraction of propranolol in isolated perfused liver when perfused with uremic serum. Both control and ARF livers showed a 50% reduction in CLINT when perfused with uremic serum [38]. Livers from ARF rats showed no reduction hepatic extraction when perfused with normal serum. This suggested that CYP metabolism of propranolol in uremic rats is not downregulated but that a rapidly acting inhibitory factor(s) exists in uremic serum, which directly effects CYP activity.
Later developments in molecular biology allowed the sequencing of the cytochrome P450 (CYP) gene superfamily. Probe drugs have been validated which are substrates of specific CYP isozymes which can be radioactively labeled or unlabeled. The intestinal and hepatic CYP metabolism in data from animal models of CRF are shown in Table 1A and 1B, respectively. In CRF model in rats using subtotal nephrectomy there was reduced protein expression of CYP2C6, CYP2C11, CYP3A2 and CYP activity using trimethadione as non specific probe [39]. An in vivo study in rats using breath tests with the radiolabeled probe drugs aminopyrene (CYP2C11), erythromycin (CYP3A2), and caffeine (CYP1A2) showed a 35% reduction in CYP2C11 and CYP3A2 activities and no difference in CYP1A2[40]. These results correlated with protein expression. A later study by the same investigators confirmed these findings of reduced mRNA and protein expression and activity with no change in CYP2C6, 2D, 1A2,2E1 [41]. Protein expression of CYP2C11, CYP3A1, and CYP3A2 were reduced by greater than 40% and CYP1A2 remained unchanged as shown in previous studies. The downregulation of CYP3A1 and CYP3A2 was reversed by the inducing agents phenobarbital and dexamethasone. Another study in which various rat models of acute renal failure using 5/6 nephrectomy, bilateral ureteral ligation, intraperitoneal injection of cisplatin, and intramuscular injection of glycerol on intestinal and hepatic CYP showed differing effects on CYP metabolism depending on the model[42] The hepatic CYP3A activity was decreased by 60% in partially nephrectomized and glycerol injected rats. The CYP2C activity approximately was reduced by 50% in cisplatin treated rats. Rats with bilateral ureteral ligation had no reduction CYP activity. Intestinal CYP3A was slightly increased in glycerol induced acute renal failure with no changes in the other models of renal failure. A possible explanation for the varied results from the different models of CRF may be due to differing amount of residual renal function. Another possible mechanism is varying degrees of inflammation and cytokine response in different models of renal failure. Partial nephrectomy and cisplatin models renal failure may have greater cytokine response then bilateral ureteral ligation and therefore more downregulation of CYP.
TABLE 1. TABLE 1A Animal Studies of CRF and Intestinal Metabolism and Transport.
| INTESTINE | Protein | Activity | Effect on F |
|---|---|---|---|
| TRANSPORTERS | |||
| Pgp | ↓65% Naud [83] | ↓30% Naud [83] | ↑ |
| ABCB1 | ↔ Veau [84] | ↓41% Veau [84] | |
| MRP2 | ↓60% Naud [83] | ↓25% Naud et al. | ↑ |
| ABCC2 | |||
| MRP3 | ↓35% Naud [83] | NA | ↓ |
| Oatp2 | ↔ Naud [83] | ↔ | ↔ |
| SLCO | |||
| ENZYMES | |||
| CYP1A1 | ↓40% Leblond [48] | ↓25% Leblond [48] | ↑ |
| CYP2C11 | ↔ Leblond [48] | NA | ↔ |
| CYP3A2 | ↓70% Leblond [48] | ↓25% Leblond [48] | ↑ |
| TABLE 1B Animal Studies of CRF and Hepatic CYP Metabolism and Transport | |||
| LIVER | Protein | Activity | Effect |
| TRANSPORTERS | |||
| Pgp | ↑25% Naud [80] | ↑45% [80] | ↑ Biliary Excretion |
| ABCB1 | ↔ Laouari [79] | ? | |
| MRP2 | ↔ Naud [80] | NA | NA |
| ABCC2 | ↑ 70-200% Laouari [79] | ||
| Oatp2 | ↓40% Naud [80] | NA | ↓ Biliary Excretion |
| SLCO | ↓ metabolic CL | ||
| ENZYMES | |||
| CYP1A1 | ↔ | ↔ Leblond [40,41] | ↔ Hepatic CL |
| CYP2C11 | ↓40-45% Leblond [40] Uchida [39] |
↓35% Leblond [40,41] Uchida [39] |
↓ CL |
| CYP2D | ↔ Leblond [41] | ↔ Leblond [41] | ↔ CL |
| CYP3A1 | ↓75-85% Leblond [40,41] | ↓35% Leblond [40,41] | ↓ CL |
| CYP3A2 | ↓45-65% Leblond [40,41] Uchida [39] |
↓35-50% Leblond [40,41] | ↓ CL |
| Nat 1 | ↓33% Simard [77] | ↓45% Simard [77] | ↓ CL |
| Nat 2 | ↓50% Simard [77] | ↓50% Simard [77] | ↓ CL |
? = limited data
When normal rat hepatocytes were incubated for 24 h with uremic serum from patients with advanced CRF the protein and mRNA expression of CYP2C6, 2C11,3A1, and 3A2 were reduced by 35% and the N-Demethylation of erythromycin was also reduced by 35% [43]. Follow-up studies by the same group with human uremic serum demonstrated an inhibition of the mRNA and protein expression of CYP1A2,2C11,2D1/D2,3A2, 4A1/A3 mRNA in rat hepatocytes by more than 45% [44]. CYP3A and CYP1A activities were reduced by 51% and 59%, respectively [44]. The degree of inhibition was not affected by dialysis but reversed completely after renal transplantation [44]. The degree of inhibition of CYP2D was variable. The serum fraction of 10-15kDa contained the inhibitory activity [44]. Cytokines and PTH are in that molecular weight range and cytokines have been shown to inhibit CYP expression [45,46]. The serum PTH in these patients correlated (R2 = 0.79) with the degree of CYP downregulation in rat hepatocytes incubated with that same serum [44]. Subsequent rat hepatocyte incubation studies have shown that exogenous PTH downregulates CYP3A2 and CYP2C11 protein expression by approximately 60% and this effect of PTH can be reversed by adsorption of serum using anti PTH antibodies [47]. In contrast, parathyroidectomized rats with CRF do not exhibit downregulation of hepatic CYP. In a rat model, sera from CRF rats was shown to specifically inhibit intestinal CYP specifically CYP1A1 and CYP3A2 by 43% and 71%, respectively [48]. In an ARF model of ischemia-reperfusion, renal CYP2D6 and CYP2D9 were downregulated in the kidney [49]. These studies confirm the presence of circulating inhibitory factors in uremic serum which downregulate or directly inhibit hepatic and intestinal CYP activity and identify PTH and cytokines as putative inhibitory factors.
4. Clinical Investigations: Effect of Chronic Renal Failure on Drug Disposition
Several reviews of the effect of chronic renal failure on drug disposition exist in the literature [1-11]. One of the first clinical studies to address the issue of reduced drug metabolism in renal failure used sulfisoxazole as an intravenous probe drug [50]. Sulfisoxazole is acetylated, glucuronidated, and metabolized partially by CYP2C9. A summary of clinical data showing significant alterations in nonrenal clearance in CRF for a number of drugs is shown in (Table 2) [7, 51-61]. The majority of these studies involve ESRD patients. These alterations include both substantial reductions in nonrenal clearance ranging from 30 to 90% [1-11].
TABLE 2. Effect of CRF on Nonrenal Clearance in Human Subjects.
| Drug | % Change CLnr | Enzyme | Metabolism |
|---|---|---|---|
| Decreased | |||
| Captopril | -50 | TPMT * | sulfoxidation |
| Morphine | -40 | UGT2B7 | glucuronidation |
| Procainamide | -60 | NAT-2 | acetylation |
| Imipenem | -58 | dehydropeptidase | |
| Nimodipine | -87 | CYP3A4 | deakylation |
| Verapamil | -54 | CYP3A4 | demethylation |
| Metoclopramide | -66 | CYP2D6 | deakylation, sulfation |
| Desmethyldiazepam | -63 | CYP2C9 | hydroxylation |
| Warfarin | -50 | CYP2C9 | hydroxylation |
TPMT thiopurine methyl transferase
The effect of CRF manifests as increase in F -for high extraction drugs as shown in Table 3 [7, 62-65]. The propranolol F rises 3-fold as CRF develops but is partially reversed when the patient starts dialysis [65]. The same investigators also found that the propranolol F was greater just prior to dialysis (43%) compared to one day post dialysis (34%). These findings are in agreement with the animal data discussed earlier. Another investigator was not able to reproduce these findings in stable renal failure patients with creatinine clearance of approximately 15 ml/min [66]. These patients may have had enough residual renal function to prevent the inhibition of hepatic metabolism. The systemic clearance of nicardapine is also reduced by 50% and F is increased by 90% with chronic oral dosing in patients with moderate-to-severe chronic kidney disease (average GFR 39 ml/min) not yet on dialysis [67]. These change in the pharmacokinetics of nicardapine are reversed after starting hemodialysis, implicating a dialyzable inhibitory factor [67]. The apparent discrepancy between the results of Michaud [44] and Ahmed [67] in terms of the reversibility of downregulation of CYP3A4 with dialysis may lie in differences in study design. The study by Ahmed is an in vivo clinical pharmacokinetic study of the CYP3A4 substrate nicardapine and Michaud is an ex vivo study in which uremic serum from CRF patients on dialysis incubated with rat hepatocytes expressing the rat homolog CYP3A2 [44,67]. CYP3A2 responses to human uremic serum may differ from that of CYP3A4. There may be some uremic factors which inhibit CYP3A2 but not CYP3A4. Also, there is no information in either paper on what type of dialyzers were used, the blood flow rate, or dialysis duration, all of which could impact on the clearance of uremic inhibitory factors.
TABLE 3. Effect of CRF on Bioavailability in Human Subjects.
| Propranolol | +300% | CYP2D6 |
| Erythromycin | +100% | CYP3A4 |
| Propoxyphene | +100% | CYP3A4 |
| Dihydrocodeine | +70% | CYP2D6 |
| Oxprenolol | +100% | CYP2D6 |
Various phenotyping protocols have been used to assess CYP function in CRF. The cumulative recovery of 4-hydroxymephenytoin, a probe of CYP2C19, was reduced by 25% in subjects with Clcr of less than 50ml/min [68]. The formation clearance of 6-hydroxychlorzoxazone, a probe for CYP2E1, was not impacted by CRF [68]. The CYP2D6 probe drug debrisoquine recovery ratio was unchanged by CRF. In contrast another study using the CYP2D6 probe drug sparteine showed a 49% reduction in activity in CRF that correlated with the degree of renal insufficiency [69]. Our group showed a 50% reduction in CYP2C9 activity in ESRD using the S/R warfarin plasma ratio as specific probe [70]. S-warfarin is metabolized exclusively by CYP2C9, while R-warfarin is metabolized by multiple pathways. The erythromycin breath test (EBT) was used to measure CYP3A4 activity in ESRD and showed a 28% reduction [71]. Induction of CYP3A4 by rifampin was shown to overcome these effects [71].
The major hepatic conjugation reactions glucuronidation and acetylation are significantly reduced in CRF. The plasma area under the plasma concentration time curve (AUC) of zidovudine which is cleared predominantly by glucuronidation via glucuronosyltransferase (UGT2B7) was doubled in the CRF group [72]. Morphine glucuronidation, also via UGT2B7, is also significantly impaired in CRF [73]. N-acetyl-transferase (NAT-2) has been shown to exhibit genetic polymorphisms with a prevalence of rapid or slow acetylator phenotype of 50% in the African-American and Caucasian populations [74]. There were significant reductions is CLNR of isoniazid in CRF patients that were also rapid acetylators. The reduction CLNR isoniazid was more pronounced in slow acetylators with CRF. Both of these effects were reversed by transplantation [74,75]. These data suggest that with high extraction drugs that also exhibit polymorphic metabolism, poor metabolizers may be at greater risk for adverse effects of CRF on drug metabolism. These results are consistent with a recent rat study by Simard which showed a reduction in mRNA and protein expression of both Nat1 and Nat2 by 30% and reduction Nat2 activity by 50% [76]. Parathyroidectomy prevented the downregulation Nat expression and inhibition of Nat2 activity [76]. Addition of exogenous PTH to rat hepatocytes reduced Nat2 expression and activity suggesting that PTH may be a circulating inhibitory factor effecting NAT-2 activity in humans.
5. The Effect of Chronic Renal Failure on Drug Transport
Drug transport and metabolism are intimately connected. Drug uptake and efflux from intestinal, renal, and hepatic cells controls the size of the intracellular pool of drug and availability of substrate for CYP and other drug metabolizing enzymes. Drug uptake across the sinusoidal membrane of hepatocytes mediated by transporters such OATP may be rate limiting on hepatic elimination of drugs [77]. The Pgp and the multi-drug resistance associated protein (MRP2) are efflux transporters in the cannalicular membrane of hepatocytes and luminal membranes of the renal proximal tubules and intestinal enterocyts and are responsible for extrusion of drugs[10]. Pgp is responsible for the efflux of neutral and cationic drugs many of which are also substrates of CYP3A4 [10]. In fact the transcription factor PXR acts as xenobiotic sensor and controls the joint upregulation of Pgp and CYP3A4 [78]. MRP-2 transports anionic glucuronide, sulfate, and glutathione-conjugated compounds [10].
The effects of CRF on drug transport have been studied only recently. A more complete review of the effects of CRF and ARF on intestinal and hepatic drug transport can be found in Sun [10] and are shown in Table 1A and 1B, respectively. In one study by Laouari, CRF in 80% nephtrectomized rats showed a 70-200% increase in protein expression and mRNA for MRP2 in both kidney and liver with no change in Pgp in either organ [79]. These results are in contrast to study by Naud that showed rats with chronic renal failure developed an increase in protein and mRNA expression of hepatic Pgp of 25% and 40%, respectively [80]. CRF increased hepatic MRP2 mRNA expression by 40% and did not alter hepatic MRP protein expression[80]. Oatp2 showed a decrease in protein expression 35% with no change in mRNA expression of the uptake transporter. Isolated rat hepatocytes were incubated with serum from uremic rats and this reproduced the in vivo protein expression data showing an increase in Pgp, decrease in Oatp2 and no change MRP2. The isolated hepatocyte data differed from the in vivo data in that it did not show change in mRNA levels of any of the transporters [80]. Naud speculated that the degree of CRF in their rat model was greater in their study compared to Laouari and that may have explained the different expression of Pgp and MRP2. Another study in rat model of glycerol-induced, acute renal failure showed an 2.5-fold increase in protein expression of renal Pgp but no change in liver and brain [81]. However, there was a suppression of Pgp activity in all three organs using rhodamine 123 as probe substrate. CRF also reduced the protein expression of rat renal organic cation transporter (OCT2) by 35% and inhibited transport activity, which was reversed by testosterone [82].
There is also data in regard to intestinal transport. In a study by Naud, CRF rats showed reduced intestinal Pgp transport function and protein expression without a change in mRNA[83]. Veau observed a decrease in intestinal Pgp function with no change protein expression or mRNA levels [84]. Rat enterocytes incubated with rat uremic serum reproduced the reduction in MRP2 and Pgp protein expression and reduction Pgp activity, which implicated a role circulating uremic factors [83]. Decrease in protein expression without a change in mRNA suggests that post-translational mechanisms such as protein degradation and not downregulation of transcription are responsible for the loss in drug tansporter function. The discrepancies in the findings were attributed to technical differences in isolation of rat enterocytes. Another possible mechanism is circulating uremic factors acting as competitive inhibitors of transport function. These findings could explain the increased bioavailability of drugs in CRF.
Recent studies have shown the effect of known uremic toxins on uptake transport and metabolism of drugs. In the basolateral proximal tubular membrane of the kidney are organic anion transporter (OAT1 and 3) OCT. The sinusoidal membrane of hepatocytes contains the uptake transporter OATP and OCT. The uremic toxin, such as 3-carboxy-4-methyl-5-propyl-2-furan-propanoic acid (CMPF) has been shown to directly inhibit the uptake of erythromycin by Oatp2 in freshly isolated rat hepatocytes [85]. Another uremic toxin, indoxyl sulfate, inhibits the enzymatic breakdown of erythromycin [85]. Use of the oral adsorbent AST-120, which binds uremic toxins such as indoxyl sulfate in rats with CRF showed prevention of downregulation of OAT1 in the kidney[86]. Uptake of renal uremic toxins have been shown to be mediated rOat1/hOAT1 and rOat3/hOAT3 on the basolateral membrane of the proximal tubules [87,88]. Both OAT1 and OAT3 contribute equally to the renal uptake indoxyl sulfate, OAT3 to CMPF uptake, and OAT1 to indolacetate and hippurate uptake [88].
6. Effect of Altered Drug Transport on Interpretation of Pharmacokinetic Data in CRF
There is a complex interaction between drug transport and metabolism and CRF may simultaneously perturb both processes leading to difficulties in interpretation pharmacokinetic data. As shown by Frassetto, the erythromycin breath test may be confounded by CRF induced changes in the drug transporters OATP) and Pgp which control the intracellular pool of erythromycin in the hepatocytes [89]. Nolin showed an increase in EBT post hemodialysis compared to predialysis indicating a dialyzable uremic factor may be inhibiting erythromycin metabolism[90]. The confounding role of transport effects on EBT are illustrated in this important clinical study by Nolin [91]. This group showed no change in midazolam IV and oral clearance in ESRD patients using midazolam as specific CYP3A4 probe. An earlier study by Vinik also showed no change unbound clearance of IV midazolam in CRF patients consistent with the later findings [92]. This indicates intestinal and hepatic CYP3A4 are not altered in the well dialyzed ESRD patient [91]. The OATP and Pgp probe drug fexofenadine was given to these same patients and there was 63% reduction in the oral clearance. Since fexofenadine is not a substrate of CYP3A4 and largely excreted unchanged in the bile these results indicate reduced hepatic Pgp and/or OATP activity [91]. This suggests the mechanism for reduced EBT is inhibition of OATP mediated uptake of erythromycin leading to reduced intracellular pool of erythromycin. Theoretically, increased hepatic Pgp mediated efflux of erythromycin would also decrease the intracellular pool of erythromycin and reduce the EBT but that would be not be consistent with the reduced fexofenadine oral clearance observed by Nolin [91]. Another level of complexity is added with intestinal Pgp and OATP which can also effect oral clearance by altering F. Intestinal Pgp mediates the efflux of drugs into the intestinal lumen reducing drug absorption. It is inhibited by CRF which would tend to increase F and therefore decrease the apparent oral clearance (CL/F) of fexofenadine, Eq (5). Intestinal OATP facilitates uptake of drug and inhibition of OATP by CRF would reduce F and therefore increase the apparent oral clearance of fexofenadine, Eq (5).
7. Regulatory Issues
The FDA is currently revising its guidance for pharmacokinetic studies in chronic renal failure. A concept paper is currently available on line at the FDA website at http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4351b1-01-FDA.pdf. The impact of the 1998 guidance is reviewed by Huang which showed an increase in the renal impairment studies and dosage adjustment recommendations based on PK changes from 1996-1997 to 2003-2007 [93]. There is a recognition by the FDA of the effect of CRF on drug metabolism and transport. The previous guidance had recommended PK studies in renal patients for drugs with a low therapeutic index (TI) which are cleared predominantly by nonrenal mechanisms. The current concept paper recommends at least one abbreviated PK study in ESRD patients. Given that the current data suggests that circulating uremic toxins which inhibit drug transport and metabolism can be cleared partially by dialysis, advanced chronic kidney disease GFR < 20 not yet on dialysis may be the most seriously effected and should be included in an abbreviated PK study.
8. Conclusions
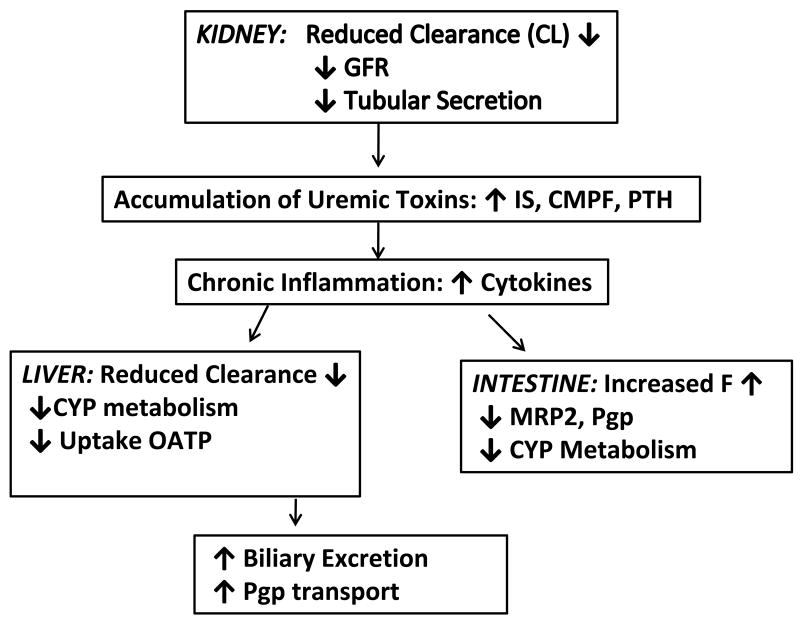
There is abundant evidence that the nonrenal clearance, protein binding, and volume of distribution of drugs are altered in chronic renal failure, increasing the risk of adverse drug reactions. The majority of the data are in patients with ESRD. Data spanning four decades have shown reduction in nonrenal clearance of drugs extracted predominantly by the liver. In the past 10 years mechanisms for this reduction of nonrenal clearance of drugs have been elucidated. The pharmacologic and physiological effects of CRF on intestinal and hepatic drug metabolism and transport are summarized in Figure 1. Downregulation of hepatic and intestinal CYP by circulating uremic factors have been shown in animal models particularly CYP2C and CYP3A iszoymes. Human studies have shown a reduction in CYP2C9 and CYP2C19 activity using serum warfarin S/R ratio and mephenytoin. CYP3A4 activity was not reduced in ESRD patients using midazolam as a probe substrate but clearance of the transport probe fexofenadine was diminished indicating reduced in OATP mediated hepatic uptake or reduced Pgp efflux. EBT in ESRD is confounded by transport effects probably due to reduced OATP mediated hepatic uptake of erythromycin. More recently animal models of CRF have shown effects on hepatic and intestinal Oatp2, MRP2, and Pgp that can have significant impact on drug disposition and metabolism. The effect of altered transport effects on warfarin metabolism have not been studied. Sun has proposed a scheme for predicting the relative importance of transport effects vs enzyme effects based on the permeability and solubility in the biopharmaceutical drug classification [10].
Figure 1.
Summary of Physiological and Pharmacologic Effects of CRF on Drug Metabolism and Transport.
9. Expert Opinion
It is clear that the effects of CRF on drug metabolism and transport are real and are clinically significant, based on many years of pharmacokinetic studies showing major alterations in nonrenal clearance in patients with CRF. Mechanisms point to circulating inhibitory factors some of which are dialyzable. Patients with advanced CRF Stage IV (GFR 15-29) and Stage V (0-15 ml/min) may behave differently depending whether or not they are on dialysis since these factors may be partially removed by dialysis. Estimating the magnitude of these nonrenal effects is difficult without empirical studies. Caution should be exercised in dosing the drugs in Tables 2 and 3, which show significant reductions in nonrenal clearance and increased bioavailability. Careful titration from the lowest dose should be attempted. Education of practitioners about the existence of this phenomenon is important.
From a regulatory standpoint, these findings would argue for the need of pharmacokinetic studies to evaluate nonrenal clearance in CRF patients with varying degrees of renal impairment particularly when the drug under development is a substrate of CYP2C9, CYP3A4, and NAT-2. Multiple animal as well as human studies have consistently shown that CYP2C9, CYP2C19, CYP3A4 and NAT-2 and the rat homologs are suppressed by CRF and that these effects are clinically significant. Animal studies by Simard showed downregulation of hepatic Nat1 and Nat2 with reduction in Nat2 activity by 50% which appeared to mediated at least partially by PTH [77]. The recent work by Nolin suggests the previous EBT results were confounded by transport effects and that CYP3A4 activity is not reduced in well dialyzed hemodialysis patients when midazolam is used as a probe [86]. Patients with advanced CRF not yet on dialysis may behave differently and studies in this population are needed. There is less animal and human evidence concerning transport by OATP, MRP2, or Pgp but preliminary studies suggest that CRF may perturb the activity of these transporters and significantly alter drug disposition. The FDA concept paper recommends PK studies for all drugs in ESRD. The scheme proposed by Sun [10] based on permeability and solubility may be useful in directing clinical studies based on whether transport or enzyme effects predominate. If the drug under development is BCS Class III where transport effects predominate and the drug is transported by MRP2, Pgp, and OATP then full pharmacokinetic (PK) studies are required in all stages of CKD particularly if the drug has low TI [87]. If the drug is Class I, where enzyme effects predominate, and the drug is a substrate of CYP2C9, CYP2C19, and NAT-2 then full PK studies should be done in all stages of CKD especially stage IV-V and the drug has low TI.
Abbreviations
- ARF
Acute Renal Failure
- AUC
area under the concentration time curve
- BCS
Biopharmaceutical Classification System
- CKD
Chronic Kidney Disease
- CL
clearance
- CLH
hepatic clearance
- CLHTOT
total hepatic clearance
- ClINT
intrinsic hepatic clearance
- CLNR
non-renal clearance
- CMPF
3-carboxy-4-methyl-5-propyl-2-furan-propanoic acid
- CRF
Chronic Renal Failure
- CYP
cyotochrome P450
- E
extraction ratio
- EH
hepatic extraction ratio
- ESRD
end stage renal disease
- F
bioavailability
- Fu
unbound fraction
- MRP-2
multi-drug resistance protein-2
- NAT-2
N-acetyltransferase 2
- OAT
organic anion transporter
- OATP
organic anion transporting polypeptide
- OCT
organic cation transporter
- Pgp
P-glycoprotein
- PK
pharmacokinetics
- PTH
parathyroid hormone
- Q
blood flow rate
- QH
hepatic blood flow
- TI
therapeutic index
- TPMT
thiopurine methyl transferase
- VD
volume of distribution
Footnotes
Declaration of Interest: The authors state no conflict of interests and have received no payment in the preparation of this manuscript.
Bibliography
- 1.Reidenberg MM. Chapter 3, Drug metabolism in uremia, in Renal function and drug action. Philadelphia: Saunders; 1971. pp. 19–32. [Google Scholar]; First Book to address the effect of renal failure on drug metabolism pathways
- 2.Gibson TP. Renal disease and drug metabolism: an overview. Am J Kidney Dis. 1986;8(1):7–17. doi: 10.1016/s0272-6386(86)80148-2. [DOI] [PubMed] [Google Scholar]
- 3.Touchette MA, Slaughter RL. The effect of renal failure on hepatic drug clearance. DICP Ann Pharmacother. 1991;25:1214–24. doi: 10.1177/106002809102501111. [DOI] [PubMed] [Google Scholar]
- 4.Elston AC, Bayliss MK, Park GR. Effect of renal failure on drug metabolism by the liver. Brit J Anaesth. 1993;71:282–290. doi: 10.1093/bja/71.2.282. [DOI] [PubMed] [Google Scholar]
- 5.Talbert RL. Drug dosing in renal insufficiency. J Clin Pharmacol. 1994;34:99–110. doi: 10.1002/j.1552-4604.1994.tb03973.x. [DOI] [PubMed] [Google Scholar]
- 6.Lam YW, Banerji S, Hatfield C, Talbert RL. Principles of drug administration in renal insufficiency. Clin Pharmacokinet. 1997;32:30–57. doi: 10.2165/00003088-199732010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Dreisbach AW, Lertora JJL. The effect of chronic renal failure on hepatic metabolism and drug disposition. Seminars Dialysis. 2003;169:45–50. doi: 10.1046/j.1525-139x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 8.Nolin TD, Frye RF, Matzke GR. Hepatic drug metabolism and transport in patients with kidney disease. Am J Kidney Dis. 2003;42:906–925. doi: 10.1016/j.ajkd.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Pichette V, Leblond FA. Drug metabolism in chronic renal failure. Curr Drug Metab. 2003;4:91–103. doi: 10.2174/1389200033489532. [DOI] [PubMed] [Google Scholar]
- 10.Sun H, Frasseto L, Benet LZ. Effects of renal failure on drug transport and metabolism. Pharmacol Therap. 2006;109:1–11. doi: 10.1016/j.pharmthera.2005.05.010. [DOI] [PubMed] [Google Scholar]; Important review of CRF and drug transport and BCS scheme
- 11.Nolin TD, Naud J, Leblond FA, et al. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther. 2008;83(6):898–903. doi: 10.1038/clpt.2008.59. [DOI] [PubMed] [Google Scholar]
- 12.Rowland M, Tozer TN. Clinical Pharmacokinetics. 2nd. Malvern, PA: Lea and Febiger; 1989. Chapter 11 Elimination; pp. 148–176. [Google Scholar]
- 13.Reidenberg MM, Drayer DE. Alterations of drug protein binding in renal disease. Clin Pharmacokinet. 1984;9(suppl 1):18–26. doi: 10.2165/00003088-198400091-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ochs HR, Rauh HW, Greenblatt DJ, et al. Chlorazepate dipotassium and Diazepam in renal insufficiency: serum concentrations and protein binding of diazepam and desmethyldiazepam. Nephron. 1984;37:100–104. doi: 10.1159/000183222. [DOI] [PubMed] [Google Scholar]
- 15.Andreasen F. Protein binding of drugs in plasma from patients with acute renal failure. Acta Pharmacol Toxicol. 1973;32:417–429. doi: 10.1111/j.1600-0773.1973.tb01488.x. [DOI] [PubMed] [Google Scholar]; First review of the altered protein binding in ARF
- 16.Hooper WD, Bochner F, Eadie MJ. Plasma protein binding of diphenylhydantion: effects of sex hormones, renal and hepatic disease. Clin Pharmacol Ther. 1991;49:457–467. doi: 10.1002/cpt1974153276. [DOI] [PubMed] [Google Scholar]
- 17.Reidenberg MM, Odar-Cederlof I, Von Bahr C, et al. Protein binding of diphenylhydantoin and desmethylimipramine in plasma from patients with poor renal function. N Eng J Med. 1971;285:264–267. doi: 10.1056/NEJM197107292850506. [DOI] [PubMed] [Google Scholar]
- 18.Farrell PC, Grib NL, Fry DL, et al. A comparision of in vitro and in vivo solute-protein binding interactions in normal and uraemic subjects. Trans Am Soc Artfic Intern Organs. 1972;18:268–276. doi: 10.1097/00002480-197201000-00067. [DOI] [PubMed] [Google Scholar]
- 19.Lowenthal DT, Briggs WA, Lev G. Kinetics of salicylate elimination in anephric Patients. J Clin Invest. 1974;54:1221–1226. doi: 10.1172/JCI107865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreasen F, Jakobsen P. Determination of frusemide in blood plasma and its binding to proteins in normal plasma and in plasma of patients with acute renal Failure. Acta Pharmacol Toxicol. 1974;35:49–57. doi: 10.1111/j.1600-0773.1974.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 21.Rane A, Villeneuve JP, Stone WJ, et al. Plasma binding and distribution of frusemide in the nephrotic syndrome and uraemia. Clin Pharmacol Ther. 1978;24:199–207. doi: 10.1002/cpt1978242199. [DOI] [PubMed] [Google Scholar]
- 22.Bachmann K, Shapiro R, Mackiewicz J. Influence of renal dysfunction on warfarin plasma protein binding. J Clin Pharmacol. 1976;16:168–172. [PubMed] [Google Scholar]
- 23.Belpaire FM, Bogaert MG, Mussche MM. Influence of renal failure on the protein binding of drugs in animals and man. Eur J Clin Pharmacol. 1977;11:27–32. doi: 10.1007/BF00561784. 1977. [DOI] [PubMed] [Google Scholar]
- 24.Haughey DB, Kraft CJ, Matzke GR, et al. Protein binding of disopyramide and elevated alpha-1-acid glycoprotein concentrations in serum obtained from dialysis patients and renal transplant recipients. Am J Nephrol. 1985;5:35–39. doi: 10.1159/000166900. [DOI] [PubMed] [Google Scholar]
- 25.Tan CC, Lee HS, Ti TY, Lee EJ. Pharmacokinetics of intravenous vancomycin in patients with end-stage renal failure. Ther Drug Monit. 1990;12:29–34. doi: 10.1097/00007691-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Koch-Weser J, Sellers EM. Binding of drugs to serum albumin. N Eng J Med. 1976;294(2 parts):311–316. 526–531. doi: 10.1056/NEJM197602052940605. [DOI] [PubMed] [Google Scholar]
- 27.Dromgoole SH. The binding capacity of albumin and renal disease. J Pharmacol Exp Ther. 1974;191:318–323. [PubMed] [Google Scholar]
- 28.Anders MW. Metabolism of drugs by the kidney. Kidney Int. 1980;18:636–647. doi: 10.1038/ki.1980.181. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson GR, Shand DG. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975;18:377–390. doi: 10.1002/cpt1975184377. [DOI] [PubMed] [Google Scholar]; Landmark paper in the pharmacokinetics of liver drug clearance
- 30.Yuan R, Venitz J. Effect of chronic renal failure on the disposition of highly hepatically metabolized drugs. Int J Clin Pharmacol Ther. 2000;38:245–253. doi: 10.5414/cpp38245. [DOI] [PubMed] [Google Scholar]; An excellent kinetic treatment on the effects PPB and ER on clearance
- 31.Leblanc M, Roy LF, Villeneuve JP, et al. Liver blood flow in chronic hemodialysis patients. Nephron. 1996;73:396–402. doi: 10.1159/000189099. [DOI] [PubMed] [Google Scholar]
- 32.Amidon GL, Lennernas H, Shah VP, Crison J. A theoretical basis for a biopharmaceutical drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 33.Leber HW, Schutterle G. Oxidative drug metabolism in liver microsomes from uremic rats. Kidney Int. 1972;2:152–158. doi: 10.1038/ki.1972.85. [DOI] [PubMed] [Google Scholar]; Early in vitro work showing reduced CYP activity in renal failure
- 34.VanPeer AP, Belpaire FM. Hepatic oxidative drug metabolism in rats with experimental renal failure. Arch Int Pharmacodyn Ther. 1977;228:180–183. [PubMed] [Google Scholar]
- 35.Leber HW, Gleumes L, Schutterle G. Enzyme induction in the uremic liver. Kidney Int. 1978;13(suppl 8):S-43–S-48. [PubMed] [Google Scholar]
- 36.Patterson SE, Cohn VH. Hepatic drug metabolism in rats with experimental chronic renal failure. Biochem Pharmacol. 1984;33(5):711–716. doi: 10.1016/0006-2952(84)90451-9. [DOI] [PubMed] [Google Scholar]
- 37.Terao N, Shen DD. Effect of experimental renal failure on the disposition kinetics of l-propranolol in rats. J Pharmacol Exp Ther. 1983;227(2):295–301. [PubMed] [Google Scholar]
- 38.Terao N, She DD. Reduced extraction of l-propranolol by perfused rat liver in the presence of uremic blood. J Pharmacol Exp Ther. 1985;233:277–284. [PubMed] [Google Scholar]; First evidence of rapidly circulating uremic inhibitory factor in vitro
- 39.Uchida N, Kurata N, Shimada K, et al. Changes of hepatic microsomal oxidative drug metabolizing enzymes in chronic renal failure (CRF) rats by partial nephrectomy. Jpn J Pharmacol. 1995;68:431–439. doi: 10.1254/jjp.68.431. [DOI] [PubMed] [Google Scholar]
- 40.Leblond FA, Groux L, Villeneuve JP, Pichette V. Decreased in vivo metabolism of drugs in chronic renal failure. Drug Metab Dispos. 2000;(11):1317–1320. [PubMed] [Google Scholar]; First in vivo CYP study showing reduced activity, protein expression in CRF
- 41.Leblond F, Geuvin C, Demers C, et al. Down regulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol. 2001;12:326–332. doi: 10.1681/ASN.V122326. [DOI] [PubMed] [Google Scholar]
- 42.Okabe H, Hasunuma M, Hashimoto Y. The hepatic and intestinal metabolic activities of P450 in rats with surgery- and drug-induced renal dysfunction. Pharm Res. 2003;20(10):1591–1594. doi: 10.1023/a:1026131216669. [DOI] [PubMed] [Google Scholar]; Varying CYP activity of different animal models of CRF
- 43.Geuvin C, Michaud J, Naud J, et al. Down-regulation of hepatic cytochrome P450 in chronic renal failure. Br J Pharmacol. 2002;137:1039–1046. doi: 10.1038/sj.bjp.0704951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaud J, Dube P, Naud J, et al. Effects of serum from patients with chronic renal failure on rat hepatic cytochrome P450. Br J Pharmacol. 2005;144:1067–1077. doi: 10.1038/sj.bjp.0706138. [DOI] [PMC free article] [PubMed] [Google Scholar]; Downregulation reproduced by uremic serum
- 45.Renton KW. Cytochrome P450 regulation and drug biotransformation during inflammation and infection. Curr Drug Metabol. 2004;5:235–243. doi: 10.2174/1389200043335559. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Razzak Z, Loyer P, Fautrel A. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Molec Pharmacol. 1993;44:707–715. [PubMed] [Google Scholar]
- 47.Michaud J, Naud J, Choinard J, et al. Role of parathyroid hormone in the downregulation of liver cytochrome P450 in chronic renal failure. 2006;17:3041–3048. doi: 10.1681/ASN.2006010035. [DOI] [PubMed] [Google Scholar]; First paper to show PTH as a putative CYP inhibitory factor
- 48.Leblond FA, Pertucci M, Dube P, et al. Downregulation of intestinal cytochrome P450 in chronic renal failure. J Am Soc Nephrol. 2002;13:1579–15. doi: 10.1097/01.asn.0000017575.50319.77. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida T, Kurella M, Beato F, et al. Monitoring changes in gene expression in renal ischemia-reperfusion in the rat. Kidney Int. 2002;61:1646–1654. doi: 10.1046/j.1523-1755.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- 50.Reidenberg MM, Kostenbauder H, Adams WP. Rate of drug metabolism in obese volunteers before and during starvation and azotemic patients. Metabolism. 1969;18(3):209–213. doi: 10.1016/0026-0495(69)90040-7. [DOI] [PubMed] [Google Scholar]
- 51.Duchin KL, Pierides AM, Herald A, et al. Elimination kinetics of captopril in patients with renal failure. Kidney Int. 1984;25:942–947. doi: 10.1038/ki.1984.114. [DOI] [PubMed] [Google Scholar]
- 52.Kripalani KJ, McKinstry DN, Singhvi SM, et al. Disposition of captopril in normal subjects. Clin Pharmacol Ther. 1980;27:636–641. doi: 10.1038/clpt.1980.90. [DOI] [PubMed] [Google Scholar]
- 53.Gibson TP, Demetriades JL, Bland JA. Imipenem/cilastin: Pharmacokinetic profile in renal insufficiency. Am J Med. 1985;78:54–61. doi: 10.1016/0002-9343(85)90102-0. [DOI] [PubMed] [Google Scholar]
- 54.Gibson TP, Atkinson AJ, Jr, Matusik E, et al. Kinetics of procainamide and N-acetylprocainamide in renal failure. Kidney Int. 1977;12:422–429. doi: 10.1038/ki.1977.133. [DOI] [PubMed] [Google Scholar]
- 55.Lehman CR, Heironimus JD, Collins CB, et al. Metochlopramide kinetics in patients with impaired renal function and clearance by hemodialysis. Clin Pharmacol Ther. 1985;37:284–289. doi: 10.1038/clpt.1985.41. [DOI] [PubMed] [Google Scholar]
- 56.Bateman DN. Clinical pharmacokinetics of metoclopramide. Clin Pharmacokinet. 1983;8:523–529. doi: 10.2165/00003088-198308060-00003. [DOI] [PubMed] [Google Scholar]
- 57.Ochs HR, Rauh HW, Greenblatt DJ, Kaschell HJ. Clorazepate dipotassium and diazepam in renal insufficiency: Serum concentrations and protein binding of diazepam and desmethyldiazepam. Nephron. 1984;37:100–104. doi: 10.1159/000183222. [DOI] [PubMed] [Google Scholar]
- 58.Ochs HR, Greenblatt DJ, Kaschell HJ, et al. Diazepam kinetics in patients with renal isufficiency or hyperthyroidism. Br J Clin Pharmacol. 1981;12:829–832. doi: 10.1111/j.1365-2125.1981.tb01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirch W, Ramsch KD, Duhrsen U, Ohnhaus EE. Clinical pharmacokinetics of nimodipine in normal and impaired renal function. Int J Clin Pharmacol Res. 1984;4:381–384. [PubMed] [Google Scholar]
- 60.Storstein L, Larsen A, Midtbo K, Saevareid L. Pharmacokinetics of calcium blockers in patients with renal insufficiency and in geriatric patients. Acta Med Scand. 1984;216(suppl 681):25–30. doi: 10.1111/j.0954-6820.1984.tb08673.x. [DOI] [PubMed] [Google Scholar]
- 61.Mooy J, Schols M, Baak MV, et al. Pharmacokinetics of verapamil in patients with renal failure. Eur J Clin Pharamcol. 1985;28:405–410. doi: 10.1007/BF00544358. [DOI] [PubMed] [Google Scholar]
- 62.Gibson TP, Giacomini KM, Briggs WA, et al. Propoxyphene in normal subjects and anephric patients. J Clin Pharmacol. 1978;18:106–109. doi: 10.1002/j.1552-4604.1978.tb02429.x. [DOI] [PubMed] [Google Scholar]
- 63.Kanfer A, Stamatakis G, Torlotin JC, et al. Changes in erythromycin clearance induced by renal failure. Clin Neprhol. 1987;27:147–150. [PubMed] [Google Scholar]
- 64.Wellling PG, Craig WA. Pharmacokinetics of intravenous erythromycin. J Pharm Sci. 1987;67:147–150. doi: 10.1002/jps.2600670809. [DOI] [PubMed] [Google Scholar]
- 65.Bianchetti G, Graziani G, Brancaccio D, et al. Pharmacokinetics and the effects of propranolol in terminal uremic patients and in patients undergoing regular dialysis treatment. Clin Pharmacokinet. 1976;1:373–84. doi: 10.2165/00003088-197601050-00004. [DOI] [PubMed] [Google Scholar]; First to show that dialysis could partially reverse CYP inhbition
- 66.Wood AJJ, Vestal RE, Spannuth CL, et al. Propranolol disposition in renal failure. Br J Clin Pharmacol. 1980;10:561–566. doi: 10.1111/j.1365-2125.1980.tb00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed JH, Grant AC, Rodger RSC, et al. Inhibitory effect of uraemia on hepatic drug clearance and metabolism of nicardapine. Br J Clin Pharmacol. 1991;32(1):57–62. doi: 10.1111/j.1365-2125.1991.tb05613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frye RF, Matzke GR, Alexander ACM, et al. Effect of renal insufficiency on CYP. Clin Pharmacol Ther. 1996;59:155. doi: 10.1016/S0009-9236(96)90180-0. Abstract. [DOI] [PubMed] [Google Scholar]
- 69.Kevorkian JP, Michel C, Hofmann U, et al. Assessment of individual CYP2D6 in extensive metabolizers with renal failure: Comparison of sparteine and dextromethorphan. Clin Pharmacol Ther. 1996;59:583–592. doi: 10.1016/S0009-9236(96)90187-3. [DOI] [PubMed] [Google Scholar]
- 70.Dreisbach AW, Japa S, Gebrekal AB, Mowry SE, Lertora JJ, Kamath BL, Rettie AE. Cytochrome P4502C9 activity in end-stage renal disease. Clin Pharmacol Ther. 2003;73:475–7. doi: 10.1016/s0009-9236(03)00015-8. [DOI] [PubMed] [Google Scholar]; Human study showing downregulation CYP2C9 in ESRD
- 71.Dowling TC, Briglia AE, Fink JC. Characterization of hepatic cytochrome P4503A activity in patients with end-stage renal disease. Clin Pharmacol Ther. 2003;73:427–434. doi: 10.1016/s0009-9236(03)00056-0. [DOI] [PubMed] [Google Scholar]; Human study showing reduced of CYP3A4 activity in ESRD
- 72.Singlas E, Pioger JC, Taburet AM, et al. Zidovudine disposition in patients with severe renal impairment: influence of hemodialysis. Clin Pharmacol Ther. 1989;46:190–197. doi: 10.1038/clpt.1989.125. [DOI] [PubMed] [Google Scholar]
- 73.Osborne R, Joel S, Grebenik K, et al. The pharmacokinetics of morphine and Morphine glucuronides in kidney failure. Clin Pharmacol Ther. 1993;54:158–167. doi: 10.1038/clpt.1993.127. [DOI] [PubMed] [Google Scholar]
- 74.Reidenberg MM, Levy M, Drayer DE, et al. Acetylator phenotype in idiopathic systemic lupus etythematosus. Arthritis Rheum. 1992;23:569–73. doi: 10.1002/art.1780230508. [DOI] [PubMed] [Google Scholar]
- 75.Kim YG, Shin JG, Shin SG, et al. Decreased acetylation of isoniazid in chronic renal failure. Clin Pharmacol Ther. 1993;54:612–20. doi: 10.1038/clpt.1993.198. [DOI] [PubMed] [Google Scholar]; Effect of ESRD on acetylation, impact of phenotype, transplantation
- 76.Simard E, Naud J, Michaud J, et al. Downregulation of hepatic acetylation of drugs in chronic renal failure. J Am Soc Nephrol. 2008;19 doi: 10.1681/ASN.2007090974. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamazaki M, Akiyama S, Nihisgaki R, Sugiyama Uptake is rate limiting in the overall hepatic elimination of pravastatin at steady-state in rats. Pharm Res. 1996;13:1559–1564. doi: 10.1023/a:1016044032571. [DOI] [PubMed] [Google Scholar]; Hepatic uptake may be rate limiting to hepatic metabolism and excretion
- 78.Wang H, LeCluyse Role of orphan nuclear receptors in the regulation of drug-metabolizing enzymes. Clin Pharmacokinet. 2003;42(15):1331–1357. doi: 10.2165/00003088-200342150-00003. [DOI] [PubMed] [Google Scholar]
- 79.Laoari D, Yang R, Veau C, et al. Two apical multidrug transporters, Pgp and MRP2, are altered by chronic renal failure. Am J Physiol. 2001;280:F636–F645. doi: 10.1152/ajprenal.2001.280.4.F636. [DOI] [PubMed] [Google Scholar]
- 80.Naud J, Michaud J, Leblond FA, et al. Effects of chronic renal failure on liver drug transporters. Drug Metab Disp. 2008;36:124–128. doi: 10.1124/dmd.107.018192. [DOI] [PubMed] [Google Scholar]
- 81.Huang ZH, Murakami T, Okochi A. Expression and function of P-glycoprotein in rats with glycerol-induced acute renal failure. Eur J Pharmacol. 2000;406:453–460. doi: 10.1016/s0014-2999(00)00699-3. [DOI] [PubMed] [Google Scholar]
- 82.Ji L, Masuda S, Saito H, Inui KI. Down-regulation of rat organic cation transporter rOCT2 by 5/6 nephrectomy. Kid Int. 2002;62:514–524. doi: 10.1046/j.1523-1755.2002.00464.x. [DOI] [PubMed] [Google Scholar]
- 83.Naud J, Michaud J, Boisvert C, et al. Down-regulation of intestinal drug transporters in chronic renal failure in rats. J Pharmacol Exp Ther. 2007;320:978–985. doi: 10.1124/jpet.106.112631. [DOI] [PubMed] [Google Scholar]; CRF downregulation of intestinal drug transport
- 84.Veau C, Leroy C, Banide H, et al. Effect of chronic renal failure on the expression and function of rat intestinal P-glycoprotein in drug excretion. Nephrol Dial Transplant. 2001;16:1607–1614. doi: 10.1093/ndt/16.8.1607. [DOI] [PubMed] [Google Scholar]
- 85.Sun H, Huang Y, Frassetto L, Benet LZ. Effects of uremic toxins on hepatic uptake and metabolism of erythromycin. Drug Metab Disp. 2004;32:1239–1246. doi: 10.1124/dmd.104.000521. [DOI] [PubMed] [Google Scholar]; Effect of uremic toxins on hepatic drug transport
- 86.Aoyama I, Eonomot A, Niwa T. Effects of oral adsorbent on gene expression in uremic rat kidney:cDNA array analysis. Am J Kidney Dis. 41(S1):S8–S14. doi: 10.1053/ajkd.2003.50075. [DOI] [PubMed] [Google Scholar]
- 87.Deguchi T, Ohtsuki S, Otagiri M, et al. Major role organic anion transporter 3 in the transport of indoxyl sulfate in the kidney. Kidney Int. 2002;61:1760–1768. doi: 10.1046/j.1523-1755.2002.00318.x. [DOI] [PubMed] [Google Scholar]
- 88.Deguchi T, Kusuhara H, Takadate A, et al. Characterization of uremic toxin transport by organic anion transporters in the kidney. Kidney Int. 2004;65:162–174. doi: 10.1111/j.1523-1755.2004.00354.x. [DOI] [PubMed] [Google Scholar]; In vitro effects of uremic toxins on transport
- 89.Frassetto LA, Poon S, Tsourounis C, et al. Effects of uptake and efflux transporter inhibition on erythromycin breath test resuts. Clin Pharmacol Ther. 2007;81:828–832. doi: 10.1038/sj.clpt.6100148. [DOI] [PubMed] [Google Scholar]; First clinical paper demonstrating confounding effects of transport on EBT in healthy human subjects.
- 90.Nolin TD, Appiah K, Kendrick SA, et al. Hemodialysis acutely improves hepatic CYP3A4 metabolic activity. J Am Soc Nephrol. 2006;17:2363–2367. doi: 10.1681/ASN.2006060610. [DOI] [PubMed] [Google Scholar]; Human study showing partial reversal CYP3A4 inhbition by dialysis
- 91.Nolin TD, Frye RF, Sadr H, et al. The pharmacokinetics of fexofenadine but not midazolam are altered in End-stage Renal Disease. Clin Pharm Therap. 2008;83(S1):S58. Abstract. [Google Scholar]; Fist clinical paper using midazolam and fexofenadine probes in ESRD demonstrating reduced OATP but preserved CYP3A4 activity.
- 92.Vinik HR, Reves JG, Greenblatt DJ, et al. The pharmacokinetics of midazolam in chronic renal failure patients. Anethesiology. 1983;59:390–394. doi: 10.1097/00000542-198311000-00005. [DOI] [PubMed] [Google Scholar]; First paper showing preserved midazolam clearance in CRF
- 93.Huang S, Abraham S, Apparaju S, et al. Impact of the 1998 renal guidance on recent new drug application submissions. Clin Pharm Therap. 2008;83(S1):S85. Abstract. [Google Scholar]