Abstract
Fear of eye gaze is common in social anxiety disorder (SAD) and may represent an evolutionarily-conserved submissive behavior. SAD subjects and healthy volunteers showed significant differences in neural activity in amygdala, fusiform, insula, anterior cingulate and prefrontal cortex in response to direct versus averted gaze. Neural response to direct gaze may identify brain regions important in the pathophysiology of SAD.
Keywords: fMRI, brain imaging, social phobia
1. Introduction
Fear of eye gaze is a common and clinically significant symptom of social anxiety disorder (SAD). It also may be a manifestation of submissive behaviors evolutionarily conserved across group-living species, such as avoidance of direct gaze and other social threats from dominant individuals (Gilbert, 2001). Threatening facial expressions have been shown to activate fear neurocircuitry preferentially in SAD (Stein et al., 2002; AmIr et al., 2005; Phan et al., 2006), as have faces of greater emotional intensity (Yoon et al., 2007), but eye gaze stimuli have been little studied. This study assessed neural activityin SAD, using fMRI with face photo stimuli showing simulated eye motion into direct or indirect (averted) gaze.
The goal of this study was to develop fMRI response to gaze direction as a novel biomarker, which, if associated with SAD, could be used in animal models and humans to improve understanding of the causes and treatment of this disorder. We hypothesized that in SAD patients, processing of faces with direct eye gaze would preferentially activate fear circuitry structures such as the amygdala and insula, associated frontal regions (rostral anterior cingulate and medial prefrontal cortex), and core areas of visual face processing (e.g. fusiform gyrus).
2. Methods
Five subjects with SAD, and five healthy comparison group (HC) subjects with no lifetime psychiatric diagnoses participated in the study. SAD, generalized type, was the principal diagnosis for all SAD subjects, and lifetime comorbidity was limited to one SAD subject with current dysthymia and one SAD subject with current dysthymia and generalized anxiety disorder. DSM –IV diagnoses were made by clinical interview and independently confirmed by Structured Clinical Interview for DSM-IV (First et al., 2002) and medical examination including urine toxicology screen. SAD subjects had been free of psychotropic medication for >5 months. SAD subjects had significantly elevated scores on the Liebowitz Socal Anxiety Scale (Heimberg et al., 1999) (81.0 (S.D.=14.4) vs. 18.0 (S.D.=18.6), t=5.8, df=8, P=0.001), but they did not differ significantly in respect to mean age (28.4. (S.D.=2.3), range 24–31 vs. 24.0 (S.D.=2.0) range 22–27 years), gender (3 of 5 male vs. 4 of 5 male), or race (2 White, 2 Asian, 1 Hispanic vs. 1 White, 2 Asian, 1 Hispanic, 1 African-American).
Subjects were scanned on a 1.5 T MRI System (General Electric, Signa Twin Speed). Images were acquired using a T2* weighted gradient echo sequence: TE = 40 ms, TR = 2 secs, flip angle = 60 deg, in plane resolution = 64 × 64, voxel size = 2.97 × 2.97 × 4.5 mm with a FOV = 190 mm and slice separation of 0mm. Simultaneous images were acquired on 24 contiguous slices oriented along the frontal plane. Each imaging series required 80 complete head volume.
Functional data were preprocessed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK). Individual subject functional data were spatially normalized using MNI transformation with automated registration procedures (Maldjian et al., 2003). Voxel-wise tests were carried out at P=0.01 whole brain level of significance.
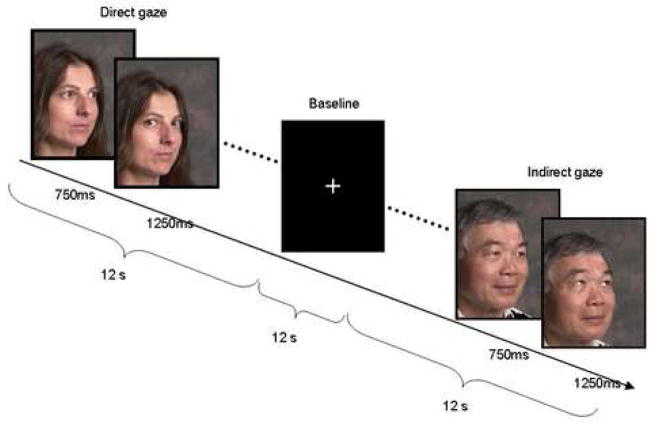
Eye gaze stimuli created by the authors consisted of images of faces with neutral expressions displaying direct or indirect gaze, modified from Kawashima et al. (1999). Faces of 18 individuals, half of each gender, representing several racial groups, were each presented three times with direct and three times with indirect gaze. All faces were displayed against a neutral background, with the chin aligned 30 degrees from the frontal plane. Each face trial was a sequence of two photographs, beginning with a 750 msec image showing eye gaze aligned with the 30-degree angle of the face. In the “indirect gaze” trial the first image was followed by a 1250 msec image of the same face identically aligned, but with eyes gazing upward (see Figure 1). The “direct gaze” trial differed in that eyes in the second image align directly with the subject, giving the illusion that gaze shifts toward the subject. Thus the direct and indirect stimuli vary only in direction of apparent movement of gaze.
Figure 1.
Example of experimental stimuli and presentation times. Direct and Indirect blocks, each consisting of 6 face pairs, were presented in randomized order.
We utilized a multi-epoch block design in which subjects viewed stimuli for 12 seconds (6 trials, 6 acquisitions) alternating with an interval of viewing crosshair for 12 seconds (6 acquisitions). Signal was averaged for each of the conditions. Subjects used a keypad to report for each face whether gaze was directed at the subject or away. Three imaging series included a total of 9 blocks with direct gaze stimuli and 9 blocks with indirect gaze stimuli, presented in random order.
Subjects viewed the stimuli through goggles (Avotec Silent Vision™ SV-4021 Fiber Optic Visual System, Avotec, Inc., Stuart, FL). Goggles were equipped with an eye-tracking device (Avotec Real Eye™ RE-4501 Fiber Optic Eye Imaging System (Avotec, Inc., Stuart, FL) combined with iViewX Tracking System (SensoMotoric Instruments, Inc., Boston, MA), which continually recorded gaze position at a sample rate of 60 Hz through the use of simultaneous pupil and corneal reflex tracking. The eye-tracking device was triggered simultaneously with the scanner. The device was calibrated for each subject using 9 fixation points, and drift correction was performed before each imaging series.
Eye tracking data analysis utilized the iView X (SensoMotoric Instrument, Inc., Boston, MA) bundled analysis package for fixation analysis. Pictorial analysis was performed for each stimulus block, and was overlayed onto a sample stimulus image, giving scanpaths and eye fixations displayed with raindrop analysis (length of fixation directly proportional to diameter of circle). During post-processing, a rectangular “object” was created to encompass the eye region, which was previously aligned for all stimulus images during the stimuli development phase. Fixation analyses were further processed using Matlab (The MathWorks, Inc., Natick, MA) to give fixation duration and position for each stimulus condition. This analysis reveals the number and length of the subject’s gaze fixations within the defined object area for each condition. Eye fixations were defined as eye position maintained within a 20-pixel diameter region for at least 50 ms. Percent of fixations in the eye region was calculated by dividing fixations within the eye region by total number of fixations.
3. Results
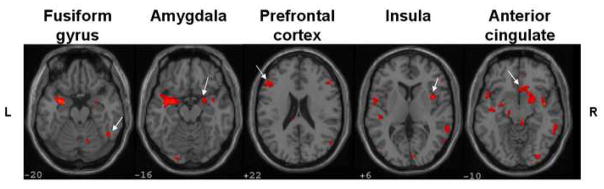
Figure 2 illustrates functional imaging data comparing scans of five SAD and five matched HC subjects analyzed with SPM2 for activityin response to direct > indirect gaze stimuli. Slices were selected to illustrate five ROIs that had been selected a priori based on study hypotheses. SAD subjects demonstrated significantly greater activity in amygdala, fusiform gyrus, insula, anterior cingulate and prefrontal cortex.
Figure 2.
SPM2 analysis of fMRI data, showing significant group differences [(Patient Direct > Indirect) > (Control Direct > Indirect), P < 0.01, k = 10] in hypothesized regions of interest.
Eye tracking results showed a trend for the mean difference in percentage of fixations in the eye regions (for indirect - direct gaze stimuli) to be higher for SAD patients compared to HC subjects (10.2% (S.D.=5.3) vs. 4.6% (S.D.=3.9); t=1.9, df=8, P=0.09). This suggests that SAD patients may have avoided direct gaze more than indirect gaze, compared to HC subjects. SAD patients had a nonsignificantly lower percentage of fixations in the eye region in response to both indirect (59% (S.D.=24.0) vs. 69.4% (S.D.=21.6)) and direct gaze stimuli (48.8% (S.D.=26.9 vs. 64.8 (S.D.=24.4)).
4. Discussion
These observations are the first to document differences in neural activity associated with eye gaze behaviors in SAD. They support the hypotheses of preferential activation of fear circuitry structures such as the amygdala and insula, associated frontal regions (rostral anterior cingulate and medial prefrontal cortex), and core areas of visual face processing (e.g. fusiform gyrus) in SAD in response to direct gaze. They confirm feasibility of this direct versus indirect gaze paradigm for mapping of brain activity patterns in response to varying degrees of eye contact, and the use of an in-scanner eye-tracking device to determine gaze direction and fixations within the target eye region. Eye tracking findings did not differ significantly between groups in this small sample, but the direction of the nonsignificant differences was consistent with the hypothesis that SAD patients show greater gaze aversion in response to direct versus indirect gaze, consistent with prior findings that SAD subjects avoid viewing the eye region of faces (Horley et al., 2003).
The differences in activity of threat neurocircuitry structures in SAD subjects in response to direct eye gaze could represent an endophenotype useful for further elucidation of the pathophysiology of SAD and its treatment. Future studies could examine whether response to direct gaze has utility as a predictor or biological marker of treatment response in SAD, and its specificity. Because avoidance of direct gaze appears to be an evolutionarily conserved submissive behavior, the paradigm used in this study could be modified for study in nonhuman primates as a model of human social anxiety.
The principal limitation of this study is small sample size, which limits generalizability of the findings and yields inadequate power for testing interactions of neural responses with demographic, clinical, and behavioral variables, and interactions between brain regions. Additionally, in this small sample we cannot rule out the possibility that the observed group differences in neural activity are a consequence of group differences in time fixating on direct and indirect gazes.
Acknowledgments
This study was supported in part by an Anxiety Disorders Association of America Junior Faculty Grant to Justine Kent and NIMH K02 MH064842 to Franklin Schneier. The authors thank Melissa Sy for her assistance in preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller L. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry. 2005;57:975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders – Patient Edition (SCID-I/P, 11/2002 revision) 2002. [Google Scholar]
- Gilbert P. Evolution and social anxiety: The role of attraction, social competition, and social hierarchies. Psychiatric Clinics of North America. 2001;24:723–752. doi: 10.1016/s0193-953x(05)70260-4. [DOI] [PubMed] [Google Scholar]
- Heimberg RG, Horner KJ, Juster HR, Safren SA, Brown EJ, Schneier FR, Liebowitz MR. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychological Medicine. 1999;29:199–212. doi: 10.1017/s0033291798007879. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, Gordon E. Face to face: visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Research: Neuroimaging. 2004;27:43–53. doi: 10.1016/j.psychres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Sugiura M, Kato T, Nakamura A, Hatano K, Ito K, Fukuda H, Kojima S, Nakamura K. The human amygdala plays an important role in gaze monitoring. A PET study. Brain. 1999;122:779–783. doi: 10.1093/brain/122.4.779. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. (WFU Pickatlas, version 2.4) [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, Brown GC. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: A 4-Tesla functional MRI study. Psychiatry Research: Neuroimaging. 2007;154:93–98. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]