Abstract
Male Sprague Dawley rats were allowed to self-administer cocaine (0.5mg/kg) during 90 minute sessions for a period of 15 days. On day 16, rats were either held abstinent in their home cage environment or experienced an extinction session in which the active lever had no programmed consequences. Facilitating N-methyl-d-aspartate (NMDA) receptor activity with the coagonist D-serine (100 mg/kg i.p.) before or following the extinction session significantly reduced the subsequent cocaine-primed reinstatement of drug-seeking behavior tested on day 17. D-serine significantly reduced drug-primed reinstatement only when combined with extinction, and its effectiveness when administered following the training session suggested that an enhancement of consolidation of extinction learning had occurred. In contrast, D-serine treatment did not reduce sucrose-primed reinstatement, indicating that the beneficial effects of this adjunct pharmacotherapy with extinction training were specific to an addictive substance (cocaine) and did not generalize to a natural reward (sucrose).
Keywords: self-administration, cocaine, NMDA receptor, glycine coagonist, addiction, extinction
Introduction
The propensity for relapse constitutes a crucial component in the disease of drug addiction. Exposure to drug associated environments, discrete conditioned stimuli, and drug ingestion elicit craving in human addicts (Ehrman et al., 1992; Foltin and Haney, 2000), which is thought to promote relapse. In preclinical studies, “craving” is commonly investigated using the rodent extinction/reinstatement model to assess drug-seeking behavior. In this model, rats respond for drug infusions in a distinct context and/or in the presence of discrete conditioned stimuli. The animals then undergo extinction training in the absence of drug and cues, resulting in decreased response rates. Extinction is a form of new learning that counters the expression of conditioned response (reviewed in Myers & Davis, 2002). Extinction also suppresses the possible contribution of several exogenous variables like habit, stress, and exploratory behavior toward lever pressing and produces neuroadaptive changes (Sutton et al., 2003). Once a criterion level of extinction is attained, the animals are tested for reinstatement of drug seeking. Reinstatement refers to resumption of drug seeking following exposure to either diffuse contextual cues (Ciccocioppo et al., 2001), proximal conditioned stimuli (Meil & See, 1996), inducers of stress/anxiety (Shaham & Stewart, 1995), or re-exposure to the drug itself (de Wit & Stewart, 1981). The advantage of this model is that the factors that contribute to increase in craving and relapse in a clinical situation have been shown to reliably reinstate the drug-seeking behavior in extinguished animals (Shalev et al., 2002). Thus, it has been argued that a reasonably strong case can be made in support of validity of this model for addiction in humans (Epstein et al., 2006).
Although the neurobiological mechanisms underlying evoked reinstatement have been the subject of intense investigation (recently reviewed in Feltenstein & See 2008), the question of how extinction training leads to a reduction of instrumental responding for an appetitive stimulus is largely uncharacterized. As extinction is recognized as a new learning process, it requires the recruitment of synaptic plasticity mechanisms required for learning and memory. Investigations in cellular models of learning and memory (long term potentiation-LTP and long term depression-LTD) have shown that many of the forms of synaptic plasticity are N-methyl-d-aspartate receptor (NMDAR) dependent and require the subsequent cascade of events triggered by the calcium influx which include insertion (LTP) and removal (LTD) of AMPA receptor (AMPAR) from post synaptic membranes (Malenka & Bear 2004). We have reported that NMDAR activity during extinction is required in order to observe a subsequent reduction in cocaine-primed reinstatement (Kelamangalath et al., 2007). Having previously shown that an NMDAR antagonist was effective in blocking the influence of extinction on drug-primed reinstatement response, our current report investigates the potential for a coagonist at the glycine regulatory site of the NMDAR to facilitate the effects of extinction and thereby further reduce the reinstatement to drug-seeking following cocaine priming. We also investigate whether the involvement of the NMDAR dependent component of extinction is unique for extinction from drug seeking, or is applicable to a natural reward as well. A sucrose self-administration, extinction and reinstatement protocol is utilized to investigate this issue. In order to enhance NMDAR function during extinction, we administered D-serine which is an endogenous coagonist at the strychnine-insensitive glycine site of the NMDAR complex (Schell et al., 1995), and also has been described as a full agonist at this site (Furukawa and Gouaux, 2003). The effects of facilitating extinction with agents which act at the strychnine-insensitive glycine site of the NMDAR have not been described using the self-administration extinction/reinstatement model, but this rationale for reducing the occurrence of relapse has recently been supported by findings employing the cocaine-induced conditioned place preference model (Botreau et al., 2006; Paolone et al., 2008). In this current report we describe the effectiveness of the NMDA receptor coagonist D-serine, when combined with extinction training, to inhibit cocaine-primed reinstatement of drug-seeking behavior. In contrast, the advantage of facilitating the NMDAR function during extinction to reduce reinstatement responsiveness was not observed in the sucrose-trained, extinguished animals.
Materials and Methods
Animals and Jugular catheterization protocol
Male Sprague-Dawley rats (Harlan) weighed approximately 300 g at the beginning of the study and were implanted with jugular catheters as previously described (Kelamangalath et al., 2007). All procedures followed the Guide for the Care and Use of Laboratory Animals and were approved by the IACUC at the University of Georgia.
Self-administration environment
The operant chambers (Med associates) were equipped with 2 lever and a light positioned above each lever. Each chamber had a rod grid floor, a house light, a speaker/tone generator (2.9 kHz, 10 dB above ambient) and was located inside an enclosure equipped with a ventilation fan. A syringe pump was located outside each enclosure and cocaine infusions were delivered via a liquid swivel and PE-50 tubing protected by a metal spring (drug tether). Cocaine hydrochloride (NIDA) was dissolved in normal saline at 4 mg cocaine/ml of solution, and each infusion delivered a volume of 0.125 ml/kg body weight (0.5 mg/kg/infusion).
The self-administration of sucrose pellets was assessed in the same environment except the operant chambers were equipped with external pellet dispensers containing 45 mg plain sucrose pellets.
Self-administration protocol (cocaine) days 1-15
Rats were allowed to self-administer cocaine for one 90 minute session each day. Upon entry into the self-administration environment, the house light was illuminated and the ventilation fan activated. In addition to triggering an infusion, pressing the active lever turned off the house light and a lever light/tone (CS complex) were turned on for 30 seconds. Additional responses on the active lever during this 30 second period had no programmed consequences. Therefore, the end of the CS complex also signals the end of 30 second time out period. Rats were initially trained for 12 days on an FR-1 (fixed ratio schedule-1) schedule, in which each active lever press outside the timeout period triggered the programmed consequences. For the last 3 days of self-administration training, an FR-3 schedule was imposed, requiring 3 active lever presses outside the time out period to trigger an infusion and the CS complex. The FR3 schedule was imposed to increase the lever pressing behavior of animals as previously described (Kelamangalath et al., 2007).
Self-administration protocol (sucrose pellets) days 1-15
A separate group of rats having access to food and water ad libitum were allowed to self-administer sucrose pellets for one 90 minute session on each day under the same conditions described above for cocaine, except that a response on the active lever resulted in the delivery of a single 45mg sucrose pellet in the chamber receptacle, rather than a cocaine infusion. As with cocaine self-administration, the training period consisted of initial FR-1 sessions for first 12 days and FR-3 sessions for the last 3 days.
Extinction protocol for cocaine self-administered animals (day 16)
After the 15 days of self-administration, the animals were divided into 5 groups (balanced for cocaine intake): 1) extinguished (saline), 2) extinguished (D-serine pre extinction), 3) extinguished (D-serine post extinction), 4) abstinent (saline) and 5) abstinent (D-serine). All groups received their respective treatments via i.p. injections, while in their home cage. Both the extinguished group 1 and the abstinent group 4 received an injection of saline (1ml/kg). Extinguished group 2 and abstinent group 5 received an injection of D-serine (100 mg/kg). Extinguished group 3 received an injection of D-serine (100 mg/kg) post extinction. Group 5 received D-serine i.p. (100mg/kg). Groups 1 and 2 underwent extinction training 2-3 hours following their respective treatments, whereas groups 4 and 5 rats remained in their home cages. Group 3 rats received an injection of D-serine immediately after the extinction session. During extinction training (day 16), the animals were attached to the drug tether but were otherwise exposed only to the environment stimuli (i.e. diffuse, contextual cues) of the operant chamber. Responses on the active lever had no programmed consequences during the 90 minute session.
Extinction protocol for sucrose pellet self-administered animals (day 16)
After 15 days of sucrose pellet self-administration session, the animals were divided into 3 groups (balanced for sucrose intake): 1) extinguished (saline), 2) extinguished (D-serine pre extinction), and 3) abstinent (saline). Animals received their respective treatments via i.p. injections, while in their home cage environments, 2-3 hours prior to extinction. On day 16, groups 1 and 2 were taken to the operant chamber environment for the extinction training, in which responses on the active lever had no programmed consequences during the 90 minute session. Group 3 (abstinent) animals remained in their home cages.
Reinstatement test in cocaine self-administering animals (day 17)
All of the animals (including the abstinent animals), were placed back in the operant chambers for reinstatement testing on day 17. The reinstatement test session conditions were similar to an extinction session in that the animals were exposed only to the contextual cues of the operant chamber environment, and active lever responding was not reinforced by the contingent availability of either the CS complex or the drug. Extinction responding to the drug-taking environment was assessed from active lever presses during the first 10 minutes of the session. Reinstatement following drug priming was assessed by a single, noncontingent i.v. infusion of cocaine (0.5 mg/kg) at time=80 minutes. Drug-seeking behavior elicited by cocaine was quantified from the number of responses on the active lever for 30 minutes following the drug priming event (t=80-110 min) as compared with the 30 minutes prior to that event (t=50-80 min). We have previously described the dose-response results and reported the lack of effect of saline priming using this noncontingent i.v. priming protocol with cocaine (Kelamangalath et al., 2007).
Reinstatement tests in sucrose pellet self-administering animals (day 17)
Extinction responding to the sucrose-taking environment was assessed from active lever presses during the first 10 minutes of the session. Reinstatement following sucrose priming was assessed by five, noncontingent sucrose pellets delivered at 10 second intervals at time=80 minutes. Drug-seeking behavior elicited by sucrose was again quantified from the number of responses on the active lever for 30 minutes following the pellet priming event (t=80-110 min) as compared with the 30 minutes prior to that event (t=50-80 min).
Drugs
Cocaine hydrochloride was obtained from NIDA (RTI). The NMDAR coagonist D-serine (Sigma, St. Louis) was administered in the home cage environment approximately 2-3 hours prior to the extinction sessions on protocol days 16 to reduce any state-dependent or acute locomotor effects in the pre extinction treatment group. For the post extinction group, the drug was administered immediately after the extinction session. Although other investigators have administered D-serine at 600-800 mg/kg in a novel object recognition test (Karasawa et al., 2008) or in a reversal learning task (Duffy et al., 2008) to demonstrate enhanced learning/memory, we have chosen a much lower dose to avoid any possible nephrotoxic effects (Williams et al., 2003). D-serine given i.p. at 50 mg/kg has been shown to significantly activate the hippocampus in fMRI studies (Panizzutti et al., 2005), and a dose of 100 mg/kg has been shown to enhance learning (Stouffer et al., 2004). Five 100 mg/kg doses were administered in our previous study over five days without consequence (Kelamangalath et al., 2007). Importantly, one study has demonstrated that 8 hours following administration of 160 mg/kg i.p. D-serine, brain (cortex) tissue levels of the compound were not significantly increased (Smith et al., 2009). This indicates that the reinstatement tests conducted more than 24 hrs following D-serine administration in this current report were not likely to have been influenced by elevated D-serine levels in the brain at the time of testing.
Statistics
The number of active lever presses, infusions, and inactive lever presses were recorded for each session and the two factors taken into consideration for 2-way ANOVA were: 1) trial (pre vs. post priming responses) and 2) treatment. Abstinent groups were also compared with the extinction groups, and planned comparisons were performed using unpaired ‘t’ tests (two tailed). A value of p<.05 was taken as significant, from either the post hoc test or planned comparisons. Statistics were done using SigmaStat software (version 3.1).
Results
Self-administration and extinction of reward-seeking behavior
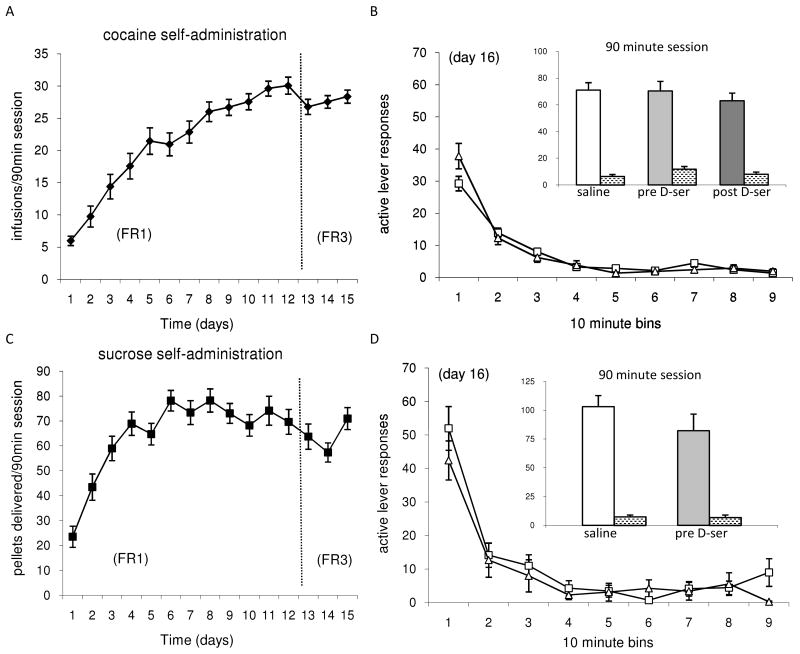
Animals typically achieved stable self-administration by day 10 of training, and switching from the FR-1 to the FR-3 schedule did not significantly alter the number of earned infusions/pellets per session (Figure 1A & C). On day 16, the animals were subjected to either an extinction session in which the active lever had no programmed consequences (Figure 1B & D), or they were kept abstinent in their home cage environment.
Figure 1.
A. Cocaine self-administration training (days1-15): The results are the mean ± SEM (n=78) of cocaine infusions earned daily during the 90 minute self-administration sessions. B. Extinction training of cocaine self-administered animals (day 16): Data are the mean ± SEM of active and inactive lever responses in 10 min bins for the single 90 min extinction session. Treatment with D-serine prior to extinction (n=15, open triangles) did not influence the extinction learning process (p>.05) as compared to the saline treated control group (n=19, open squares). The inset shows the total number of active (solid bars) and inactive (stippled bars) lever responses for the indicated treatments over the entire 90 minute extinction session. C. Sucrose pellet self-administration training (days1-15): The results are the mean ± SEM (n=21) of sucrose pellets ingested daily during the 90 minute self-administration sessions. D. Extinction training of sucrose self-administered animals (day 16): Data are the mean ± SEM of active and inactive lever responses in 10 min bins for the single 90 min extinction session. Treatment with D-serine prior to extinction (n=7, open triangles) did not influence the extinction learning process (p>.05) as compared to the saline treated control group (n=7, open squares). The inset shows the total number of active (solid bars) and inactive (stippled bars) lever responses for the indicated treatments over the entire 90 minute extinction session.
Extinction of cocaine-seeking behavior
The role of NMDARs in the extinction process was evaluated in three groups of extinguished rats (Fig. 1B): one group treated with D-serine (a coagonist at the glycine site of NMDARs) at a dose of 100 mg/kg i.p. prior to the extinction session (pre D-ser); a control group treated with saline (1 ml/kg) i.p. prior to the extinction session; and a third extinction group, in which animals were treated with D-serine at a dose of 100 mg/kg i.p. immediately after the extinction session (post-D-ser). D-serine treatment prior to extinction (facilitating the NMDAR activity during the extinction) training did not enhance the extinction learning process within the session for the pre D-ser group, as compared to either the saline treated control group or the post-D-ser group (Fig. 1B inset).
Extinction of sucrose-seeking behavior
As described above for cocaine animals, the role of NMDARs in the extinction process was evaluated in two groups of extinguished rats: one group treated with D-serine at a dose of 100 mg/kg i.p. prior to the extinction session, and a control group treated with saline (1 ml/kg) i.p. prior to the extinction session (Fig. 1D). D-serine treatment administered prior to the extinction session did not enhance the extinction learning process as compared to the saline treated controls (Fig. 1D inset).
Resumption of reward-seeking behavior
The evaluation of reward-seeking behavior within this single extinction session illustrates that the majority of lever pressing activity occurs during the initial ten minutes in the operant chamber, suggesting that environmental contextual cues are evoking this response (Fig. 1B & D). Following this initial burst of extinction responding, active lever pressing diminished rapidly by time=30-40 minutes and remained low for the remainder of the 90 minute session. Therefore, the reinstatement test involved the noncontingent administration of either a single cocaine infusion or sucrose pellet priming event, delivered at time=80 minutes into the 120 minute session. This allowed a temporal distinction to be made during the reinstatement test between the reward-seeking induced by reintroduction to the operant chamber environment (i.e. initial activity during time=0-10 minutes) vs. reward-seeking induced by priming.
D-serine effects on resumption of cocaine-seeking
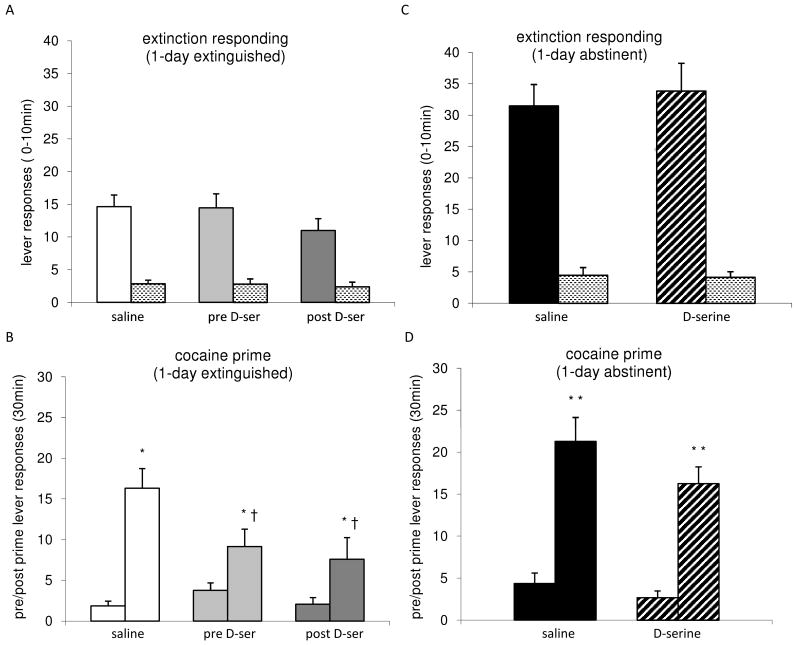
Animals underwent either abstinence or an extinction session (day 16), and were then tested for the resumption of drug-seeking behavior upon re-exposure to the operant chamber environment (initial extinction responding) and to a noncontingent i.v. prime with cocaine during the reinstatement test session. The resumption of drug-seeking induced by diffuse environmental cues was assessed during the first ten minutes of the test session conducted on day 17 (Figs 2A & C). Among the extinguished groups (Fig. 2A), the initial extinction responding observed in the saline treated control group and the groups treated with D-serine were not significantly different (p>.05, one way ANOVA), suggesting that facilitation of NMDARs does not affect this measure of cocaine-seeking. As expected, responding on the active lever in extinguished (saline and both D-serine) groups during this period was significantly decreased (p<.001, unpaired ‘t’ test) compared to the responding of the abstinent (saline and D-serine) groups kept in the home cage environment for one day (i.e. Fig. 2A vs. 2C).
Figure 2.
Extinction responding and cocaine-primed reinstatement in extinguished and abstinent animals. A. Data show the mean ± SEM of the lever presses for the initial 10 minutes of reinstatement (day 17) in the 1-day extinguished animals (solid bars-active lever; stippled bars-inactive lever for the indicated treatment groups: saline, n=19; pre D-serine, n=15; post D-serine, n=15). B. Data are the mean ± SEM of the active lever responses in 1-day extinguished animals for 30 minutes before (left bar) and after (right bar) the cocaine prime among indicated treatment groups. Within each group, the post prime response was significantly greater than the pre prime response (* p<.05). Between the groups, the cocaine prime responses of the pre D-serine (n=13) and post D-ser (n=15) treated animals were significantly lower compared to the saline (n=16) treated rats († p<.05). C) Data show the mean ± SEM of the lever presses for the initial10 minutes of reinstatement (day 17) in the 1-day abstinent animals (solid/striped bars-active lever; stippled bars-inactive lever for the indicated treatment groups: saline abstinent, n=15; D-serine abstinent, n=14). D) Data shows the mean ± SEM of the active lever responses in 1-day abstinent animals for 30 minutes before (left bar) and after (right bar) the drug prime among the indicated treatment groups. Within each group, the post prime response was significantly greater than the pre prime response (** p<.01). The cocaine prime response of the D-serine treated animals (n=12) was not significantly different from the saline treated rats (n=14).
Cocaine-induced resumption of drug-seeking was tested at time=80 minutes in the reinstatement test session, when a single, noncontingent i.v. cocaine infusion (0.5mg/kg prime) was delivered. The active lever responding during the next 30 minutes was measured as an indication of the reinstatement of drug-seeking behavior evoked by cocaine (Fig. 2B & D). Inactive lever presses averaged less than 1 during the post prime period (data not shown). The cocaine-seeking behavior among the extinguished animals was analyzed by a two-way ANOVA and showed an effect of trial, Ftrial (1, 82)=32.4 and treatment, Ftreatment (2, 82)=3 as well as an interaction of trial and treatment, Finteraction (2,82)=4.3. Within trial, the post hoc analysis showed that this noncontingent priming dose of cocaine significantly reinstated the drug-seeking behavior among all three extinguished groups (*saline, p<.001, t=5.9; *D-serine pre treatment group, p<.05, t=2; and *D-serine post treatment group, p<.05, t=2.2). The effect of treatment was evaluated with post hoc analysis of post prime responses and reinstatement in the saline treated control group was significantly greater than that of either the pre D-serine (†p<.05, t=3.5) or post D-serine groups (†p<.05, t=2.8). This indicates that facilitating the NMDAR activity either during extinction or immediately following extinction can enhance the effects of extinction in reducing the drug-primed reinstatement.
The resumption of cocaine-induced drug-seeking in the two abstinent groups (saline and D-serine) analyzed by a two-way ANOVA confirmed an effect of trial, Ftrial (1,48)=58.1 but not an effect of treatment. Within trial, the post-prime response was found to be significantly greater than the pre prime response for both the saline abstinent (*p<.001, t=5.4) and the D-serine abstinent (*p<.001, t=5.8) groups (Fig. 2D). A single day of extinction training alone was not effective in reducing the reinstatement response, as a planned comparison between the saline extinction group and the saline abstinent group (Fig. 2B vs. 2D) was not significant (p>.05, unpaired ‘t’ test). In contrast, planned comparisons of the post prime responses between both the pre D-serine and post D-serine extinguished groups with the D-serine treated abstinent group showed significance (p<.05, t=2.4 for pre D-ser and p<.05, t=2.44 for post D-ser). This demonstrates the advantage of combining D-serine treatment as an adjunctive pharmacotherapy with an extinction session vs. D-serine treatment with abstinence. Also, the post prime response of the D-serine treated abstinent group was not significantly different vs. the saline treated abstinent group (Fig. 2D), indicating that D-serine treatment in the absence of an extinction session does not affect lever pressing measured in response to drug-primed reinstatement. Finally, there was no significant difference observed between the post prime responses of the group treated with D-serine prior to extinction and the group treated with D-serine immediately following extinction (Fig. 2B). This demonstrates that facilitating NMDAR function during the extinction session does not result in increased effectiveness to reduce cocaine-primed reinstatement of drug-seeking behavior as compared to facilitating NMDAR function following the extinction session.
D-serine effects on resumption of sucrose-seeking
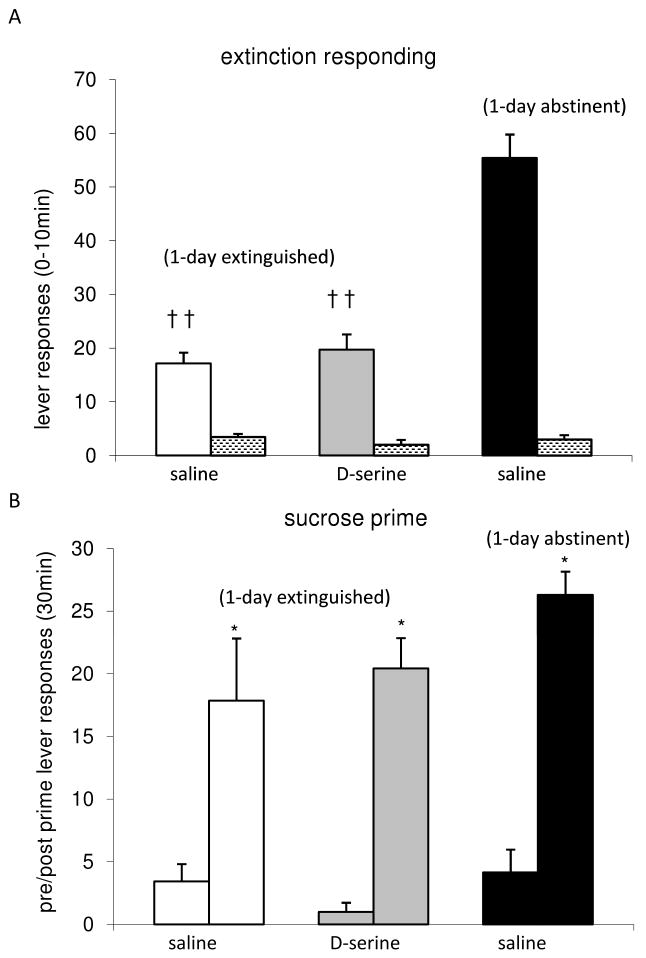
Extinction responding was assessed in rats trained to self-administer sucrose pellets after a one day withdrawal period (either extinguished or abstinent on day 16). During the reinstatement test (day 17), active lever presses recorded over the first 10 minutes of the session were significantly decreased (††p<0.001, unpaired ‘t’ test) in the extinguished (saline and D-serine treated) groups of rats vs. the abstinent saline treated group (Fig. 3A). This indicates that for sucrose self-administration, as observed with the cocaine animals, one day of extinction training was sufficient to significantly diminish responding to the environmental cues of the operant chamber in comparison to an abstinent group. The saline and D-serine extinguished groups did not significantly differ from each other in extinction responding (p>.05, one way ANOVA), suggesting that facilitation of NMDARs does not affect this measure of sucrose-seeking (Fig. 3A).
Figure 3.
Extinction responding and sucrose-primed reinstatement in extinguished and abstinent animals. A. Data show the mean ± SEM of the lever presses for the initial 10 minutes of reinstatement (day 17) in the 1-day extinguished and 1-day abstinent animals (solid bars-active lever; stippled bars-inactive lever for the indicated treatment, n=7 for all groups. Active lever responses among the extinguished animals were significantly lower as compared to that of the saline abstinent group (†† p<.001). B. Data are the mean ± SEM of the active lever responses for 30 minute bin before (left bar) and after (right bar) the sucrose prime among the indicated treatment groups. Within each group, the post prime response was significantly greater than the pre prime response (* p<.05). Between the groups, the post prime responses of the extinguished groups were not significantly different from the saline treated abstinent group.
Sucrose-induced resumption of drug-seeking was tested at time=80 minutes of the reinstatement test session, using a total of five noncontingent deliveries of sucrose (5 pellet prime). The active lever responding during t=80-110 was measured as an indication of the reinstatement of sucrose-seeking behavior evoked by sucrose pellets (Fig. 3B). Inactive lever presses averaged less than 1 during the post prime periods (data not shown). Planned comparisons between the post prime responses of the saline extinguished and the saline abstinent groups were not significantly different (p>.05, unpaired ‘t’ tests), indicating that one day of extinction was sub maximal. Analysis of the sucrose-prime data among the extinguished animals by two-way ANOVA confirmed an effect of trial, Ftrial (1,24)=12.65 but not an effect of treatment. The post prime responses were significantly greater than the pre prime responses for both the saline treated (*p<.05, t=2.8) and the D-serine treated (*p<.001, t=7.7) extinguished groups. This indicates that the noncontingent delivery of sucrose was able to reinstate sucrose-seeking behavior. However, in contrast with the ability of D-serine to enhance the effectiveness of extinction in reducing cocaine-primed reinstatement (Fig. 2B), the facilitation of NMDAR activity during extinction did not affect the sucrose-primed reinstatement response (Fig. 3B).
Discussion
In this study, we have found that facilitating NMDAR activity either during, or immediately following, an extinction session can enhance the effectiveness of such training to reduce subsequent cocaine-primed reinstatement. This enhancement was not observed when assessing extinction responding induced by reintroduction to diffuse environmental cues of the drug-taking environment, suggesting a dissociation between the underlying neurobiological mechanisms that mediate various forms of reinstatement of drug-seeking behavior. D-serine treatment administered independent of an extinction session (D-ser abstinent group) did not significantly affect the reinstatement response, demonstrating that D-serine is effective in reducing cocaine-primed reinstatement only when combined with an extinction session. Finally, the benefit of D-serine treatment to reduce primed reinstatement was specific to cocaine, and was not evident when a natural appetitive food reward was tested.
Extinction likely involves new learning (Pavlov, 1927; Bouton et al., 2004), and at the molecular level, both NMDAR (Falls et al., 1992) and non-NMDAR (i.e. L-type voltage gated calcium channel) dependent forms of synaptic plasticity (Cain et al., 2002) are thought to contribute to this type of learning. The mechanisms involved in the learning and memory of extinction and the mechanisms involved in the effects of extinction on reinstatement have been extensively investigated using paradigms such as fear conditioning, inhibitory avoidance, spatial navigation and conditioned taste aversion (Cammarota et al., 2005). For example, it has been shown that extinction of conditioned fear responses can be facilitated by injections of the partial NMDAR coagonist D-cycloserine (Walker et al., 2002), which acts at the strychnine-insensitive glycine site of the NMDAR (Watson et al., 1990). Another study reported that D-cycloserine, administered either prior to or immediately after extinction, significantly reduced reinstatement, but not renewal (Ledgerwood et al., 2003). Such results suggest that facilitating NMDAR activity is an effective means to enhance some forms of extinction learning.
In a previous report using a cocaine self-administration protocol, we found that systemic administration of the NMDAR antagonist (±) CPP prior to an extinction session did not impair the acquisition of extinction, but inhibited the recall of that extinction on a drug-free reinstatement test day (Kelamangalath et al., 2007). When administration of a learning/memory disruptor prior to the session does not affect the extinction responding within the session, but subsequently affects performance later when tested for recall, the disruptors are thought to likely be preventing the consolidation of the memory of extinction learning (Quirk and Mueller, 2007). However, the expectation that facilitation of NMDAR activity with the coagonist D-serine would act to enhance the recall of extinction and thus reduce reinstatement was not met in our 2007 study, possibly due to an overtraining effect of the 5-day extinction protocol. Additional preliminary experiments involving 2-day extinction training also showed a “floor effect” of extinction when the animals were tested for reinstatement to a noncontingent drug prime (data not shown), thus a single extinction session was chosen, in order to produce a partial reduction of responding. A single priming dose of cocaine was administered i.v. in the same manner that resulted in significant cocaine-primed reinstatement in our previous report (Kelamangalath et al., 2007). As this reinstatement test session was not reinforced and was therefore otherwise equivalent to an extinction session, additional days of reinstatement testing with multiple priming doses of cocaine were not performed.
In the current study, we have utilized the 1-day, sub maximal extinction protocol in order to investigate the potential enhancing effects of D-serine on extinction and then assessed the cocaine-primed reinstatement of drug-seeking behavior. The NMDAR coagonist D-serine significantly reduced cocaine-primed reinstatement when administered either prior to or immediately after the extinction session. Since facilitating NMDAR-mediated mechanisms during extinction learning provided no additional advantage as compared with D-serine treatment post extinction, the results are consistent with a role for D-serine in enhancing the consolidation of extinction memory. The effectiveness of pre session administration can be attributed to the bioavailability of D-serine in the brain, which extends for several hours following i.p. administration. Regarding the interpretation of the effectiveness of the post extinction session administration of D-serine, the relatively long duration of the extinction session (90 minutes) before D-serine was administered is not consistent with another potential strategy that has also been proposed for the treatment of relapse, an interference in the reconsolidation of drug-taking memories (Taylor et al., 2008). The most parsimonious explanation for the results described in this report is that D-serine can act to enhance the consolidation of extinction memory and thereby inhibit the ability of cocaine-priming to reinstate drug-seeking behavior during the reinstatement test conducted the following day.
Finally, the extinction/reinstatement protocol employed for cocaine self-administration was adapted for use in examining operant behavior for a natural reward (sucrose). Interestingly, in contrast to our results obtained with cocaine, D-serine did not significantly enhance the effects of 1-day extinction to reduce sucrose-primed reinstatement of sucrose-seeking behavior. It has been shown that the recall of extinction using different unconditioned stimuli can involve different mechanisms. For example, in the conditioned taste aversion model, the recall of extinction is not mediated by NMDARs (Berman and Dudai, 2001). In contrast, NMDARs are important for the recall of extinction in a conditioned fear model (Santini et al., 2001). Hence using two different forms of rewarding stimuli (cocaine and sucrose self-administration models) in the operant task might be recruiting different molecular mechanisms and their distinct neural circuitry for the consolidation of their respective extinction memories. In addition, differences in neuroadaptations reported following cocaine vs. sucrose self-administration could be informative. Changes in glutamatergic neurotransmission have been reported in cocaine self-administering animals and not observed in sucrose self-administering animals (Sutton et al., 2003). In that study, animals self-administering cocaine, but not those self-administering sucrose, revealed a deficiency in glutamate receptor expression, which was reversed by extinction training. Perhaps the D-serine administered in our experiments resulted in an enhancement in consolidation of extinction memory processes that involved glutamatergic mechanisms. These observations indicate that specificity exists for the actions of D-serine (and the role of NMDAR-dependent learning/memory processes), that may be related to the addictive potential of the reward. In sum, our observations using the rodent self-administration model provide support for the involvement of NMDAR-mediated mechanisms in the effect of extinction to reduce cocaine-primed reinstatement. The findings from this preclinical study support the hypothesis that facilitating NMDAR-mediated mechanisms via enhanced D-serine activity during extinction is a promising adjunct pharmacotherapy to combine with behavioral approaches aimed towards preventing the occurrence of relapse in humans.
Acknowledgments
We thank Michael Stramiello for critiquing an earlier version of the manuscript. This work supported by the National Institutes of Health (DA016302) to JJW and the Interdisciplinary Toxicology Program (stipend award) to LK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berman DE, Dudai Y. Memory Extinction, Learning Anew, and Learning the New: Dissociations in the Molecular Machinery of Learning in Cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- Botreau F, Paolone G, Stewart J. D-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behavioural Brain Research. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and Behavioral Processes in Extinction. Learning and Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. L-Type Voltage-Gated Calcium Channels Are Required for Extinction, But Not for Acquisition or Expression, of Conditional Fear in Mice. J Neurosci. 2002;22:9113–9121. doi: 10.1523/JNEUROSCI.22-20-09113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LRM, Barros DM, Vianna MRM, Izquierdo LA, Medina JH, Izquierdo I. Retrieval and the Extinction of Memory. Cellular and Molecular Neurobiology. 2005;25:465–474. doi: 10.1007/s10571-005-4009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: Reversal by D1 antagonists. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deWit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Duffy S, Labri V, Roder JC. D-Serine Augments NMDA-NR2B Receptor-Dependent Hippocampal Long-Term Depression and Spatial Reversal Learning. Neuropsychopharmacology. 2008;33:1004–1018. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls W, Miserendino M, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. British Journal of Pharmacology. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO Journal. 2003;22:2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A, Chiba Y. Effect of systemic administration of D-serine on the levels of L- and D-serine in several brain areas and periphery of rat. European Journal of Pharmacology. 2004;495:153–158. doi: 10.1016/j.ejphar.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Karasawaa Ji, Hashimotob K, Chaki S. D-Serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behavioural Brain Research. 2008;186:78–83. doi: 10.1016/j.bbr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kelamangalath L, Swant J, Stramiello M, Wagner JJ. The effects of extinction training in reducing the reinstatement of drug-seeking behavior: Involvement of NMDA receptors. Behavioural Brain Research. 2007;185:119–128. doi: 10.1016/j.bbr.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, C J. Effects of D-cycloserine on extinction of conditioned freezing. Behavioral Neuroscience. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An Embarrassment of Riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behavioural Pharmacology. 1996;7:754–763. [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and Neural Analysis of Extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Panizzutti R, Rausch M, Zurbrügg S, Baumann D, Beckmann N, Rudin M. The pharmacological stimulation of NMDA receptors via co-agonist site: an fMRI study in the rat brain. Neurosci Lett. 2005;380:111–115. doi: 10.1016/j.neulet.2005.01.062. [DOI] [PubMed] [Google Scholar]
- Paolone G, Botreau F, Stewart J. The facilitative effects of d -cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. Oxford university press; Oxford UK: 1927. [Google Scholar]
- Quirk GJ, Mueller D. Neural Mechanisms of Extinction Learning and Retrieval. Neuropsychopharmacology. 2007;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, OB CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of Extinction Learning Involves Transfer from NMDA-Independent to NMDA-Dependent Memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder S. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of Relapse to Heroin and Cocaine Seeking: A Review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Smith SM, Uslaner JM, Yao L, Mullins CM, Surles NO, Huszar SL, McNaughton CH, Pascarella DM, Kandebo M, Hinchliffe RM, Sparey T, Brandon NJ, Jones B, Venkatraman S, Young MB, Sachs N, Jacobson MA, Hutson PH. The Behavioral and Neurochemical Effects of a Novel D-Amino Acid Oxidase Inhibitor Compound 8 [4H-Thieno [3,2-b]pyrrole-5-carboxylic Acid] and D-Serine. J Pharmacol Exp Ther. 2009;328:921–930. doi: 10.1124/jpet.108.147884. [DOI] [PubMed] [Google Scholar]
- Stouffer EM, Petri HL, Devan B. Effect of D-serine on a delayed match-to-place task for the water maze. Behavioural Brain Research. 2004;152:447–452. doi: 10.1016/j.bbr.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Olaussona Peter, Quinna JJ, Torregrossaa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56:186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of Conditioned Fear Extinction by Systemic Administration or Intra-Amygdala Infusions of D-Cycloserine as Assessed with Fear-Potentiated Startle in Rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GB, Bolanowski MA, Baganoff MP, Deppeler CL, Lanthorn TH. D-Cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed inXenopus oocytes. Brain Research. 1990;510:158–160. doi: 10.1016/0006-8993(90)90745-w. [DOI] [PubMed] [Google Scholar]
- Williams RE, Jacobsen M, Lock EA. 1H NMR Pattern Recognition and 31P NMR Studies with D-Serine in Rat Urine and Kidney, Time- and Dose-Related Metabolic Effects. Chemical Research in Toxicology. 2003;16:1207–1216. doi: 10.1021/tx030019q. [DOI] [PubMed] [Google Scholar]