Abstract
Muc4 is a heterodimeric membrane mucin implicated in epithelial differentiation and tumor progression. It is expressed from a single gene as a 300 kDa precursor protein which is cleaved in the endoplasmic reticulum to its two subunits. Our previous work has shown that Muc4 is regulated by TGFβ, which represses the precursor cleavage. Working with Muc4-transfected A375 tumor cells, we now show that Muc4 undergoes proteosomal degradation. Proteosome inhibitors prolong the life of the precursor, shunt the Muc4 into cytoplasmic aggresomes, increase the level of Muc4 associated with the endoplasmic reticulum chaperones calnexin and calreticulin and increase the levels of ubiquitinated Muc4. Most importantly, proteosome inhibitors repress the TGFβ inhibition of Muc4 expression. These results suggest a model in which TGFβ inhibits precursor cleavage, shunting the precursor into the proteosomal degradation pathway. Thus, the cells have evolved a mechanism to use the quality control pathway for glycoproteins to control the quantity of the protein produced.
INTRODUCTION
The membrane mucin Muc4 has been implicated in tumor progression and resistance to therapies by several mechanisms. First, the mucin can act as an anti-adhesive, sterically blocking the approach of other cells [Komatsu et al., 1997, 1999] and even repressing antibody binding to the cell surface of tumors [Komatsu et al., 1999; Price-Schiavi et al., 2002]. By this anti-recognition mechanism Muc4 on tumor cells can block immune cell killing of the tumor cells [Komatsu et al., 1999] and inhibit Herceptin binding and action [Price-Schiavi et al., 2002; Nagy et al., 2005]. Second, Muc4 can bind as an unorthodox intramembrane ligand via an EGF-like domain to the receptor tyrosine kinase ErbB2 [Carraway et al., 1999]. Complex formation between Muc4 and ErbB2 occurs soon after the two proteins are synthesized and can influence the localization, phosphorylation and signaling of the ErbB2 in both polarized epithelial [Ramsauer et al., 2006] and tumor [Funes et al., 2006] cells. In conjunction with the ErbB3 ligand neuregulin, Muc4 can promote signaling through the phosphoinositide 3-kinase/Akt pathway which supports tumor progression [Funes et al., 2006]. Finally, Muc4 can act as an anti-apoptotic [Komatsu et al., 2001; Hu et al., 2003], promoting the survival of tumor cells and repressing their susceptibility to therapeutic drugs [Hu et al., 2003].
The multiple roles of Muc4, protecting normal epithelia from external insults, but also promoting tumor progression, suggest that the mucin must be tightly regulated in epithelia, but that the regulation is lost in tumors. In the mammary gland we have shown that Muc4 is regulated post-translationally by TGFβ [Price-Schiavi et al., 1998, 2000; Soto et al., 2003]. This regulation is lost in mammary tumors as they lose their responsiveness to the growth factor. Our previous work has shown that TGFβ regulates Muc4 via the SMAD pathway [Soto et al., 2003]. What is unclear is how this regulation acts on the mucin. In previous studies on mammary epithelial cells we showed that TGFβ inhibits Muc4 processing [Price-Schiavi et al., 2000; Soto et al., 2003]. Rat Muc4 is composed of two subunits, the mucin subunit ASGP-1 and the transmembrane subunit ASGP-2, encoded by a single gene [Sheng et al., 1992]. The 9 kb transcript from this gene [Sheng et al., 1992; Wu et al., 1994] is translated as a 300 kDa N-glycosylated precursor [Sheng et al., 1990]. This precursor pMuc4 is cleaved into the two subunits early in its transit to the cell surface, before O-glycosylation of ASGP-1 [Sheng et al., 1990]. TGFβ acts by repressing the cleavage of the precursor to the mature heterodimeric form [Price-Schiavi et al., 2000].
This scenario raised an interesting possibility, that failure to cleave the precursor results in its transit to the proteosome and degradation. To test this mechanism, we used a tumor cell line developed previously, which has been stably transfected with a tetracycline-regulated Muc4 construct. Using that cell line, we demonstrated that TGFβ blocks the expression of both Muc4 and its precursor. We further demonstrated proteosomal degradation of Muc4 using proteosome inhibitors and that these inhibitors can block TGFβ downregulation of Muc4. These findings support a novel regulatory mechanism in which TGFβ represses cleavage of the Muc4 precursor, which is then targeted for degradation by the proteosome.
MATERIALS AND METHODS
Cell lines and cell cultures
A375 melanoma cells stably transfected with a tetracycline-responsive inducible construct for Muc4 were previously described [Komatsu et al., 1997]. Cells were cultivated to 70% confluence in DMEM supplemented with 10% fetal calf serum, 100 IU/ml penicillin, 0.3 mg/ml hygromycin, 0.8 mg/ml G418 and 2 μg/ml of tetracycline. To induce expression of Muc4, the cells were washed three times with antibiotic-free medium and then cultivated with tetracycline-free medium for two subsequent days before treatment. Fisher 344 rats were used in accordance with the National Institutes of Health Guide and Association for Research in Vision and Ophthalmology for the Care and Use of Laboratory Animals. The MAT-B1 subline ascites cells of the 13762 rat mammary adenocarcinoma were maintained and isolated from peritoneal fluid of 4 to 6 month old female rats as previously described [Carraway et al., 1976]. Shortly after isolation from the peritoneal cavity, cells were washed three times with warmed (37°C) phosphate-buffered saline (PBS) and collected by centrifugation at 800 × g. Cells were resuspended in medium and plated on plastic six well cell culture dishes. Rat corneal epithelial cells were isolated from Fisher 344 rats and maintained in culture as previously described [Lomako et al., 2005].
Antibodies
Antibodies used during this study were developed in our laboratory: mAb 4F12 recognizing an extracellular epitope of ASGP-2 [Rossi et al., 1996], polyclonal C-pep raised in rabbits and directed against a peptide from the C-terminal cytoplasmic domain of ASGP-2 [Rossi et al., 1996] and polyclonal anti-ASGP-2 against isolated ASGP-2 [Sheng et al., 1989]. Polyclonal antiubiquitin and monoclonal anti-β-actin antibodies were from Sigma. Anti-calnexin and anti-calreticulin were from Stresgen Bioreagents.
Immunoblotting
To analyze cellular proteins by immunoblotting, cell collection, lysis and immunoblotting were performed as described previously [Komatsu et al., 1997]. Briefly, collected cells were solubilized for 5 min in boiling 2% sodium dodecyl sulfate (SDS). Extracts were clarified by centrifugation at 14,000 × g for 10 min, and 30 μg protein was subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. Membranes were probed for 2 h with primary antibodies diluted in 1% bovine serum albumin (BSA)-Tris-buffered saline/0.02% Tween-20 (TTBS), washed three times with TTBS and subsequently incubated for 1 hr with appropriate secondary antibody-horseradish peroxide-conjugates (Pierce, Rockford, IL) followed by protein visualization by the Renaissance™ Enhanced Chemiluminescence Kit (NEN Life Science Products, Inc., Boston, MA). In each case β-actin was used as a control for protein loading.
Immunoprecipitation
For immunoprecipitation the ubiqitinylated or radioactively labeled protein ice-cold-PBS-washed cells were extracted for 5 min with boiling modified RIPA buffer (1% SDS, 150 mM NaCl, 1% Nonidet p-40, 0.5% deoxycholate, 50 mM Tris/HCl, pH 8.0) [Rossi et al., 1996]. Extracts were clarified by centrifugation and diluted 10 fold with SDS-free RIPA buffer containing a cocktail of proteinase inhibitors ( Sigma-Aldrich). For complex formation between Muc4 and ER chaperones, cells were lysed in RIPA buffer (0.1% SDS, 150 mM NaCl, 1% Nonidet p-40, 0.5% deoxycholate, 50 mM Tris/HCL, pH 8.0, containing a cocktail of proteinase inhibitors). 1 ml of extract was added to 30 μl protein G/A-agarose beads with bound antibodies. Samples were rotated overnight at 4°C and washed five times with cold RIPA buffer, and immunoadsorbed proteins were eluted for 5 min with boiling SDS-PAGE sample buffer. Immunoprecipitated proteins were analyzed by immunoblotting with appropriate antibodies.
Immunofluorescence analysis
Adherent Muc4-transfected A375 cells were grown on 6 mm Transwel-Clear™ inserts (Corning, Costar) for 1 day and then for 48 hours after induction of Muc4 expression. Cells were washed three times with Dulbecco’s PBS, pH 7.4, fixed in 4% formaldehyde in PBS for 20 min and, when indicated, permeabilized by two five minute treatments with 0.2% Triton X-100 in PBS. Aldehyde groups were quenched with 50 mM ammonium chloride and, after one 5 min wash with PBS and two subsequent washes with 1% BSA in PBS, cells were incubated for 1 h with indicated primary antibodies diluted in BSA-PBS. The cells were rinsed for 5 min twice with BSA-PBS, twice with PBS and incubated in the dark for 1 h with specific secondary antibodies coupled to Alexa Fluor 488 or Texas Red (Molecular Probes, Eugene, OR) and subsequently washed three times with PBS. Membranes containing the cells were excised from the inserts and mounted with a Prolong Antifade Kit (Molecular Probes) on microscope glass slides. To distinguish between membrane and intracellular localization of Muc4, nonpermeabilized cells were labeled first with mAb 4F12. The cells were then permeabilized, and intracellular Muc4 protein was labeled. The fluorescence labeling was examined by confocal microscopy using an LSM 510 microscope (Carl Zeiss, GmbH, Germany) equipped with two laser sources. Cells were analyzed using 100x oil immersion objectives. The images were collected and processed using LSM 510 software obtained from Zeiss.
Pulse-chase labeling of A375 cells expressing Muc4
A375 melanoma cells stably transfected with tetracycline-responsive inducible Muc4 vector were grown to 70% confluence. Two days after induction of the expression of Muc4, cells were washed three times with PBS, starved for 2 h in Cys/Met-free DMEM essential medium supplemented with 100 IU/ml penicillin, 0.3 mg/ml hygromycin, and 0.8 mg/ml G418 (starvation medium) and subsequently incubated in starvation medium containing 100 μCi/ml of 35S EXPRESS Protein Labeling Mix (NEN Life Science). Labeled cells were washed three times with ice-cold PBS and lysed in modified RIPA buffer. Extracts were clarified by centrifugation at 10,000 rpm, and cell lysates were immunoprecipitated with polyclonal Ab against ASGP-2. Protein released from the polyclonal Ab-A/G agarose beads by boiling for 5 min with SDS-sample buffer was analyzed by SDS-PAGE and fluorography.
RESULTS
TGFb represses expression of Muc4 in A375 tumor cells
Our previous studies on the repression of Muc4 expression by TGFβ were done on primary rat mammary epithelial cells [Price-Schiavi et al., 1998, 2000; Soto et al., 2003]. However, studies on these primary cells are very time-consuming and expensive. To further investigate the mechanism by which TGFβ acts on Muc4, we have turned to a cell line, A375 melanoma, transfected with Muc4, in which the Muc4 is under the regulation of a tetracycline-dependent promoter [Komatsu et al., 1997]. To determine whether TGFβ represses Muc4 expression in the transfected A375 cells in a similar manner to that previously observed with the mammary epithelial cells, we treated the A375 cells expressing Muc4 with TGFβ for 20 hr at concentrations similar to those used with the mammary cells. As shown in Figure 1A, TGFβ has a pronounced inhibitory effect on Muc4 expression in the A375 cells. Moreover, the delayed timing of this effect (Figure 1B) is consistent with our previous observations that TGFβ is acting on the precursor of Muc4 [Price-Schiavi et al., 2000] and its effect is only observed when the Muc4 has had sufficient time to undergo degradation. This mechanism of action of TGFβ on Muc4 synthesis is further supported by pulse-chase analyses, which show that label incorporation into the transmembrane subunit of Muc4 is blocked and that precursor is degraded in the TGFβ-treated cells (Figure 1C).
Fig. 1.
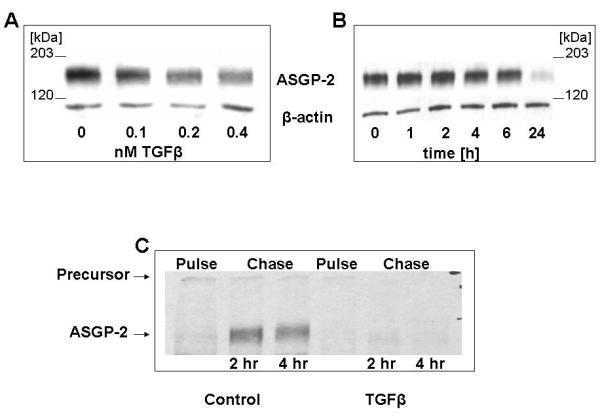
TGFβ repression of Muc4 expression in tumor cells. (A) A375 cells were treated with tetracycline for 48 hr to activate Muc4 expression, then treated with TGFβ at the concentrations shown for 24 hr. Muc4 was analyzed by immunoblotting. (B) Muc4-expressing A375 cells were treated with 0.4 nM TGFβ for the periods shown, then analyzed by immunoblotting. (C) A375 cells with or without TGFβ treatment were pulse-chase labeled for the periods shown and lysed for immunoprecipitation. The lysates were analyzed by SDS PAGE and fluorography.
Muc4 degradation by proteosomal pathway
Since precursor cleavage of Muc4 is suggested to occur in the endoplasmic reticulum [Sheng et al., 1990], we propose that TGFβ regulation of Muc4 might involve proteosomal degradation. To determine whether Muc4 undergoes significant proteosomal degradation, we have treated two different cell types expressing Muc4 with proteosomal inhibitors and analyzed their Muc4 content. As noted above, one of these, the A375, cells have been transfected with Muc4 to induce overexpression. The MAT-B1 13762 rat ascites mammary adenocarcinoma cells have endogenous levels of Muc4. As shown in Figure 2A, treatment of the cells with two different proteosome inhibitors resulted in increased Muc4 expression, indicating that Muc4 is subject to proteosome degradation. These results show that proteosome degradation of Muc4 is occurring in cancer cells with both endogenous and transfected Muc4. A similar effect has also been shown in isolated corneal epithelial cells (data not shown). Importantly, pulse-chase analyses of the A375 cells indicated that proteosome inhibitor repressed the degradation of the Muc4 precursor, as shown previously in mammary epithelial cells [Price-Schiavi et al., 2000] (Figure 2B).
Fig. 2.
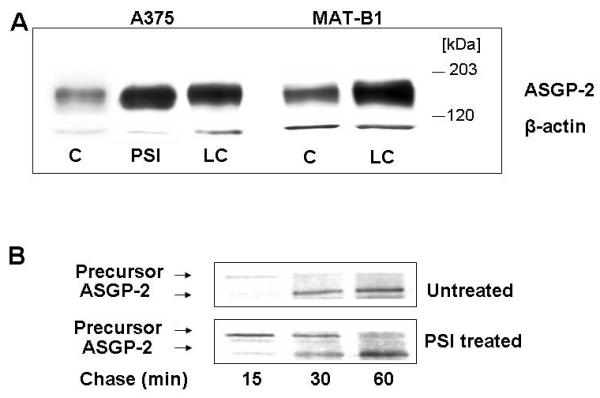
Effect of proteosome inhibitors on Muc4 in tumor cells. (A) A375 and 13762 MAT-B1 cell cultures were treated for 4 hours in medium containing 0.01 μM PSI or 5 μM lactacystin (LC) and analyzed by immunoblotting. (B) A375 cells with or without PSI treatment were pulse-chase labeled for the periods shown and lysed for immunoprecipitation. The lysates were analyzed by SDS PAGE and fluorography.
Previous studies by others have shown that inhibition of proteosome degradation results in proteins from the endoplasmic reticulum being accumulated in aggresomes in the cell cytoplasm [Johnston et al., 1998; Kopito 2000]. Therefore, we measured incorporation of Muc4 into aggresomes analyzed by two color confocal microscopy. Cell surface Muc4 was first labeled on intact cells by a green-labeled antibody against the extracellular domain of Muc4. The cells were then permeabilized, and the cytoplasmic Muc4 was labeled with a red-labeled antibody against the cytoplasmic domain of Muc4. Figure 3 shows that cells treated with proteosome inhibitor have a substantial accumulation of Muc4 in cytoplasmic structures.
Fig. 3.
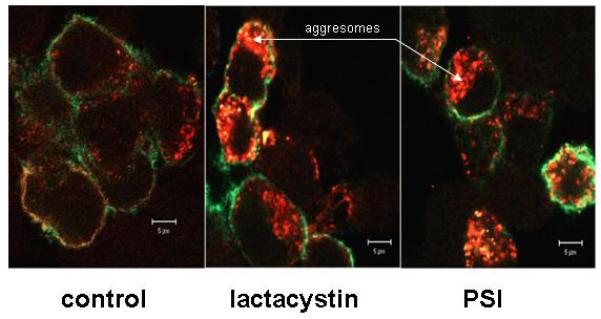
Proteosome inhibition relocalizes Muc4 in A375 cells to intracellular compartments. Two-color immunofluorescence analysis. A375 cells were grown in tetracycline-containing medium for one day after seeding on Transwell-Clear inserts and then on tetracycline-free medium for the next two days (cells “on”). Cells were then treated with 5 μM lactacystin or 0.01 μM PSI for 4 h. Cell surface Muc4 was labeled on non-permeabilized cells with 4F12 mAb directed to an extracellular epitope of Muc4, followed by anti-mouse Alexa-Fluor (green). Subsequently, cells were permeabilized with 0.2% Triton X100, and intracellular Muc4 was labeled with polyclonal anti C-pep antibody followed by Alexa-Texas Red anti-rabbit antibody (red).
Endoplasmic reticulum degradation pathway for Muc4
Glycoproteins undergoing degradation in the endoplasmic reticulum interact with chaperones which are involved in the processing of N-linked oligosaccharides [Ellgaard and Helenius, 2003]. To investigate the components which interact with Muc4, we treated the A375 cells with the mannosidase inhibitor deoxymannojirimycin (DMJ) to promote Muc4 degradation by the proteosomal degradation pathway, as shown in Figure 4A. The effect of the DMJ on the Muc4 degradation was reversed by proteosome inhibitor. To determine whether the chaperones calnexin and calreticulin are involved in the Muc4 degradation, cells were treated with or without the mannosidase inhibitor deoxynojirimycin (DNJ), then lysed and immunoprecipitated with anti-calnexin or anti-calreticulin. Immunoblots of the immunoprecipitates with anti-Muc4 showed clearly that interaction of Muc4 with both of these chaperones is increased by the DNJ treatment (Figure 4B).
Fig. 4.
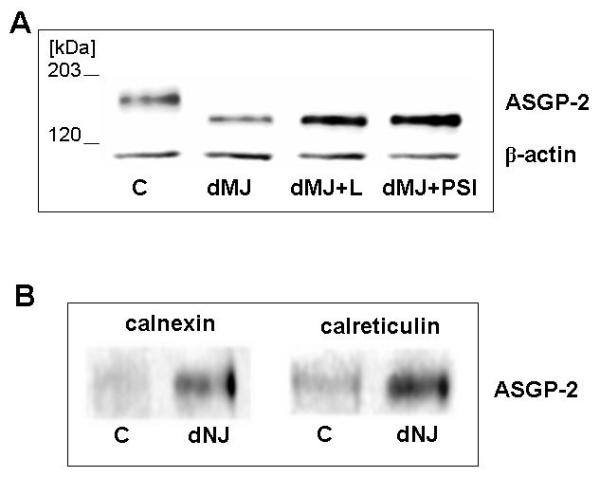
Effect of mannosidase inhibitors on Muc4 proteosomal degradation and association with ER chaperones. (A) A375 cells were grown overnight in the presence of 100 μg/ml deoxymannojirimycin (dMJ) and treated for an additional 4 h in the presence of proteasome inhibitors. SDS-extracted proteins were subjected to SDS-PAGE and immunobloting with 4F12 mAb. (B) A375 cells were grown in the presence of dNJ (0.5 mM) and analyzed for chaperone-Muc4 complexes by immunoprecipitation with antibodies against the chaperones. The immunoprecipitates were analyzed by immunoblotting with anti-Muc4.
Ubiquitination of Muc4
Proteosomal degradation of glycoproteins from the endoplasmic reticulum requires that they be ubiquitinated for recognition by the proteosome [Weissman, 2001; Pickart and Cohen, 2004]. Muc4 ubiquitination was analyzed by sequential immunoprecipitation and immunoblotting with anti-ubiquitin and anti-Muc4 in lysates from A375, MAT-B1. Levels of ubiquitinated Muc4 are increased in both tumor cell types by treatment with proteosome inhibitors (Fig. 5), though the effects appear less pronounced in the ascites cells. A similar effect was observed in isolated corneal epithelial cells (data not shown)
Fig. 5.
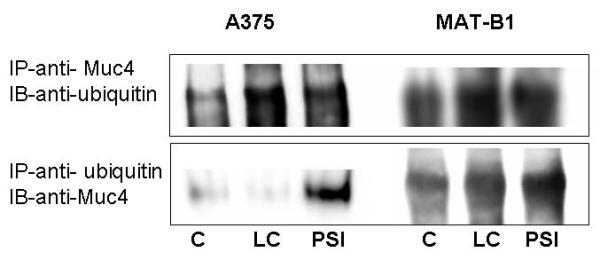
Muc4/SMC is ubiquitinated in A375 cells. Protein from RIPA extracts of A375 or 13762 MAT-B1 ascites cells treated with or without proteosome inhibitors was subjected to sequential immunoprecipitation and immunoblot analyses with anti-ubiquitin and anti-Muc4. (A) Lysates immunoprecipitated with anti-ASGP-2 polyclonal antibody coupled to A/G-agarose and immunoprecipitates immunoblotted with anti-ubiquitin antibody. (B) Lysates immunoprecipitated with anti-ubiquitin antibody coupled to A/G-agarose and immunoprecipitates immunoblotted with 4F12 mAb.
Proteosome inhibitor reverses the effect of TGFb on Muc4 expression
If TGFβ is repressing Muc4 expression by enhancing proteosomal degradation, then proteosome inhibitor should block the TGFβ effect and restore the Muc4 expression. Figure 6A shows that the proteosome inhibitor PSI is effective in preventing the loss of Muc4 due to TGFβ.
Fig. 6.
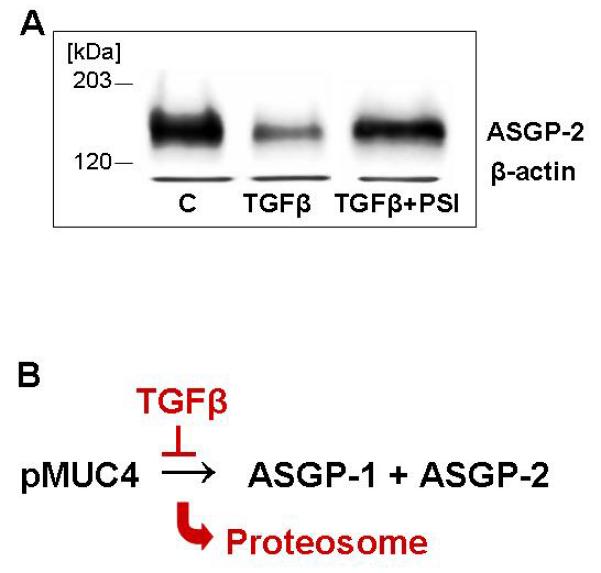
Reversal of TGFβ inhibition of Muc4 expression by proteosome inhibitor. (A) A375 cells treated with or without TGF-β and proteosome inhibitor were analyzed for Muc4 by immunoblotting. (B) Model for TGFβ regulation of Muc4 by proteosomal degradation.
DISCUSSION
Proteosomal degradation is used for both quality and quantity control for cellular proteins [Ellgaard and Helenius, 2003; Wojcikiewicz, 2004]. The need for quality control for Muc4 is obvious. It is a complex glycoprotein with numerous disulfides and multiple domains, such as the epidermal growth factor domains [Sheng et al., 1992], which require precise folding. Quantity control is also important for membrane mucins. The anti-adhesive properties of these molecules are critical for protection of epithelial cell surfaces, but overproduction can potentially compromise cell-cell interactions [Komatsu et al., 1997, 1999]. Membrane mucin overexpression is frequent in some carcinomas, contributing to their dissemination and metastasis [Komatsu et al., 2000]. We have shown previously that TGFβ is a key regulator of Muc4 in mammary epithelial cells [Price-Schiavi et al., 1998, 2000; Soto et al., 2003]. This regulatory mechanism is lost in mammary tumors with tumor progression due to loss of responsiveness to TGFβ [Price-Schiavi et al., 2000]. Our current work on the A375 and ascites tumor cells suggests that this is a general mechanism for regulating Muc4. An important question is how TGFβ acts on Muc4. Our previous work suggested that TGFβ repressed processing of the precursor of Muc4 to its two subunits in the endoplasmic reticulum. Our present work indicates that the precursor is then targeted to the proteosome for proteosomal degradation (Figure 6B), possibly because the precursor is unable to fold correctly without cleavage. These results suggest that a quantity control mechanism for Muc4 has evolved from the general quality control mechanism.
In summary, our work provides the first evidence for a role for proteosomal degradation in regulating mucin production in cells, offering a route for both the quality and quantity control necessary to maintenance of the protective epithelial barrier.
ACKNOWLEDGMENTS
This research was supported in part by grants CA52498 and EY12343 from the National Institutes of Health and by the Sylvester Comprehensive Cancer Center of the University of Miami. Confocal microscopy was performed at the University of Miami Core Analytical Imaging Facility under the supervision of Brigitte Shaw.
REFERENCES
- Carraway KL, Fogle DD, Chesnut RW, Huggins JW, Carraway CAC. Ecto-enzymes of mammary gland and its tumors. Lectin inhibition of 5′-nucleotidase of the 13762 rat mammary ascites carcinoma. J Biol Chem. 1976;251:6173–6178. [PubMed] [Google Scholar]
- Carraway KL, III, Rossi EA, Komatsu M, Price-Schiavi SA, Huang D, Guy PM, Carvajal ME, Fregien N, Carraway CAC, Carraway KL. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem. 1999;274:5263–5266. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Funes M, Miller JK, Lai C, Carraway KL, III, Sweeney C. The mucin Muc4 potentiates neuregulin signaling by increasing the cell surface populations of ErbB2 and ErbB3. J Biol Chem. 2006;281:19310–19319. doi: 10.1074/jbc.M603225200. [DOI] [PubMed] [Google Scholar]
- Hu Y-P, Haq B, Carraway KL, Savaraj N, Lampidis T. Multidrug resistance correlates with overexpression of muc4 but inversely with P-gp and MRP in transfected human melanoma cells. Biochem Pharmacol. 2003;65:1419–1425. doi: 10.1016/s0006-2952(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Carraway CAC, Fregien NL, Carraway KL. Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J Biol Chem. 1997;272:33245–33254. doi: 10.1074/jbc.272.52.33245. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Jepson S, Arango ME, Carraway CAC, Carraway KL. Muc4/Sialomucin Complex, an intramembrane modulator of ErbB2/HER2/Neu, potentiates primary tumor growth and suppresses apoptosis in a xenotransplanted tumor. Oncogene. 2001;20:461–470. doi: 10.1038/sj.onc.1204106. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Tatum L, Altman NH, Carraway CAC, Carraway KL. Potentiation of metastasis by cell surface sialomucin complex (rat muc4), a multifunctional anti-adhesive glycoprotein. Int J Cancer. 2000;87:480–486. doi: 10.1002/1097-0215(20000815)87:4<480::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Yee L, Carraway KL. Overexpression of sialomucin complex, a rat homolog of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999;59:2229–2236. [PubMed] [Google Scholar]
- Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- Lomako J, Lomako W, Carraway CAC, Carraway KL. Non-apoptotic desquamation of cells from corneal epithelium: putative role for Muc4/sialomucin complex in cell release and survival. J Cell Physiol. 2005;202:115–124. doi: 10.1002/jcp.20101. [DOI] [PubMed] [Google Scholar]
- Nagy P, Friedländer E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM. Decreased accessibility and lack of activation of erbB2 in a Herceptin-resistant, MUC-4-expressing breast cancer cell line. Cancer Res. 2005;65:473–482. [PubMed] [Google Scholar]
- Pickart CM, Cohen RE. Proteasomes and their kin: proteases in the machine age. Nature Rev Mol Cell Biol. 2004;5:177–187. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- Price-Schiavi SA, Carraway CAC, Fregien N, Carraway KL. Post-transcriptional regulation of a milk membrane protein, the sialomucin complex (ascites sialoglycoprotein (ASGP)-1/ASGP-2, rat Muc4) by TGFβ. J Biol Chem. 1998;273:35228–35237. doi: 10.1074/jbc.273.52.35228. [DOI] [PubMed] [Google Scholar]
- Price-Schiavi SA, Jepson S, Li P, Arango M, Rudland PS, Yee L, Carraway KL. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99:783–791. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- Price-Schiavi SA, Zhu X, Aquinin R, Carraway KL. Sialomucin complex (rat muc4) is regulated by transforming growth factor in mammary gland by a novel post-translational mechanism. J Biol Chem. 2000;275:17800–17807. doi: 10.1074/jbc.275.23.17800. [DOI] [PubMed] [Google Scholar]
- Ramsauer VP, Pino V, Farooq A, Carraway CAC, Salas PJI, Carraway KL. Muc4-ErbB2 complex formation and signaling in polarized CACO-2 epithelial cells indicate that Muc4 acts as an unorthodox ligand for ErbB2. Mol Biol Cell. 2006;17:2931–2941. doi: 10.1091/mbc.E05-09-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi EA, McNeer R, Price-Schiavi SA, Komatsu M, Van den Brande JMH, Thompson JF, Carraway CAC, Fregien NL, Carraway KL. Sialomucin complex, a heterodimeric glycoprotein complex: Expression as a soluble, secretable form in lactating mammary gland and colon. J Biol Chem. 1996;271:33476–33485. doi: 10.1074/jbc.271.52.33476. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Hull SR, Carraway KL. Biosynthesis of the cell surface sialomucin complex of ascites 13762 rat mammary adenocarcinoma cells from a high Mr precursor. J Biol Chem. 1990;265:8505–8510. [PubMed] [Google Scholar]
- Sheng Z, Vanderpuye OA, Hull SR, Carraway CAC, Carraway KL. Topography and microfilament core association of a cell surface glycoprotein of ascites tumor cell microvilli. J Cell Biochem. 1989;40:453–466. doi: 10.1002/jcb.240400406. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Wu K, Carraway KL, Fregien N. Molecular cloning of the transmembrane component of the 13762 mammary adenocarcinoma sialomucin complex: A new member of the epidermal growth factor superfamily. J Biol Chem. 1992;267:16341–16346. [PubMed] [Google Scholar]
- Soto P, Price-Schiavi SA, Carraway KL. SMAD2 and SMAD7 involvement in the post-translational regulation of Muc4 via the transforming growth factor-β and interferon-γ pathways in rat mammary epithelial cells. J Biol Chem. 2003;278:20338–20344. doi: 10.1074/jbc.M301886200. [DOI] [PubMed] [Google Scholar]
- Weissman AM. Themes and variations on ubiquitylation. Nature Rev Mol Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJH. Regulated ubiquitination of proteins in GPCR-initiated signaling pathways. Trends Pharm Sci. 2004;25:35–41. doi: 10.1016/j.tips.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Wu K, Fregien N, Carraway KL. Molecular cloning and sequencing of the mucin subunit of a heterodimeric, bifunctional cell surface glycoprotein complex of ascites rat mammary adenocarcinoma cells. J Biol Chem. 1994;269:11950–11955. [PubMed] [Google Scholar]


