Abstract
This manuscript details a validated liquid chromatography–atmospheric pressure chemical ionization-tandem mass spectrometry (LC–APCI-MS–MS) method for the quantification of methadone and its metabolites 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) and 2-ethyl-5-methyl-3,3-diphenylpyroline (EMDP) in 0.5 mL human breast milk. Limits of detection were 5 ng/mL for methadone and EDDP, and 10 ng/mL for EMDP. Linearity ranged from 10 to 500 ng/mL for all analytes. Breast milk is a complex biological fluid, necessitating several specimen preparation steps to separate methadone and metabolites from the lipophilic matrix. Recoveries were 66–97% following protein precipitation and solid-phase extraction with minimal matrix effect. Acceptable accuracy (89–101%) and precision (15–20% RSD) were achieved for all analytes. This is the first LC–APCI-MS–MS method for the sensitive and specific detection of methadone, EDDP, and EMDP in human breast milk. The method proved suitable for quantification of methadone and metabolites in breast milk of methadone-maintained opiate-dependent women.
Introduction
Methadone, a synthetic opiate, is the only replacement pharmacotherapy approved for opiate addiction in pregnant and lactating women in the U.S (1). Because of methadone's lipophilic nature, the drug is transferable from mother to infant via breast milk. Previously, lactating women maintained on greater than 20 mg/day of methadone were discouraged from breast-feeding, based on recommendations of the American Academy of Pediatrics (AAP) (2). Current treatment practices frequently include maintenance of opiate-dependent women in the perinatal period on methadone doses greater than 100 mg/day (3). In 2002, the AAP changed the recommendations, stating it was safe to breast-feed infants whose mothers were on methadone doses higher than 20 mg/day (4). Also, according to the U.S. Department of Health and Human Services (DHHS), methadone pharmacotherapy is not contraindicated in nursing mothers because of the importance of breast-feeding in establishing mother-infant bonding (5).
Methadone is a lipid soluble, weakly basic (pKa 8.25) compound that is highly protein bound. Methadone undergoes dealkylation to form its major metabolite 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) and a minor metabolite, 2-ethyl-5-methyl-3,3-diphenylpyroline (EMDP). Methadol, another minor metabolite, is formed by the reduction of methadone. EDDP, EMDP, and methadol are thought to be inactive metabolites.
Breast milk is a complex and variable biological matrix that requires extensive specimen cleanup to separate drugs from lipophilic components of the fluid and reduce matrix effects. The composition of breast milk changes during the course of a feeding, with higher lipid concentrations in the hindmilk (last two-thirds of a feed) as compared to the foremilk (first one-third of a feed) (6). Colostrum, produced in the days immediately after birth, and immature milk have a higher protein content and less fat, as compared to mature milk, present two to three weeks postpartum (7).
There are limited data on neonatal methadone exposure from breast milk (3,6,8,9). No analytical method quantifies methadone, EDDP and EMDP in this matrix. Maternal methadone doses of 10–180 mg/day yielded breast milk concentrations of 20–570 ng/mL by gas chromatography–mass spectrometry (GC–MS) (8-12) or liquid chromatography (LC) and photodiode array (3) or UV detection (6).
The aim of the present study was to develop and validate a sensitive and accurate LC–MS–MS method for measuring methadone and metabolites in human breast milk. This assay will be utilized to further our understanding of the transfer of methadone and metabolites from breast milk of methadone-maintained women to their infants.
Materials and Methods
Chemicals
(±)-Methadone, EDDP, (EMDP, methadone-d9, and EDDP-d3 perchlorate were purchased from Cerilliant™ (Austin, TX). All standards were > 99.9% pure, as described by the manufacturer. Reagent-grade ammonium acetate and formic acid were obtained from Sigma Chemical Co. (Milwaukee, WI). All other solvents were HPLC grade. Drug-free breast milk was obtained from local breast-feeding volunteers (n = 2) and verified as negative within our laboratory.
Calibration standards, internal standards, and quality control samples
Stock standard solutions (0.1 mg/mL) of native analytes were prepared in methanol. Dilution of stock solutions with water yielded working solutions of 200–10,000 ng/mL. Methadone-d9 and EDDP-d3 working solutions of 200 ng/mL in water were prepared from methanolic stock internal standard solutions. Methadone-d9 was the internal standard for methadone and EMDP and EDDP-d3 for EDDP. Methadone-d9 was chosen as the internal standard for EMDP due to a closer relative retention time. Quality control samples were prepared from different stock vials than were used for calibrator solutions.
Calibration curves were constructed by fortifying 0.5 mL of blank breast milk with working solutions of methadone, EDDP, and EMDP. Six calibrators at 10, 25, 50, 125, 250, and 500 ng/mL were prepared fresh daily.
Specimen preparation
One-half milliliter of breast milk was transferred to a polypropylene centrifuge tube, 25 mL internal standard solution was added, and the tube was vortex mixed for approximately 30 s. After chilling in an ice bath, 1 mL of chilled methanol was added dropwise while vortex mixing. Specimens were centrifuged at 6831 * g for 10 min. The organic supernatant phase was decanted into a clean glass tube. Solvent volume was reduced under N2 at 37°C to approximately 0.5 mL using a TurboVap® LV (Zymark, Hopkington, MA). In preparation for solid-phase extraction (SPE), samples were reconstituted in 2 mL of 2N sodium acetate buffer (pH 4.0) and 1 mL DI water and acidified with 60 μL concentrated phosphoric acid.
SPE was performed using manufacturers' recommended basic analytes (Phenomenex®, Torrance, CA). Reconstituted extracts were applied to preconditioned mixed-mode SPE columns with 60 mg of stationary phase and a 3-mL reservoir (Strata-X-C Cation Mixed-Mode Polymer columns). Columns were conditioned with 2 mL methanol and 2 mL deionized water prior to sample loading. After loading the samples at 0.5 mL/min, columns were washed successively with 2 mL 0.1N HCl and 2 mL of methanol, then dried under vacuum for 3 min. Analytes of interest were eluted with 2 mL of freshly prepared methanol/ammonium hydroxide (95:5). Elutes were evaporated to dryness under nitrogen at 37°C using a TurboVap LV and reconstituted in 100 μL mobile phase A. Twenty microliters was injected onto the LC–MS–MS.
Instrumentation
An LCQ Deca XP ion trap MS, equipped with an orthogonal APCI source, was interfaced to a Surveyor HPLC system (ThermoFinnigan, San Jose, CA). Data acquisition was carried out using Xcalibur™ software (version 1.2, ThermoFinnigan). The analytical column was a Phenomenex Fusion RP 80A (75 * 2.0 mm, 4 mm) fitted with a Fusion RP (4.0 * 2.0 mm) guard column. The column oven was maintained at 30°C and the autosampler tray at 15°C. Analytes were chromatograpically resolved via gradient elution. Mobile phase was 10mM ammonium formate in water with 0.001% formic acid (pH 4.5) (A) and acetonitrile (B). Flow rate was 200 mL/min. The initial gradient condition was 40% B for 2 min, increased to 90% B over 8 min, and maintained at this concentration for an additional 2 min. The column was re-equilibrated for 5 min, yielding a total run time of 17 min. HPLC flow was directed to the MS from 3 to 11 min; during the remaining time, flow was diverted to waste.
MS data were collected in positive ion mode, with the following APCI-MS parameters: corona discharge needle voltage, 4.5 kV; vaporizer temperature, 450°C; sheath gas setting (high purity nitrogen), 70 psi; no auxiliary gas; and a transfer capillary temperature of 220°C.
Identification and quantification of analytes were based on selected reaction monitoring (SRM). Precursor and product ions were established by direct infusion of individual analytes at a concentration of 5 mg/mL. Xcalibur software (version 1.2) was utilized to calculate linear regression.
Method validation
The following criteria were used to evaluate the LC–MS–MS method: limit of detection (LOD), limit of quantification (LOQ), linearity, specificity, imprecision, accuracy, recovery, carryover, stability, and matrix effects. Method validation was accomplished with four analytical runs on four different days. The LOD for each analyte was the lowest concentration yielding a signal-to-noise ratio of at least 3:1, adequate peak shape, presence of all ions and a retention time within ± 2% of the average retention time for all calibrators. The LOQ was defined as the lowest concentration with a signal-to-noise ratio of 10:1, in addition to the criteria described. Linearity was investigated by calculation of the regression line by the method of least-squares and expressed by the correlation coefficient (R2). Equal weighting was applied. Each calibrator was calculated against the full curve to ensure that quantification was within ± 20% of target. Imprecision and accuracy were evaluated using three in-house prepared quality controls spread across each analyte's linear dynamic range (20, 40, and 400 ng/mL). Imprecision (intraday n = 5 and interday n = 20) was expressed as the relative standard deviation (RSD). Accuracy of the method was calculated as the percent of expected concentration. Carryover was assessed by injecting a blank breast milk specimen following the 500 ng/mL calibrator.
Extraction efficiency was assessed with five replicates at three concentrations (20, 40, and 400 ng/mL). Human breast milk samples were fortified with standard before and after solid phase extraction. Percent expected concentrations were expressed as the mean area of the samples fortified after SPE.
Matrix effect was evaluated by comparing analyte peak area of extracted samples fortified after SPE with the analyte peak areas of neat samples prepared in mobile phase A at quality control concentrations. Matrix effect is expressed as a percentage of the mean area of the neat samples (n = 5 at three concentrations) (13).
Stability was assessed by fortifying human breast milk with analytes of interest at 40 ng/mL (n = 3). Temperature effect was examined over 24 h, at three conditions (24°C, 4°C, and −20°C). Additionally, fortified breast milk specimens were subjected to three freeze-thaw cycles.
Human breast milk collection
Breast milk specimens containing methadone and metabolites were obtained from methadone-maintained breast-feeding mothers enrolled in a comprehensive substance abuse treatment facility, the Center for Addiction and Pregnancy (CAP), and participating in a study at the Johns Hopkins Bayview Medical Center in Baltimore, MD. The Johns Hopkins University School of Medicine and NIDA Institutional Review Boards approved the study, and written informed consent was obtained from all participants. Mothers were compensated for participation.
Results
Specimen cleanup utilized methanolic protein precipitation followed by SPE. The combination of these two steps gave sufficient recovery of all analytes (> 97% for methadone, > 73% for EDDP, and > 66% for EMDP) (Table I).
Table I.
Solid-Phase Extraction (SPE) Recovery and Matrix Effect
| % SPE Extraction Recovery (n = 5) |
Matrix Effect (n = 5) |
|||||
|---|---|---|---|---|---|---|
| 20 ng/mL | 40 ng/mL | 400 ng/mL | 20 ng/mL | 40 ng/mL | 400 ng/mL | |
| Methadone | 118.0 | 97.2 | 105.2 | 96.5 | 84.8 | 74.8 |
| EDDP | 84.1 | 72.9 | 96.7 | 113.8 | 94.3 | 101.6 |
| EMDP | 80.9 | 113.1 | 66.2 | 78.2 | 78.9 | 88.7 |
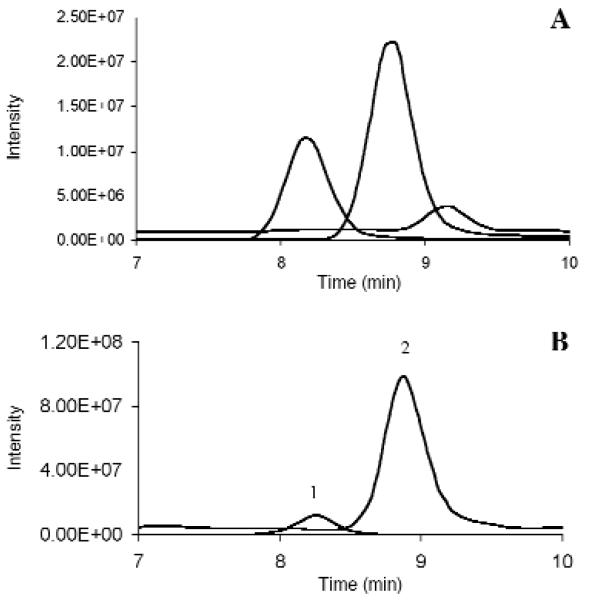
Separation of analytes of interest and their respective internal standards was achieved within 10 minutes, with relative retention times within ± 2% (Table II and Figure 1A). Precursor, product ion, collision energy (V), and retention time for each analyte are described in Table II. Relative retention times proved to be stable over the total run time.
Table II.
LC–APCI-MS–MS Parameters for the Quantification of Methadone and Metabolites in Human Breast Milk
| Analyte | (V)* | Precursor Ion |
Product Ion |
Retention Time (min) |
|---|---|---|---|---|
| Methadone | 40 | 310 | 265 | 8.8 (±1%)† |
| Methadone-d9‡ | 40 | 319 | 268 | 8.9 |
| EDDP§ | 30 | 278 | 249 | 8.1(±1%)† |
| EDDP-d3 | 30 | 281 | 249 | 8.2 |
| EMDP# | 30 | 264 | 235 | 9.4 |
(V) collision energy.
Relative retention time.
Internal standard for methadone and EMDP.
EDDP = 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine.
EMDP = 2-ethyl-5-methyl-3,3-diphenylpyroline.
Figure 1.
Chromatogram representative of LOQs at 20 ng/mL for methadone, EDDP, and EMDP from extracted breast milk (A) and chromatogram of a representative breast milk specimen collected on day four postpartum from a mother maintained on methadone 50 mg/day; calculated concentration of methadone (109.2 ng/mL) and EDDP (39.0 ng/mL) (B) Peak identification: 1, EDDP; 2, methadone; and 3, EMDP.
LODs, LOQs, and linearity are detailed in Table III. LODs were 5.0 ng/mL for methadone and EDDP and 10 ng/mL for EMDP. The linear dynamic ranged from 10.0 to 500 ng/mL for methadone, EDDP, and EMDP with correlation coefficients of > 0.99 (R2, equal weighting factor).
Table III.
Limits of Detection (LOD, n = 4) and Quantification (LOQ, n = 4) and Calibration Curve Results for Methadone and Metabolites in Breast Milk by LC–APCI-MS–MS
| Compound | Internal Standard |
LOD (ng/mL) |
LOQ (ng/mL) |
Equation | R2 |
|---|---|---|---|---|---|
| Methadone | MT*-d9 | 5.0 | 10.0 | y = 0.0169x + 0.2023 | 0.998 |
| EDDP | EDDP-d3 | 5.0 | 10.0 | y = 0.0153x + 0.2996 | 0.996 |
| EMDP | MT-d9 | 10.0 | 10.0 | y = 0.0019x + 0.1234 | 0.999 |
Abbreviations: MT, methadone; EDDP, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine; and EMDP, 2-ethyl-5-methyl-3,3-diphenylpyroline.
Imprecision was evaluated over the linear dynamic range at three concentrations (20, 40, and 400 ng/mL) of methadone, EDDP, and EMDP (Table IV). Intraday imprecision calculated from repeated analysis (n = 5) was < 15% for all analytes. Interday imprecision (n = 20) was < 15% for methadone, EDDP, and EMDP, except for 400 ng/mL EMDP, which was < 20%. Accuracy was based on percent difference from target concentration and was 89% to 101% for all analytes at all concentrations (Table IV). Methadone and metabolites were stable (less than 5% variability) for 24 h at 4 and −80°C, except for EDDP at ambient temperature, which decreased by 14% (Table V). There was no significant suppression or enhancement of methadone, EDDP, or EMDP with LC–APCI-MS–MS analysis because of the complex breast milk matrix.
Table IV.
Imprecision and Accuracy of Methadone and Metabolites in Breast Milk as Determined by LC–APCI-MS–MS
| Intraday (n = 5) |
Interday (n = 20) |
|||||
|---|---|---|---|---|---|---|
| Analyte (%expected) |
Concentration | Mean (ng/mL) |
Imprecision (ng/mL) |
Mean (RSD) |
Imprecision (ng/mL) |
Accuracy (RSD) |
| Methadone | 20 | 19.2 | 14.4 | 19.6 | 10.5 | 98.0 |
| 40 | 38.9 | 11.1 | 40.4 | 9.2 | 101.0 | |
| 400 | 401.5 | 7.9 | 388.3 | 12.3 | 97.0 | |
| EDDP | 20 | 18.0 | 7.8 | 17.9 | 8.7 | 89.0 |
| 40 | 35.3 | 5.4 | 35.8 | 8.3 | 90.0 | |
| 400 | 351.7 | 5.6 | 367.7 | 10.5 | 92.0 | |
| EMDP | 20 | 20.1 | 7.8 | 20.3 | 12.4 | 101.0 |
| 40 | 42.0 | 10.0 | 40.2 | 10.0 | 100.0 | |
Table V.
Stability of Methadone and Metabolites at a Concentration of 400 ng/mL (% found) at Certain Parameters
| 24°C, 24 h | 4°C, 24 h | Three Freeze- Thaw Cycles |
|
|---|---|---|---|
| Methadone | 100.2 | 101.4 | 109.2 |
| EDDP | 85.9 | 95.9 | 98.3 |
| EMDP | 108.9 | 103.0 | 99.7 |
As proof of this method, a breast milk specimen collected four days postpartum at peak plasma methadone concentration, approximately 3 h after oral methadone dosing, is shown in Figure 1B. The woman was maintained on a daily 50-mg methadone dose, and her breast milk contained 109 ng/mL methadone and 39 ng/mL EDDP.
Discussion
This LC–MS–MS method proved to be selective and sensitive for the detection of methadone, EDDP, and EMDP in human breast milk following specimen preparation and concentration. Methadone and metabolites have been analyzed by LC–tandem MS in a variety of biological matrices including urine (14), plasma (6,15,16), hair (17), and meconium (18); however, this is the first LC–MS–MS analysis of methadone in breast milk.
Extraction of drugs from breast milk is an analytical challenge because of its high protein and fat content and changing composition during the postpartum period. In addition, there is a variable composition during the feeding period. Previously published breast milk methods used liquid–liquid extraction (3,6,8,12) or SPE (9). Our procedure employed an initial protein precipitation by 1 mL of chilled methanol prior to SPE. Flow through the SPE columns was irregular when breast milk was applied directly because of high lipid and protein content of the matrix. Samples flowed freely via gravity through the SPE columns after methanolic protein precipitation. Addition of ambient temperature methanol and acetonitrile was investigated but insufficient, leading to poor recovery of analytes. The sample preparation step was simple, efficient and minimized matrix effects (Table I). Accuracy of the method yielded RSD < 20% for all analytes.
Linearity for this method ranged from 10 to 500 ng/mL for all analytes using 0.5 mL of breast milk. Begg et al. (3) and McCarthy et al. (9) both reported LOQs of 10 ng/mL for methadone, but 1 mL of breast milk was required. Specimen-sparing techniques are important during the early stages of breast-feeding when milk production is limited.
Conclusions
Methanolic protein precipitation followed by SPE in combination with LC–APCI-MS–MS detection offers sufficient analytical sensitivity, selectivity and simultaneous quantification of methadone and its two primary metabolites, EDDP and EMDP, in a complex breast milk matrix. Good recovery was achieved for methadone and EDDP, but it was lower for EMDP. This method will be useful for the quantification of methadone and metabolites in breast milk from mothers maintained on methadone after pregnancy. Accurate and sensitive measurements of methadone and metabolites in breast milk may help to elucidate the relationship between maternal methadone dose and infant exposure during breast-feeding.
Acknowledgments
This study was supported by Grant K08 DA00495 from the National Institute on Health and the National Institute on Drug Abuse Extramural Research Grant awarded to Dr. Lauren Jansson and National Institute on Drug Abuse intramural funds. We would like to acknowledge the staff at the Center for Addiction and Pregnancy and postpartum wards of the Johns Hopkins Bayview Medical Center for assistance during the course of the study.
References
- 1.Jansson LM, Velez M, Harrow C. Methadone maintenance and lactation: a review of the literature and current management guidelines. J. Hum. Lact. 2004;20:62–71. doi: 10.1177/0890334403261027. [DOI] [PubMed] [Google Scholar]
- 2.Kauffman RE, Banner W, Jr., Berlin CM, Jr., Blumer JL, Gormer RL, Lambert GH, Wilson GS. The transfer of drugs and other chemicals into human milk. Pediatrics. 1994;93:137–150. [Google Scholar]
- 3.Begg EJ, Malpas TJ, Hackett LP, Ilett KF. Distribution of R- and S-methadone into human milk during multiple, medium to high oral dosing. Br. J. Clin. Pharmacol. 2001;52:681–685. doi: 10.1046/j.1365-2125.2001.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ressel G. AAP updates statement for transfer of drugs and other chemicals into breast milk. Am. Fam. Physician. 2002;65:979–980. [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services . Treatment Improvement Protocol Series: Improving Treatment for Drug Exposed Infants. 1993. Center for Substance Abuse Treatment. (Report #95-3056). [Google Scholar]
- 6.Wojnar-Horton RE, Kristensen JH, Yapp P, Ilett KF, Dusci LJ, Hackett LP. Methadone distribution and excretion into breast milk of clients in a methadone maintenance program. Br. J. Clin. Pharmacol. 1997;44:543–547. doi: 10.1046/j.1365-2125.1997.t01-1-00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennet P. Drugs and Human Lactation. 2nd ed. Elsevier Science; Amsterdam, The Netherlands: 1996. [Google Scholar]
- 8.Blinick G, Inturrisi CE, Jerez E, Wallach RC. Methadone assays in pregnant women and progeny. Am. J. Obstet. Gynecol. 1975;121:617–621. doi: 10.1016/0002-9378(75)90461-5. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy JJ, Posey BL. Methadone levels in human milk. J. Hum. Lact. 2000;16:115–120. doi: 10.1177/089033440001600206. [DOI] [PubMed] [Google Scholar]
- 10.Kreek MJ, Schecter A, Gutjahr CL, Bowen D, Field F, Queenan J, Merkatz I. Analyses of methadone and other drugs in maternal and neonatal body fluids: use in evaluation of symptoms in a neonate of mother maintained on methadone. Am. J. Drug Alcohol Abuse. 1974;1:409–419. doi: 10.3109/00952997409011033. [DOI] [PubMed] [Google Scholar]
- 11.Kreek MJ. Methadone disposition during the perinatal period in humans. Pharmacol. Biochem. Behav. 1979;11(Suppl):7–13. [PubMed] [Google Scholar]
- 12.Geraghty B, Graham EA, Logan B, Weiss EL. Methadone levels in breast milk. J. Hum. Lact. 1997;13:227–230. doi: 10.1177/089033449701300312. [DOI] [PubMed] [Google Scholar]
- 13.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC–MS/MS. Anal. Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 14.Dams R, Murphy CM, Lambert WE, Huestis MA. Urine drug testing for opioids, cocaine, and metabolites by direct injection liquid chromatography/tandem mass spectrometry. Rapid Comm. Mass Spectrom. 2003;17:1665–1670. doi: 10.1002/rcm.1098. [DOI] [PubMed] [Google Scholar]
- 15.Fleishaker JC. Models and methods for predicting drug transfer into human milk. Adv. Drug Deliv. Rev. 2003;55:643–652. doi: 10.1016/s0169-409x(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 16.Rook EJ, Hillebrand MJ, Rosing H, van Ree JM, Beijen JH. The quantitative analysis of heroin, methadone and their metabolites and the simultaneous detection of cocaine, acetylcodeine and their metabolites in human plasma by high-performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B. 2005;824:213–221. doi: 10.1016/j.jchromb.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 17.Kelly T, Doble P, Dawson M. Chiral analysis of methadone and its major metabolites (EDDP and EMDP) by liquid chromatography–mass spectrometry. J. Chromatogr. B. 2005;814:315–323. doi: 10.1016/j.jchromb.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 20.Choo RE, Murphy CM, Jones HE, Huestis MA. Determination of methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, 2-ethyl-5-methyl-3,3-diphenylpyraline and methadol in meconium by liquid chromatography atmospheric pressure chemical ionization tandem mass spectrometry. J. Chromatogr. B. 2005;814:369–373. doi: 10.1016/j.jchromb.2004.10.068. [DOI] [PubMed] [Google Scholar]