Abstract
Purpose
This study aimed to test the hypothesis that elevated expression of antiapoptotic Bcl-2 family proteins predicts a poor therapeutic response of oropharyngeal squamous cell carcinoma (OPSCC) to concurrent platinum-based chemoradiation therapy.
Experimental Design
Levels of Bcl-2, Bcl-XL, and Bcl-w were determined and correlated with resistance to cisplatin in a large panel of cell lines derived from squamous cell carcinoma of the head and neck (HNSCC). Univariate and multivariate analyses were used to evaluate the relationship between Bcl-2 and Bcl-XL expression and disease-free survival following chemoradiation therapy in a uniformly treated cohort of patients with OPSCC.
Results
In HNSCC cell lines, high endogenous Bcl-2 expression was associated with increased cisplatin resistance, and experimental overexpression of Bcl-2 promoted cisplatin resistance. In patients, tumors positive for Bcl-2 before treatment had greater risk of treatment failure (hazard ratio, 5.99; 95% confidence interval, 1.73−20.8; P = 0.0014). In contrast, endogenous Bcl-XL showed no correlation either with cisplatin sensitivity in the cell line panel in vitro, or with risk of recurrence in vivo (hazard ratio, 1.28; 95% confidence interval, 0.39 − 4.19; P = 0.68). Associations between Bcl-2 expression and other clinical characteristics did not account for the predictive value of Bcl-2.
Conclusions
Immunohistochemical assessment of Bcl-2 in pretreatment biopsy specimens can predict response of advanced OPSCC to concurrent platinum-based chemoradiation. As treatments targeting Bcl-2 and its family members become available, this immunohistochemical assessment could help personalize therapy by identifying a subpopulation of patients with a poor prognosis who might benefit from such treatments.
Identifying pretreatment molecular markers that can predict response to therapy is of great interest in head and neck oncology and is required to develop personalized treatments that maximize survival while minimizing morbidity. Numerous approaches have been examined in head and neck squamous cell carcinoma (HNSCC), including the use of individual and combined markers and global genomic strategies, with some limited success (1). In oropharyngeal squamous cell carcinoma (OPSCC), for example, infection with human papilloma virus (HPV) is associated with improved survival (2–6), whereas high levels of the epidermal growth factor receptor are associated with treatment resistance (6). Nevertheless, neither of these markers has sufficient predictive power to individualize treatment selection and direct therapy. Global genomic approaches that identify prognostic gene signatures have not cleared the important hurdle of reproducibility, because different genomic marker sets share few genes (7–9). Consequently, additional markers of response are needed to predict outcome, guide the intensity and choice of therapy, and point the way to novel therapeutic targets.
Recent studies on the in vitro response to genotoxic agents in HNSCC have described cellular pathways related to treatment resistance and identified potential markers of therapeutic response. One pathway thought to be important for cellular survival in HNSCC involves two members of the p53 family, TAp73 and ΔNp63α (Fig. 1A; ref. 10). TAp73 isoforms, which share many of the proapoptotic functions of p53, are highly expressed in cultured HNSCC cells and primary tumors, unlike in the normal epithelial cells of origin (11). Genotoxic treatments of these tumor cells activate a TAp73-dependent apoptotic transcriptional program that leads to cell death even in the absence of functional p53 (12). Indeed, the association of some p53 mutants with treatment resistance in HNSCC is postulated to arise from their inhibition of p73-dependent apoptosis (13).
Fig. 1.

Roles of Bcl-2 family members in inhibiting cell death after cisplatin treatment of cell lines derived from HNSCC. A, diagram summarizing how genotoxic therapy such as chemoradiation affects the p53 family members p63 and p73 in HNSCC, and the downstream inhibition of p73-induced apoptosis by antiapoptotic members of the Bcl-2 family. B through D, scatter plots of cisplatin IC50 versus endogenous mRNA levels of Bcl-2 family members for 19 cell lines derived from HNSCC. Logarithmic horizontal axis allows display of cell lines differing by several orders of magnitude in expression. Regression lines of IC50 against logarithm of mRNA level are plotted, along with correlation coefficients and significance. B, Bcl-2; C, Bcl-XL; D, Bcl-w. Bcl-2 mRNA levels displayed in this figure were highly correlated with protein expression seen on Western blots; cf. Supplementary Fig. S3.
Translational Relevance.
Previous studies have reported conflicting outcomes regarding the role of Bcl-2 family members in predicting treatment outcome in squamous cell carcinoma of the head and neck (HNSCC). Our prior studies had identified Bcl-2 as critical for survival of cell lines derived from HNSCC. In this study we first showed that high Bcl-2 expression is specifically correlated with resistance to cisplatin among these cell lines. In a uniformly treated cohort of patients with advanced oropharyngeal squamous cell carcinoma, we showed that elevated pretreatment Bcl-2 levels predict therapeutic resistance to platinum-based concurrent chemoradiation. This study defines a novel subgroup of patients who will require more aggressive or novel therapies to achieve survival rates comparable to patients having Bcl-2–negative tumors. Furthermore, the elevated levels of Bcl-2 found in this subgroup of patients represent an attractive therapeutic target for small-molecule Bcl-2 inhibitors currently being developed.
We have found (11, 14) that the proapoptotic effects of TAp73 are often kept in check by ΔNp63α, the predominant form of p63 in normal epithelia and in HNSCC (15). ΔNp63α, normally restricted to basal cells of squamous epithelia, is highly expressed in most HNSCC (15, 16). In HNSCC where ΔNp63α inhibits TAp73 function, platinum-based chemotherapeutic agents have been proposed to act by inducing degradation of ΔNp63α (17) and phosphorylation of TAp73 (18), allowing expression of the TAp73-induced proapoptotic pathway and cell death. Consistent with ΔNp63α as a target of platinum agents, one study showed that HNSCC not expressing ΔNp63α are resistant to such chemotherapy (19).
This key role of ΔN63α in tumor cell survival (11, 14, 18) led us to ask how HNSCC cells that do not express antiapoptotic ΔNp63α manage to survive. Neither TAp73 nor downstream apoptotic triggers like p53-up-regulated modulator of apoptosis are typically lost in HNSCC (20). Rather we found that cell lines deficient in ΔNp63α overexpress the antiapoptotic protein Bcl-2, which acts downstream of TAp73 to block cell death (Fig. 1A; ref. 14). Based on these findings, we hypothesized that if high Bcl-2 allows HNSCC cells to stay alive in the absence of endogenous ΔNp63α, high Bcl-2 might also enhance survival of cells subjected to chemotherapy that leads to loss of ΔNp63α.
Antiapoptotic members of the Bcl-2 family such as Bcl-2, Bcl-XL, and Bcl-w control the integrity of the outer mitochondrial membrane, thereby regulating the susceptibility to apoptosis through the intrinsic pathway (21). In numerous studies, elevated expression of antiapoptotic Bcl-2 family members has been associated with resistance to chemotherapy (reviewed in ref. 22). In HNSCC, both Bcl-2 and Bcl-XL expression have been associated with resistance to genotoxic therapy, although there have been inconsistent reports about the relative contribution of each protein, with several studies presenting conflicting results (23–25).
In the present study we examined how these proapoptotic and antiapoptotic proteins correlate with cisplatin resistance in vitro and, in a well-characterized cohort of patients having OPSCC, with clinical outcome following chemoradiation treatment. Consistent with our model, in vitro resistance to cisplatin correlated positively with Bcl-2 levels. In patients, high pretreatment Bcl-2 levels in tumors were strikingly correlated with poor clinical outcome. Bcl-XL, in contrast, showed little or no correlation with cisplatin sensitivity in vitro or with clinical outcome following chemoradiation treatment.
Materials and Methods
Cell lines
Cell lines derived from HNSCC were generous gifts of David Sidransky (Johns Hopkins University) or of Robert Ferris (University of Pittsburgh Cancer Institute), or were obtained from the American Type Culture Collection. Supplementary Table S1 provides information on specific cell lines. Cells were maintained at 37°C with 5% CO2 in either RPMI or DMEM supplemented with 10% fetal bovine serum and with penicillin/streptomycin.
Expression constructs
Reverse transcription PCR of human fibroblast cDNA was used to subclone cDNA for Bcl-2, Bcl-XL, or Bcl-w into the BamHI/XhoI restriction sites of the pLPC retrovirus expression vector (primer sequences available on request). Production of high-titer amphotrophic retroviral stocks and retroviral infection was done as described (14).
Protein and mRNA analysis
Methods of protein detection by Western blot, RNA extraction, cDNA preparation, and quantitative real-time PCR (qRT-PCR) were as in a previous study (14), except that HotStart-IT Taq Master Mix (USB) supplemented with SybrGreen (Invitrogen) was used for PCR. Expression plasmids for Bcl-2, Bcl-XL, Bcl-w, ΔNp63α, and TAp73β were used to prepare standard curves for qRT-PCR. PCR results are expressed as femtograms of coding-sequence DNA providing qRT-PCR results equivalent to cDNA corresponding to 50 ng total RNA. Primers for PCR of p63 and p73 (sequences available on request) were specific for their ΔN and TA forms, respectively, but did not distinguish COOH-terminal variants. The anti-p63 4A4 antibody (MS 1081; NeoMarkers) recognizes the DNA-binding domain common to all forms of p63.
Analysis of cisplatin sensitivity
Cells in 96-well plates were treated with cisplatin for 48 h, followed by analysis of cell viability with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; ref. 18). Data for MTT signal versus cisplatin concentration were analyzed with the drfit package of the R statistical software environment (http://www.r-project.org) to obtain the IC50, the concentration at which the MTT signal was reduced by half.
Patient data
Human studies approval was obtained from the Massachusetts General Hospital Internal Review Board (Partners Human Research Committee) to obtain archival tumor specimens and do retrospective chart review. Patients with squamous cell carcinoma of the oropharynx were identified in the Massachusetts General Hospital pathology database. Inclusion criteria were: (a) biopsy-proven squamous cell carcinoma of the oropharynx treated definitively with concurrent chemoradiation, with or without neck dissection; (b) no prior history of head and neck squamous cell carcinoma; (c) no prior history of head and neck irradiation; (d) pretreatment biopsy paraffin block available; (e) adequate documented clinical follow-up for at least 2 y or until a recurrence proven by biopsy; and (f) use of a platinum-based agent for chemotherapy (cisplatin or carboplatin with paclitaxel). Of 69 patients meeting the first three criteria, tumor specimens were unavailable for 13, 8 did not meet the minimum follow-up, and 10 received a different chemotherapy regimen. Results are reported for the remaining 38 patients. Entry into the study was set as the initial biopsy date (all from May 1996 to May 2005). During the course of radiation, chemotherapy was administered either as cisplatin (100 mg/m2 i.v. over 1 h) every 3 wk for up to 3 cycles, or as weekly administrations of carboplatin (area under the curve = 1.5 i.v. over 30 min) and paclitaxel (45 mg/m2 i.v. over 30 min) for up to 7 treatments as tolerated. Radiation therapy was delivered as intensity modulated radiation therapy five times weekly in daily fractions of 1.8 to 2.12 Gy for a total of 33 to 40 fractions to the gross tumor volume, or as a concomitant boost accelerated fractionation schedule (72 Gy delivered to the gross tumor volume). Lymph nodes received 45 to 60 Gy according to the level of risk. Patients were evaluated closely each 4 to 6 wk during the first 2 y of follow-up. The median follow-up since initial biopsy of the 26 patients both alive and disease free at last follow-up was 46.5 mo (range, 31–126 mo). One patient died without evidence of recurrent disease after 25 mo. Statistical analysis was done in the R environment, including the survival package for analysis of disease-free survival.
Immunohistochemistry
BenchMark XT automated tissue staining systems (Ventana Medical Systems, Inc.) were used, with protocols provided by the manufacturer. Endogenous peroxidase activity was blocked by H2O2. A combination of EDTA and boric acid in Tris buffer (CC1 reagent; Ventana) was applied to the tissues for antigen retrieval for 30 to 60 min prior to washing and primary antibody incubation. Primary antibodies and incubation times were: p63 (4A4; 1:200 dilution; 8 min), Bcl-2 prediluted antibody (Ventana; 1 h 52 min), and Bcl-XL (1:40 dilution; NeoMarkers; 32 min). Secondary antibodies were UltraView horseradish peroxidase–conjugated multimers (goat antimouse and rat Igs.; Ventana). Antigen detection was done using UltraView diaminobenzidine chromogen (Ventana). Tissues were counterstained with hematoxylin.
A pathologist blinded to outcome (WCF) used the criteria of Jackel et al. (26) to score Bcl-2 and Bcl-XL immunohistochemistry. An intensity score (0, absent; 1, weak; 2, moderate; 3, strong) and a prevalence score (0, <25% of tumor stained; 1, 25–75% stained; 2, >75% stained) were added; a total of ≥3 was called positive.
HPV analysis
HPV-16 DNA was detected by in situ hybridization–catalyzed signal amplification for biotinylated probes (GenPoint; Dako), as described previously (27). A HPV-16–positive tumor specimen provided a positive control. A pathologist blinded to outcome (WHW) scored slides as positive for HPV-16 if there was a punctate signal specific to tumor cell nuclei.
Results
Cisplatin resistance of cell lines derived from HNSCC was related to Bcl-2 expression
Our and others' previous work linked the expression of both p63 and p73 to cisplatin sensitivity in HNSCC and other tumors (Fig. 1A; refs. 10, 18). Therefore, we first examined whether their endogenous expression correlated with cisplatin sensitivity in a panel of 19 HNSCC-derived cell lines. We used real-time quantitative RT-PCR (qRT-PCR) to assay the major p63 and p73 isoforms expressed in HNSCC, ΔNp63α, and TAp73. We compared expression levels with cisplatin resistance (IC50) determined from a quantitative dose-response assay (MTT). Because ΔNp63α has been shown to promote survival of HNSCC cells and to be targeted for degradation by cisplatin (14, 17), we predicted that low ΔNp63α expression would be associated with cisplatin resistance. Indeed, low ΔNp63α expression was highly associated with cisplatin resistance (r = 0.70, P = 0.0008; Supplementary Fig. S1). Also as predicted, low endogenous expression of TAp73, an effector of cell death in response to cisplatin (28), was correlated with cisplatin resistance (Supplementary Fig. S2).
We next examined correlation of Bcl-2 with cisplatin resistance in these cell lines. We had previously shown that Bcl-2 expression correlates inversely with ΔNp63α levels and potently blocks TAp73-dependent apoptosis in HNSCC cells, suggesting that Bcl-2 expression might be a strong predictor of cisplatin resistance. As shown in Fig. 1B, we found in the present study that Bcl-2 expression was significantly correlated to cisplatin resistance among these cell lines (r = 0.72, P = 0.00055; Supplementary Table S1). We also examined expression of two other antiapoptotic members of the Bcl-2 family, Bcl-XL, and Bcl-w, which are thought to have similar target binding specificity as Bcl-2 (22). Of these, Bcl-XL has been associated in some studies with cisplatin resistance (29) and with poor outcome in HNSCC (6). In contrast to the results with Bcl-2, expression of these other antiapoptotic members of the Bcl-2 family was not correlated to cisplatin resistance (Fig. 1C and D).
To examine mechanisms that might account for this correlation between Bcl-2 expression and cisplatin resistance, we investigated the effects of overexpressing Bcl-2 in a subset of these cell lines. Figure 2A shows results of such an experiment on the JHU029 cell line, a line highly sensitive to cisplatin. Cells were infected with control retrovirus or with virus coding for Bcl-2, selected with puromycin for stably infected cells, and then subjected to cisplatin. Control cells expressed high levels of ΔNp63α and very low levels of Bcl-2 protein, as we reported previously for this cell line (14). Cisplatin treatment led to loss of ΔNp63α, consistent with a previous report in other HNSCC lines (17). The apoptotic program elicited by loss of ΔNp63α is evidenced by expression of the p53/p73 target gene NOXA and cleavage of poly ADP-ribose polymerase after exposure of control cells to cisplatin (Fig. 2A, leftmost two lanes).
Fig. 2.

Effects of Bcl-2 overexpression on cisplatin resistance of cell lines derived from HNSCC. A, cisplatin treatment led to loss of ΔNp63 and activation of upstream proapoptotic targets regardless of Bcl-2 expression, whereas Bcl-2 inhibited the downstream apoptotic program. Western blots of protein extracts taken after 21h exposure of vector-control JHU029 cells (left) or cells overexpressing Bcl-2 (right) to 5 μg/mL cisplatin. The p73 target gene NOXA is induced by cisplatin in both cases, whereas the apoptotic program, illustrated by cleavage (two bands) of poly-ADP ribose polymerase, was inhibited by Bcl-2 overexpression. B, Bcl-2 overexpression increased cisplatin resistance of JHU029 cells as assessed by MTT viability assay. MTT absorbance, normalized to vehicle-only control absorbance, in vector-control cells or cells overexpressing Bcl-2, treated with the indicated concentrations of cisplatin for 48 h; mean ± SE of four observations at each concentration. Dashed lines, shift in IC50, the concentration of cisplatin required to reduce viability to 50% of control. C, Bcl-2 overexpression increased cisplatin resistance of all cell lines examined. For each of nine cell lines, mean cisplatin IC50 in cells overexpressing Bcl-2 is plotted against mean IC50 in vector-control cells examined in parallel. Dashed line, line of identity, expected if Bcl-2 expression did not affect IC50; solid line, observed linear regression of IC50 for cells expressing Bcl-2 versus IC50 for control cells, indicating a 54% increase in resistance following Bcl-2 overexpression; P value is for difference of observed slope from line of identity.
JHU029 cells overexpressing Bcl-2 showed loss of ΔNp63α and induction of NOXA similar to that in control cells, so upstream steps in the response to cisplatin were intact despite Bcl-2 overexpression. Cells overexpressing Bcl-2, however, did not show the classic downstream sign of apoptosis, cleavage of poly ADP-ribose polymerase (Fig. 2A; compare the second and fourth lanes from the left). This result was consistent with the position of Bcl-2 along the apoptotic cascade; Bcl-2 inhibits BH3-only proapoptotic proteins from initiating mitochondrial lysis and the resulting activation of caspases, whose subsequent cleavage of poly ADP-ribose polymerase is a sign of apoptosis (22). Thus overexpression of Bcl-2 in this cell line allowed early steps in the response to cisplatin, but inhibited downstream apoptotic mechanisms.
The effect of Bcl-2 overexpression on cisplatin resistance in this sensitive cell line is shown in Fig. 2B. In this experiment, control cells and cells overexpressing Bcl-2 were exposed to a range of cisplatin concentrations covering those reached in clinical practice (30, 31), and cell viability was assessed with MTT. Bcl-2 overexpression shifted the cisplatin concentration-response curve to the right. After overexpression of Bcl-2, about half of the cells escaped death after 48 hours' exposure to 3 μg/mL of cisplatin, conditions that killed almost all control cells. We did similar studies of Bcl-2 overexpression on eight additional HNSCC cell lines, with both high and low ΔNp63α expression. As shown in Fig. 2C, overexpression of Bcl-2 led to a 50% increase in cisplatin IC50 over all nine lines tested. Thus, Bcl-2 overexpression could increase cisplatin resistance in these cell lines regardless of ΔNp63α status.
To determine whether reduction of Bcl-2 expression would decrease resistance to cisplatin, we attempted to prepare cell lines with stably reduced Bcl-2 levels by infecting cells with lentivirus coding for short hairpin RNAs targeting Bcl-2 for knockdown. In all our attempts, no cells expressing such shRNAs survived puromycin selection for infected cells, whereas puromycin-resistant cells infected with control lentiviruses were readily obtained with high efficiency (not shown). These results suggest that basal expression of endogenous Bcl-2 plays a significant role in survival of these cell lines.
Poor outcome following chemoradiation treatment of advanced OPSCC was related to Bcl-2 expression in pretreatment tumors
Based on these cell-line studies, we decided to examine whether pretreatment Bcl-2 expression might serve as a marker for poor outcome after concurrent chemoradiation of HNSCC. Because previous studies had suggested that Bcl-XL could serve as such a marker (6, 29), we chose to include Bcl-XL expression in our analysis, although Bcl-XL expression did not correlate with cisplatin resistance among the cell lines (Fig. 1C). Bcl-w was not analyzed in the tumors because it failed to correlate with cell-line cisplatin resistance and has not been reported as a marker of response in HNSCC. Given our findings in cell lines and a previous clinical report (19), we also examined expression of ΔNp63α.
Selection of patients; immunohistochemical evaluation
We examined characteristics and outcomes of patients having stage III or IV OPSCC treated with concurrent radiation and platinum-based chemotherapy, the present standard of care for such patients (32). In addition to the well-defined anatomical origin of these tumors, essentially all patients presenting with this disease receive the same treatment at our institution, minimizing pretreatment selection bias. Clinical characteristics of the patients are presented in Table 1.
Table 1.
Patient characteristics and associated recurrence hazards
| Characteristic | Total | Bcl-XL | Bcl-2 | Recurrence at last follow-up | Hazard* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | P† | Positive | Negative | P† | No | Yes | Ratio | P | |||
| Age (y) | <50 | 12 | 8 | 4 | 0.29 | 4 | 8 | 1 | 7 | 5 | 1 | 0.26 |
| 50-65 | 21 | 8 | 13 | 6 | 15 | 15 | 6 | 0.62 | ||||
| >65 | 5 | 3 | 2 | 1 | 4 | 5 | 0 | 0 | ||||
| Gender | Male | 31 | 16 | 15 | 1 | 11 | 20 | 0.084 | 20 | 11 | 1 | 0.085 |
| Female | 7 | 3 | 4 | 0 | 7 | 7 | 0 | 0 | ||||
| Site | Tongue base | 16 | 9 | 7 | 0.60 | 5 | 11 | 1 | 11 | 5 | 1 | 0.99 |
| Tonsil | 18 | 9 | 9 | 5 | 13 | 13 | 5 | 0.95 | ||||
| Other | 4 | 1 | 3 | 1 | 3 | 3 | 1 | 0.83 | ||||
| T | 1 | 8 | 6 | 2 | 0.16 | 3 | 5 | 0.95 | 5 | 3 | 1 | 0.32 |
| 2 | 18 | 8 | 10 | 5 | 13 | 15 | 3 | 0.43 | ||||
| 3 | 8 | 2 | 6 | 2 | 6 | 5 | 3 | 1.07 | ||||
| 4 | 4 | 3 | 1 | 1 | 3 | 2 | 2 | 2.04 | ||||
| N | 0 | 5 | 2 | 3 | 0.73 | 0 | 5 | 0.13 | 4 | 1 | 1 | 0.13 |
| 1 | 8 | 4 | 4 | 1 | 7 | 8 | 0 | 0 | ||||
| 2 | 23 | 11 | 12 | 10 | 13 | 13 | 10 | 2.11 | ||||
| 3 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | ||||
| Disease stage | III | 11 | 5 | 6 | 1 | 1 | 10 | 0.12 | 11 | 0 | 0 | 0.02 |
| IV | 27 | 14 | 13 | 10 | 17 | 16 | 11 | 1 | ||||
| Smoking | No | 17 | 9 | 8 | 1 | 6 | 11 | 0.49 | 13 | 4 | 1 | 0.47 |
| Yes | 21 | 10 | 11 | 5 | 16 | 14 | 7 | 1.57 | ||||
| Alcohol | No | 28 | 14 | 14 | 1 | 8 | 20 | 1 | 22 | 6 | 1 | 0.078 |
| Yes | 10 | 5 | 5 | 3 | 7 | 5 | 5 | 2.79 | ||||
| HPV | Positive | 25 | 13 | 12 | 1 | 8 | 17 | 0.72 | 20 | 5 | 0.33 | 0.054 |
| Negative | 13 | 6 | 7 | 3 | 10 | 7 | 6 | 1 | ||||
| Bcl-XL | Positive | 19 | 6 | 13 | 1 | 13 | 6 | 1.28 | 0.68 | |||
| Negative | 19 | 5 | 14 | 14 | 5 | 1 | ||||||
| Bcl-2 | Positive | 11 | 6 | 5 | 1 | 4 | 7 | 5.99 | 0.0014 | |||
| Negative | 27 | 13 | 14 | 23 | 4 | 1 | ||||||
NOTE: Clinical characteristics and immunohistochemical scoring as noted in Materials and Methods. Smoking refers to any history of smoking. Alcohol refers to a history of alcohol abuse or dependence. Of the 38 patients, all of whom were followed for at least 31 mo or until recurrence, 11 had recurrent disease.
Hazard ratios based on Cox proportional hazard analysis. Reference level of each characteristic is shown as hazard ratio of 1, with an increased hazard ratio indicating increased risk of recurrence. P values for significant differences in hazard ratios among levels of each characteristic based on log rank test.
P values by Fisher exact test for differences from independent joint distributions of each characteristic with respect to Bcl-XL or Bcl-2 staining.
To facilitate comparison with results from other institutions, we used commercially available reagents and automated equipment for immunohistochemical staining of p63, Bcl-2, and Bcl-XL in pretreatment biopsy specimens. The pathologist evaluating the staining was unaware of treatment outcomes and used a published technique, described in Materials and Methods, that combines both staining intensity and prevalence to classify a tumor as positive for an antigen. With respect to Bcl-2, particular care was taken to exclude lymphocytes from immunohistochemical scoring. Although infiltration by lymphocytes, which are Bcl-2–positive, can be a positive prognostic sign (33), we wished to restrict analysis of Bcl-2 to the tumor cells in which elevated Bcl-2 was predicted to be associated with poor outcome following genotoxic therapy.
Staining for p63 was not helpful in distinguishing among tumors
All 38 tumors showed moderate to robust staining for ΔNp63α, although the staining patterns differed substantially among tumors. Examples of ΔNp63α staining patterns are shown in Fig. 3A. Due to the large fraction of p63-positive tumor cells in all cases, we could not classify tumor ΔNp63α status in a way that provided a useful marker for treatment outcome.
Fig. 3.

Immunohistochemical staining of tumors for p63, Bcl-2, and Bcl-XL. Immunostaining for indicated antigens in brown, with hematoxylin counterstain. A, heterogeneity of staining patterns for p63 among tumors. Examples from four separate tumors. B, examples of p63, Bcl-2, and Bcl-XL staining in pretreatment biopsies of four additional tumors. H+E is conventional H&E staining. Each column, sections from a single tumor, with time to recurrence noted. From left to right: positive for Bcl-2, not Bcl-XL; positive for Bcl-2 and Bcl-XL; positive for Bcl-XL, not Bcl-2; negative for both Bcl-2 and Bcl-XL. Recurrence information only applies to panel B.
Patients with tumors expressing Bcl-2 were at greater risk of recurrence
Classification of tumors for Bcl-2 and Bcl-XL staining, in contrast, was straightforward. Half of the tumors were positive for Bcl-XL staining, and 11 of 38 (29%) were positive for Bcl-2. Unlike some other studies that found inverse correlations of these two antiapoptotic proteins among individual tumors (24, 34), we found examples of all combinations of Bcl-2 and Bcl-XL staining, as illustrated in Fig. 3B. In fact, the distributions of Bcl-2 and Bcl-XL among these tumors were independent of each other, with one half of both Bcl-2–positive and Bcl-2–negative tumors showing Bcl-XL expression (Table 1; P = 1 by Fisher exact test).
As we wished to test the hypothesis that Bcl-2 expression is a marker of poor outcome of therapy, we examined whether Bcl-2 expression was correlated to other clinical characteristics that might affect outcome. As shown in Table 1, no other clinical characteristics differed significantly (at P < 0.05) among the groups distinguished by Bcl-2 staining.
We then did Cox proportional hazard analysis on disease-free survival as a function of Bcl-2 status. Patients with pretreatment tumors staining for Bcl-2 had much shorter disease-free survival, as predicted by our model. This univariate analysis found a hazard ratio of 5.99 for patients with tumors expressing Bcl-2 (95% confidence interval, 1.73–20.8; P = 0.0014 by log-rank test). Pretreatment Bcl-XL status, in contrast, bore no relation to disease-free survival (95% confidence interval for hazard ratio, 0.39–4.19; P = 0.68). Figure 4 shows Kaplan-Meier plots illustrating this difference between Bcl-2 (Fig. 4A) and Bcl-XL (Fig. 4B) status. (Analysis of overall survival was uninformative, because only seven patients died, including one with no sign of recurrent disease.) These relations of disease-free survival to pretreatment tumor Bcl-2 and Bcl-XL status were consistent with our cell-line results (Fig. 1B and C).
Fig. 4.

Relations of recurrence to pretreatment Bcl-2 and Bcl-XL expression (cf. Table 1). Kaplan-Meier plots of disease-free survival, with times of last follow-up indicated by cross-marks. Bcl-2 expression was related to significantly increased risk of recurrence (A) whereas risk of recurrence was not related to Bcl-XL (B). See Table 1 and text for Cox proportional hazard analysis.
Of the 11 patients whose tumors recurred, recurrence was at a distant site in 4 of 7 Bcl-2 positive cases, versus 1 of 4 cases having pretreatment tumors negative for Bcl-2. This difference was not statistically significant (P = 0.55, Fisher's exact test).
In addition to Bcl-2 status, univariate Cox proportional hazard analyses for each clinical characteristic in Table 1 found no significant relations to disease-free survival (at P < 0.05) except for disease stage, with no recurrences in 10 patients presenting with stage III disease (of whom only 1 had a Bcl-2–positive tumor).
Forward stepwise multivariate analysis identified low Bcl-2, HPV positivity, disease stage III, female gender, site other than tonsil or tongue base, and age >65 y as significant positive predictors for disease-free survival. Detailed analysis of these additional predictors showed that there were no recurrences in seven female patients (none Bcl-2 positive) or in five patients >65 y (with only 1 Bcl-2 positive). Out of four patients with tumors in oropharyngeal sites other than tonsil or tongue base, the single patient with recurrence had a Bcl-2–positive tumor. Thus, multivariate analysis provided little additional predictive value over Bcl-2 expression alone, except for the already known relationship between good outcome and HPV infection.
Finally, although we had found no clinical characteristics significantly correlated to Bcl-2 status in this study, we recognized that the sample size of only 38 patients might limit the power to detect true correlations of Bcl-2 with other characteristics. Thus, we also examined three clinical characteristics that both came close to significant correlations with Bcl-2 expression and had significant or near-significant univariate relations to disease-free survival (Table 1): gender, N score, and disease stage. None of seven female patients had Bcl-2–positive tumors or recurrent disease. Ten of 11 Bcl-2–positive tumors were associated with an N score of 2, and 10 of 11 patients with Bcl-2–positive tumors presented with stage IV disease.
To examine the practical implications of interactions with these characteristics for using Bcl-2 as a marker, we repeated disease-free survival analysis while restricting analysis to males (31 patients), to N score 2 (23 patients), or to disease stage IV (28 patients). As shown in Fig. 5, Bcl-2 expression was associated with shorter disease-free survival in each of these subgroups. Thus pretreatment expression of Bcl-2 in tumors was associated with substantially increased risk of poor outcome following chemoradiation treatment of OPSCC, in a way that was not substantially related to covariation of Bcl-2 status with other clinical characteristics of the patients.
Fig. 5.
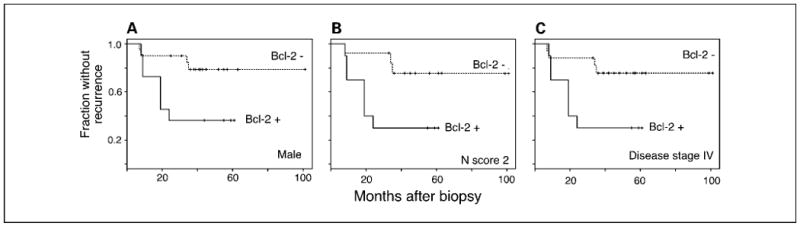
Relations of Bcl-2 expression to other prognostic indicators did not account for the observed relation between Bcl-2 and recurrence. Restricting analysis to male patients (A), patients with N score of 2 (B), or disease stage IV (C) resulted in similar relations between pretreatment Bcl-2 status and recurrence.
Discussion
Predictive value of p63
We expected that staining for p63 would be at best of limited predictive usefulness, because the vast majority of primary HNSCC express high levels of p63 as assessed by routine immunohistochemistry. Our ability to detect a correlation between p63 expression and cisplatin resistance in HSNCC cell lines may be due in part to the larger dynamic range of mRNA analysis. Furthermore, our cell-line studies showed that Bcl-2 overexpression could lead to increased cisplatin resistance even in cells expressing ΔNp63α (Fig. 2C). Thus, some tumors resistant to therapy might nevertheless be p63-positive.
Different approaches to classifying p63 expression or analysis of different HNSCC cohorts might yield more informative results. For example, use of Western blot analysis on fresh protein extracts from HNSCC primary tumors was able to correlate ΔNp63α expression with sensitivity to chemoradiation (19).
Predictive value of Bcl-2
Our results support the hypothesis, based on the underlying molecular mechanisms, that pretreatment Bcl-2 status is closely related to the success of chemoradiation treatment of OPSCC. Elevated Bcl-2 expression in the primary tumor is associated with significantly increased risk of recurrence. At the time of last follow-up, only 4 of 27 patients (15%) with tumors negative for Bcl-2 had recurrent disease, whereas nearly two thirds (7 of 11) of those with tumors positive for Bcl-2 had recurrence. In contrast, pretreatment Bcl-XL staining was not related to outcome, with about one third of patients in each Bcl-XL category having recurrence.
Relation to previous reports
The present study points a way toward understanding apparently incompatible previous reports of relations between Bcl-2 expression and patient outcomes in HNSCC, which have ranged from good prognosis through no relation to poor prognosis (24, 25, 34–41). First, we chose a well-defined subset of HNSCC with a defined anatomical source and with essentially identical treatment in all cases. A defined tumor origin in this type of study is crucial, because tumors originating from different sources may differ substantially in the selective pressures they have faced, their mutational strategies before clinical presentation, and their responses to therapy (32, 42). The type of tumor we examined posed few problems in terms of bias arising from selection of treatment. There also was at most limited relation between pretreatment Bcl-2 status and other clinical characteristics of the patients. We thus could relate differences in Bcl-2 status to differences in treatment outcome while minimizing confounding influences of other uncontrolled factors.
Second, we used a standardized immunohistochemical method and strict criteria for scoring tumors as positive for Bcl-2, combining staining intensity and prevalence into a single score (26). In evaluating stained specimens, we only scored staining of tumor cells, explicitly ignoring lymphoid tissue or Bcl-2–positive lymphocytes infiltrating the tumors.
Third, we examined the relation of Bcl-2 expression to the outcome of a treatment that should be influenced directly by the antiapoptotic effects of Bcl-2, chemoradiation. In particular, we did not include patients subjected to primary surgery with curative intent. Whereas simple correlations between a biomarker and outcome can serve prognosis regardless of the underlying mechanisms, the strongest relation between a biomarker and treatment outcome is expected for a marker directly related to the mechanism of the treatment. That was the case for the relation of Bcl-2 to protection from apoptosis in the present study.
Notably, prior studies of HNSCC that shared the strengths of the present study – an adequate number of Bcl-2–positive tumors with well defined origin, no major relation between Bcl-2 status and other clinical characteristics, Bcl-2 scores limited to tumor cells, and examining response to a standardized treatment that damaged DNA – also showed the relation we predicted and found between Bcl-2 status and treatment outcome. Examples are studies on response to primary radiation therapy of stage I and II tumors of the oral cavity, pharynx, and larynx (35) and in early-stage laryngeal tumors (25). Contrary reports of no prognostic value or a positive prognostic value of pretreatment Bcl-2 expression in HNSCC often involved studies with few Bcl-2–positive cases (43–45), not specifically examining the effects of treatments that damage DNA (24, 37, 39–41), having correlations of Bcl-2 with unrelated positive prognostic indicators (36, 38), or not explicitly excluding tumor-associated lymphoid tissue in the Bcl-2 scoring.
Differences between the patient population in the present study and those in other studies may also account for some discrepancies. For example, almost all (88%) of the patients in the study by Aebersold et al. (34) on responses of advanced oropharyngeal tumors to radiation therapy were smokers, versus only about one half (55%) in the present study. Furthermore, in the study by Aebersold et al., smoking was associated with low Bcl-2 expression, whereas we found no association of smoking history with Bcl-2. Another possibility is that Bcl-2 might be more closely related to outcome after platinum-based chemotherapy or concurrent chemoradiation than to outcome after primary radiation therapy. Although platinum-based chemotherapy and radiation both damage DNA and lead to apoptosis, differences in the intensity and nature of the DNA damage and subsequent apoptotic pathways after the two types of treatments might be associated with differences in the ability of Bcl-2 to prevent apoptosis, or combination therapy might alter apoptotic thresholds so that Bcl-2 expression is more important to tumor cell survival.
Although our results did not show a relation of Bcl-XL expression and outcome following chemoradiation therapy of OPSCC, they do not rule out roles of Bcl-XL in helping to determine treatment responses in HNSCC. Studies examining primary radiation treatment (25, 35) have shown that elevated Bcl-XL is associated with worse outcome in some forms of HNSCC. Furthermore, although we did not find a correlation of endogenous Bcl-XL expression with cisplatin resistance in cell lines derived from HNSCC, we have found that exogenous overexpression of Bcl-XL in these cell lines can increase their cisplatin resistance (not shown). It will be important to characterize the specific roles of Bcl-2 and Bcl-XL in different types of HNSCC and in the responses to different types of cytotoxic therapy. Differences in cellular mechanisms responding to radiation versus platinum-based chemotherapy, or interactions of p53 status with proapoptotic and antiapoptotic mechanisms, might explain some of the yet-unresolved differences in prognostic significance between Bcl-2 and Bcl-XL. Dynamics of subcellular localization may also play a role because Bcl-2 is constitutively membrane-bound to the mitochondria, whereas Bcl-XL and Bcl-w are cytosolic and translocate to the mitochondria during apoptosis (46).
Relation to HPV
Our identification of Bcl-2 as an important prognostic marker in advanced OPSCC complements recent studies identifying HPV as a predictor of therapeutic response and overall survival (2–6). Patients with HPV-associated HNSCC typically are younger, lack the traditional HNSCC risk factors of alcohol and tobacco, and are more likely to have a higher number or oral and vaginal sexual partners (3). There was no correlation between HPV infection and either Bcl-2 or Bcl-XL status among the patients in the present study, indicating that HPV and Bcl-2 status might be independent predictors of disease-free survival. A larger study would be required to determine the nature of interactions between Bcl-2 and HPV status for predicting outcome of OPSCC.
Implications for therapy
Patients having oropharyngeal tumors with low Bcl-2 expression typically had favorable outcomes following concurrent chemoradiation therapy, so the major outstanding clinical problem is the poor outcome typical of patients with high Bcl-2 expression. In this subset of patients, upfront surgery followed by chemoradiation may be a therapeutic option when the oropharyngeal primary is amenable to resection with acceptable morbidity. In this regard, transoral robotic surgery for base of tongue neoplasms might provide a minimally invasive approach that leads to less morbidity than classic open or endoscopic transoral laser surgical approaches (47). For patients with high Bcl-2 expression in tumors where surgical resection is impossible or would yield excess morbidity, clinically effective medical treatments must be identified. Options to explore for this subgroup include non-platin chemotherapy (e.g. taxanes or fluorouracil) either as induction therapy prior to radiation (48) or concurrent with radiation (49). Most interesting for this subset would be biologic therapies, such as anti–epidermal growth factor receptor monoclonal antibodies or small-molecule Bcl-2 inhibitors, with radiation. Retrospective analyses of Bcl-2 expression in tumor specimens from published trials of radiation therapy with or without combinations of cetuximab and cisplatin (reviewed in ref. 32) could validate and extend the results of this present study, and potentially clarify whether cetuximab can limit the apparent resistance to radiation of tumors expressing high levels of Bcl-2.
The correlation of Bcl-2 with therapeutic response to chemoradiation in advanced OPSCC is consistent with its known role as a primary regulator of apoptosis. This interpretation is supported by our in vitro data relating both endogenous Bcl-2 expression and overexpressed exogenous Bcl-2 to cisplatin resistance in HNSCC cell lines. As previously suggested, Bcl-2 may thus be an attractive therapeutic target in OPSCC. The recent solution of the structure of antiapoptotic Bcl-2 family members (reviewed in ref. 46) has resulted in the design of a number of novel small molecule inhibitors currently under active therapeutic evaluation (21, 50). Targeting Bcl-2 with these agents in the clinical trial setting may be a compelling approach in patients with OPSCC predicted to do poorly due to elevated pretreatment Bcl-2 expression when upfront resection offers unacceptable morbidity. Phase I-II studies of small molecule Bcl-2 inhibitors are early in development. Studies of these agents in patients whose head and neck squamous cancers overexpress Bcl-2 are eagerly awaited.
Supplementary Material
Acknowledgments
We thank Robert Ferris and David Sidransky for gifts of cell lines.
Grant support: Financial support was provided by the Laurence Murphy Fund, the Norman Knight Fund, and the James Kelly Fund for Head and Neck Research, a Clinical Investigator Award from the Flight Attendant Medical Research Institute (J.W. Rocco), and NIH grant DE015945 (L.W. Ellisen and J.W. Rocco).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/). W.A. Michaud, A.C. Nichols, and E.A. Mroz contributed equally to the manuscript.
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Singh B, Pfister DG. Individualized treatment selection in patients with head and neck cancer: do molecular markers meet the challenge? J Clin Oncol. 2008;26:3114–6. doi: 10.1200/JCO.2007.14.7298. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–47. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 3.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 4.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 5.Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26:3138–46. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bockmuhl U, Schluns K, Kuchler I, Petersen S, Petersen I. Genetic imbalances with impact on survival in head and neck cancer patients. Am J Pathol. 2000;157:369–75. doi: 10.1016/S0002-9440(10)64549-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belbin TJ, Singh B, Barber I, et al. Molecular classification of head and neck squamous cell carcinoma using cDNA microarrays. Cancer Res. 2002;62:1184–90. [PubMed] [Google Scholar]
- 9.Chung CH, Parker JS, Karaca G, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5:489–500. doi: 10.1016/s1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 10.Rocco JW, Ellisen LW. p63 and p73: life and death in squamous cell carcinoma. Cell Cycle. 2006;5:936–40. doi: 10.4161/cc.5.9.2716. [DOI] [PubMed] [Google Scholar]
- 11.DeYoung MP, Johannessen CM, Leong CO, et al. Tumor-specific p73 up-regulation mediates p63 dependence in squamous cell carcinoma. Cancer Res. 2006;66:9362–8. doi: 10.1158/0008-5472.CAN-06-1619. [DOI] [PubMed] [Google Scholar]
- 12.Irwin MS, Kondo K, Marin MC, Cheng LS, Hahn WC, Kaelin WG., Jr Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–10. doi: 10.1016/s1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 13.Bergamaschi D, Gasco M, Hiller L, et al. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell. 2003;3:387–402. doi: 10.1016/s1535-6108(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 14.Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Sniezek JC, Matheny KE, Westfall MD, Pietenpol JA. Dominant negative p63 isoform expression in head and neck squamous cell carcinoma. Laryngoscope. 2004;114:2063–72. doi: 10.1097/01.mlg.0000149437.35855.4b. [DOI] [PubMed] [Google Scholar]
- 16.Barbieri CE, Pietenpol JA. p63 and epithelial biology. Exp Cell Res. 2006;312:695–706. doi: 10.1016/j.yexcr.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 17.Fomenkov A, Zangen R, Huang YP, et al. RACK1 and stratifin target DNp63a for a proteasome degradation in head and neck squamous cell carcinoma cells upon DNA damage. Cell Cycle. 2004;3:1285–95. doi: 10.4161/cc.3.10.1155. [DOI] [PubMed] [Google Scholar]
- 18.Leong CO, Vidnovic N, DeYoung MP, Sgroi D, Ellisen LW. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J Clin Invest. 2007;117:1370–80. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zangen R, Ratovitski E, Sidransky D. DNp63a levels correlate with clinical tumor response to cisplatin. Cell Cycle. 2005;4:1313–5. doi: 10.4161/cc.4.10.2066. [DOI] [PubMed] [Google Scholar]
- 20.Ratovitski E, Trink B, Sidransky D. p63 and p73: teammates or adversaries? Cancer Cell. 2006;9:1–2. doi: 10.1016/j.ccr.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2008 Sep 19; doi: 10.1038/cdd.2008.137. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer JA, Trask DK, Kumar B, et al. Reversal of cisplatin resistance with a BH3 mimetic, (-)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol CancerTher. 2005;4:1096–104. doi: 10.1158/1535-7163.MCT-05-0081. [DOI] [PubMed] [Google Scholar]
- 24.Pena JC, Thompson CB, Recant W, Vokes EE, Rudin CM. Bcl-xL and Bcl-2 expression in squamous cell carcinoma of the head and neck. Cancer. 1999;85:164–70. doi: 10.1002/(sici)1097-0142(19990101)85:1<164::aid-cncr23>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Nix P, Cawkwell L, Patmore H, Greenman J, Stafford N. Bcl-2 expression predicts radiotherapy failure in laryngeal cancer. Br J Cancer. 2005;92:2185–9. doi: 10.1038/sj.bjc.6602647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackel MC, Dorudian MA, Marx D, Brinck U, Schauer A, Steiner W. Spontaneous apoptosis in laryngeal squamous cell carcinoma is independent of bcl-2 and bax protein expression. Cancer. 1999;85:591–9. [PubMed] [Google Scholar]
- 27.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9:6469–75. [PubMed] [Google Scholar]
- 28.Gong JG, Costanzo A, Yang HQ, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–9. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 29.Bauer JA, Kumar B, Cordell KG, et al. Targeting apo-ptosis to overcome cisplatin resistance: a translational study in head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:S106–8. doi: 10.1016/j.ijrobp.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sileni VC, Fosser V, Maggian P, et al. Pharmaco-kinetics and tumor concentration of intraarterial and intravenous cisplatin in patients with head and neck squamous cancer. Cancer Chemother Pharmacol. 1992;30:221–5. doi: 10.1007/BF00686317. [DOI] [PubMed] [Google Scholar]
- 31.Hecquet B, Vennin P, Fournier C, Poissonnier B. Evaluation of the pharmacological benefit and determination of the influencing factors of intraarterial cis-diamminedichloroplatinum administration in patients with uterine cervical cancer. Cancer Res. 1987;47:6134–7. [PubMed] [Google Scholar]
- 32.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 33.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 34.Aebersold DM, Kollar A, Beer KT, Laissue J, Greiner RH, Djonov V. Involvement of the hepato-cyte growth factor/scatter factor receptor c-met and of Bcl-xL in the resistance of oropharyngeal cancer to ionizing radiation. Int J Cancer. 2001;96:41–54. doi: 10.1002/1097-0215(20010220)96:1<41::aid-ijc5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 35.Gallo O, Boddi V, Calzolari A, Simonetti L, Trovati M, Bianchi S. bcl-2 protein expression correlates with recurrence and survival in early stage head and neck cancer treated by radiotherapy. Clin Cancer Res. 1996;2:261–7. [PubMed] [Google Scholar]
- 36.Wilson GD, Grover R, Richman PI, Daley FM, Saunders MI, Dische S. Bcl-2 expression correlates with favourable outcome in head and neck cancer treated by accelerated radiotherapy. Anticancer Res. 1996;16:2403–8. [PubMed] [Google Scholar]
- 37.Spafford MF, Koeppe J, Pan Z, Archer PG, Meyers AD, Franklin WA. Correlation of tumor markers p53, bcl-2, CD34, CD44H, CD44v6, and Ki-67 with survival and metastasis in laryngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1996;122:627–32. doi: 10.1001/archotol.1996.01890180035010. [DOI] [PubMed] [Google Scholar]
- 38.Giatromanolaki A, Koukourakis M, Zaramboukas T, et al. p53 and bcl-2 expression in locally advanced squamous cell head-neck cancer treated with platinum based chemotherapy and radiotherapy. Anticancer Res. 1998;18:4685–92. [PubMed] [Google Scholar]
- 39.Stoll C, Baretton G, Ahrens C, Lohrs U. Prognostic significance of apoptosis and associated factors in oral squamous cell carcinoma. Virchows Arch. 2000;436:102–8. doi: 10.1007/pl00008207. [DOI] [PubMed] [Google Scholar]
- 40.Klatka J. Prognostic value of the expression of p53 and bcl-2 in patients with laryngeal carcinoma. Eur Arch Otorhinolaryngol. 2001;258:537–41. doi: 10.1007/s004050100383. [DOI] [PubMed] [Google Scholar]
- 41.Lo Muzio L, Falaschini S, Farina A, et al. Bcl-2 as prognostic factor in head and neck squamous cell carcinoma. Oncol Res. 2005;15:249–55. doi: 10.3727/096504005776404599. [DOI] [PubMed] [Google Scholar]
- 42.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas S, Bosch FX, Klein-Kuhne W, et al. Expression von Zellzykluskomponenten in fortgeschrittenen Kopf-Hals-Karzinomen. Bedeutung fur die Prognose nach primarer akzelerierter Radiochemotherapie. HNO. 1999;47:777–86. doi: 10.1007/s001060050460. [DOI] [PubMed] [Google Scholar]
- 44.Casado S, Forteza J, Dominguez S, et al. Predictive value of P53, BCL-2, and BAX in advanced head and neck carcinoma. Am J Clin Oncol. 2002;25:588–90. doi: 10.1097/00000421-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Trask DK, Wolf GT, Bradford CR, et al. Expression of Bcl-2 family proteins in advanced laryngeal squamous cell carcinoma: correlation with response to chemotherapy and organ preservation. Laryngoscope. 2002;112:638–44. doi: 10.1097/00005537-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 47.O'Malley BW, Jr, Weinstein GS, Snyder W, Hockstein NG. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope. 2006;116:1465–72. doi: 10.1097/01.mlg.0000227184.90514.1a. [DOI] [PubMed] [Google Scholar]
- 48.Price L, Shaw HJ, Hill BT. Larynx preservation after initial non-cisplatin containing combination chemotherapy plus radiotherapy, as opposed to surgical intervention with or without radiotherapy in previously untreated advanced head and neck cancer: final analysis after 12 years follow-up. J Laryngol Otol. 1993;107:211–6. doi: 10.1017/s0022215100122662. [DOI] [PubMed] [Google Scholar]
- 49.Biete Sola A, Marruecos Querol J, Calvo Manuel FA, et al. Phase II trial: concurrent radio-chemotherapy with weekly docetaxel for advanced squamous cell carcinoma of head and neck. Clin Transl Oncol. 2007;9:244–50. doi: 10.1007/s12094-007-0047-y. [DOI] [PubMed] [Google Scholar]
- 50.Zeitlin BD, Zeitlin IJ, Nor JE. Expanding circle of inhibition: small-molecule inhibitors of Bcl-2 as anticancer cell and antiangiogenic agents. J Clin Oncol. 2008;26:4180–8. doi: 10.1200/JCO.2007.15.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


