Abstract
The startle response evoked by repeated presentation of a loud acoustic stimulus is regulated by the independent processes of sensitization and habituation. While schizophrenia is associated with information processing impairments, there is conflicting evidence regarding the existence of habituation deficits in schizophrenia patients. Recent clinical evidence, however, indicates that patients with schizophrenia display exaggerated startle sensitization and diminished habituation. Given the linkage between dopaminergic abnormalities and schizophrenia, the goal of the present investigation was to examine the effect of deleting D1 and D2-like dopamine receptors on sensitization and habituation of the acoustic startle reflex in mice. For these experiments, the acoustic startle reflex was assessed in dopamine D1, D2, and D3 receptor wild-type (WT) and knockout (KO) mice on a C57BL/6J background, using a methodology that can measure both sensitization and habituation. Mice lacking the D1 receptor gene displayed enhanced sensitization, along with a decrease in the amount of habituation that occurs in response to repetitive presentations of a startling stimulus. Conversely, the loss of the dopamine D2 or D3 receptor gene produced a sensitization deficit and a significant increase in habituation. The behavioral phenotype exhibited by D1 receptor KO mice is clearly distinct from that of the D2 and D3 receptor KO mice. The findings in D1 receptor KO mice are reminiscent of the abnormalities observed in schizophrenic patients tested in comparable startle paradigms, and indicate that D1 agonists may possess therapeutic efficacy against the information processing deficits associated with schizophrenia.
Keywords: schizophrenia, acoustic startle, sensitization, habituation, mice, dopamine receptors
A variety of species of animals exhibit a transient motor response to loud and unexpected acoustic stimuli (Dodge and Louttit, 1926; Fleshler, 1965). This motor reaction is known as the acoustic startle response, and is mediated by a short-latency four synapse circuit linking primary auditory afferents with spinal motor neurons (Davis et al., 1982). In rats, there is an initial transitory increase in the amplitude of the startle response to repetitive bursts of white noise, followed by a marked decrement of the response (Szabo and Kolta, 1967; Groves and Thompson, 1970). Similar findings have also been reported for humans (Davis and Heninger, 1972). To explain this phenomenon, Groves and Thompson (1970) proposed that two distinct and independent processes govern the behavioral response to repetitive sensory stimulation: (1) an incremental process called sensitization, and (2) a decremental process called habituation. According to this dual-process theory of response habituation, sensitization occurs at the beginning of the test session and is responsible for the transitory increase in response amplitude, whereas habituation occurs throughout the test session and is responsible for the delayed response decrement. In this theoretical framework, the process of sensitization involves stimulus-induced changes in the level of arousal and is dependent on the aversiveness of the stimulus. However, sensitization gradually wanes because the salience of the stimulus decreases with repeated presentation, leaving the habituation process unopposed.
There is considerable evidence that attentional and information-processing deficits are central features of schizophrenia (Braff, 1985; Braff and Geyer, 1990). Schizophrenic patients display a pronounced inability to filter out irrelevant stimuli (McGhie and Chapman, 1961), resulting in increased distractibility, sensory flooding, and cognitive impairment. It has been reported that schizophrenia is associated with startle reflex habituation deficits that may contribute to the sensory overload observed in these patients (Geyer and Braff, 1987). Geyer and Braff (1982) measured the eyeblink reflex component of the human acoustic startle response and found that schizophrenic patients exhibited marked habituation deficits in comparison with normal volunteers or psychiatric control patients. Likewise, Bolino et al. (1992, 1994) reported that habituation of startle evoked by electrocutaneous stimulation is impaired in schizophrenics relative to healthy controls. Other workers subsequently reported similar findings with electrocutaneously evoked startle (Taiminen et al., 2000) and acoustic startle (Parwani et al., 2000). First-episode, unmedicated schizophrenia patients have also been shown to display substantial acoustic startle reflex habituation deficits (Ludewig et al., 2003), indicating that the changes in habituation observed in earlier studies are specifically related to the psychopathology of the illness and are not attributable to medication effects or the progression of the illness. This conclusion is further supported by the observation that medication status does not appear to influence startle habituation in schizophrenic subjects (Duncan et al., 2003).
A number of other investigations found only weak startle habituation deficits in schizophrenia (Braff et al., 1992), or failed to find significant support for reduced habituation (Braff et al., 1999; Cadenhead et al., 2000; Kumari et al., 2000, 2002; Ludewig et al., 2002; Perry et al., 2002). Given these inconsistent results, it is important to note that none of the studies that reported negative findings were designed to assess habituation, but rather were optimized to detect abnormalities in prepulse inhibition of startle. Habituation was assessed in these studies over blocks of trials, with the average across the first block of trials being used as a measure of the initial level of startle responding. This methodological approach is potentially problematic because it does not take into account the possibility that sensitization may occur within the first block of trials.
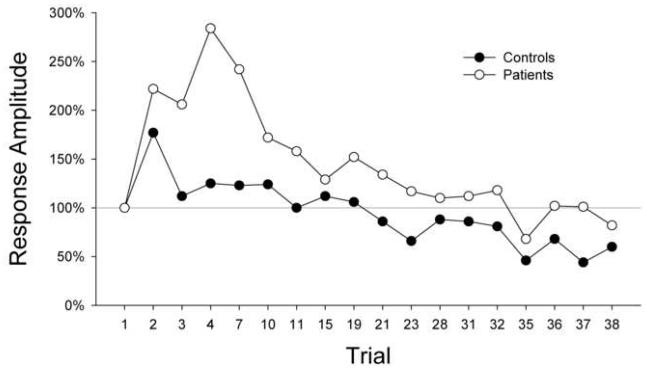
To address these concerns, Meincke et al. (2004) systematically tested schizophrenia patients for startle sensitization and habituation using a trial-by-trial analysis of startle magnitude. The magnitude of the response to the first stimulus trial was used to assess the nonhabituated startle response. This approach revealed that schizophrenia patients, when compared with healthy controls, display both exaggerated sensitization and reduced habituation (Figure 1). It is notable that these workers were unable to detect habituation deficits in schizophrenia patients if the effect of sensitization was not removed from analysis (i.e., if habituation was measured not in relation to the first trial but rather to the first block of trials). These findings confirm that schizophrenia patients show impaired habituation. Furthermore, the results provide one potential explanation for the failure of previous studies to detect habituation deficits, i.e. the exaggerated sensitization displayed by schizophrenic subjects may have masked their habituation impairments.
Figure 1.
The amplitude of the startle responses from 18 healthy control subjects and 34 patients with schizophrenia are shown as group means (percentage scores) in relation to the first trial. Schizophrenia patients displayed greater sensitization and impaired habituation relative to normal control subjects. Redrawn from data presented in Meincke et al. (2004).
Startle Habituation in Animal Models of Schizophrenia
Animal models of schizophrenia provide insight into the neurobiological substrates of the disease and can be used to screen novel compounds for antipsychotic efficacy (Geyer and Moghaddam, 2002). Given that the startle reflex is a cross-species phenomenon, assessment of startle habituation in animals has proven to be a useful tool to study the information processing and attentional disturbances in schizophrenia. One advantage of this behavioral model is that startle habituation can be assessed in humans and animals using similar testing procedures. Furthermore, the fact that these studies narrowly focus on a specific symptom of schizophrenia simplifies the construct and cross-species validation of this animal model (Geyer and Markou, 1995). A number of drugs that produce hallucinogenic effects in humans, including serotonergic hallucinogens such as lysergic acid diethylamide (LSD) and dissociative anesthetics such as phenyclidine (PCP), produce effects that mirror certain aspects of the syndrome of schizophrenia (Vollenweider and Geyer, 2001). Studies have examined the effects of these drugs on startle habituation in rodents (reviewed by Geyer and Braff, 1987). Acute administration of LSD to rats produces impaired habituation of startle evoked by tactile stimuli (Geyer et al., 1978; Braff and Geyer, 1980). PCP also reduces startle habituation when tested in rats (Geyer et al., 1984) and mice (Kokkinidis, 1986; Klamer et al., 2004). The findings with LSD and PCP confirm that these drugs can produce startle habituation deficits in rodents that mirror the clinical findings of impaired startle habituation in schizophrenia. Further, these findings demonstrate the utility of using startle habituation as a specific cross-species model of the information processing deficits in schizophrenia.
There is considerable evidence indicating that dysfunction of dopaminergic systems plays a role in schizophrenia, either as a primary causative factor (Snyder, 1973), or alternatively as a result of cortical or limbic-cortical dysfunction (Csernansky et al., 1993). Dopamine agonists exacerbate the symptoms of schizophrenia (Janowsky et al., 1973; Angrist and Gershon, 1977), and dopamine receptors are the primary targets of antipsychotic agents (Seeman and Lee, 1975). The effects of dopamine agonists and antagonists on startle habituation have been tested in rodents, but the results of these studies have been somewhat inconsistent. The nonselective dopamine agonist apomorphine produces alterations in startle reactivity in rats but does not alter habituation (Geyer et al., 1978). It has also been reported that startle habituation in rats is not influenced by administration of the D2/3 receptor agonist bromocriptine (Swerdlow et al., 2002). Conversely, the catecholamine releaser and indirect dopamine agonist (+)-amphetamine has been shown to produce habituation deficits in mice (Kokkinidis, 1986; Wang et al., 2003; Klamer et al., 2004) when administered at fairly high doses (>2.0 mg/kg). Evidence suggests that in (+)-amphetamine does not alter habituation in rats but does augment sensitization (Davis et al., 1975). The antipsychotic haloperidol, an antagonist at dopaminergic D2 receptors as well as D3 and D4 receptors, alone has no effect on startle habituation, but it can reverse the impairment of startle habituation induced by (+)-amphetamine in mice (Klamer et al., 2004).
It is not currently clear why (+)-amphetamine is capable of altering startle habituation whereas apomorphine is ineffective. It is important to note that in addition to acting as an indirect dopamine agonist, (+)-amphetamine also acts presynaptically to increase the release of norepinephrine. The relatively rapid metabolism of apomorphine in rodents may also complicate the assessment of startle habituation due to confounds associated with the time-course of drug action. Another problem with interpretation of studies using nonselective dopaminergic agents to probe startle habituation is the possibility that there may be oppositional or synergistic interactions between individual dopamine receptor subtypes, as has been demonstrated to occur for prepulse inhibition of startle (Peng et al., 1990) and in other behavioral paradigms (Dreher and Jackson, 1989; Daly and Waddington, 1992; Jutkiewicz and Bergman, 1994; Karasinska et al., 2000). Nonetheless, the results obtained with (+)-amphetamine indicate that alterations in dopaminergic tone can produce changes in startle habituation.
New Data: Startle Habituation in Dopamine Receptor Knockout Mice
An alternative approach toward characterizing the influence of dopamine receptors on startle habituation involves the use of genetically modified mouse strains. In recent years, the mouse has become the predominant mammalian model used in genetic studies of behavior and neuropharmacology (Silver, 1995). Gene-targeting methods have been used to generate a wide variety of knockout (KO) mice, and by studying these animals it is possible to determine the specific function of the deleted gene. The use of genetically engineered KO mice is more specific and thorough than pharmacological methods for blocking receptors, and is not confounded by pharmacologic factors such as ligand receptor selectivity, dose, and pharmacokinetics.
In order to probe how individual dopamine receptors contribute to startle habituation, we have compared acoustic startle reflex habituation between wild-type (WT) mice and mice genetically engineered to lack dopamine D1, D2, or D3 receptors. Sensitization and habituation were assessed in these animals using a trial-by-trial analysis approach similar to that of Meincke et al. (2004). In mice, the startle response to the first stimulus trial is often highly variable (Halberstadt and Geyer, unpublished observations); hence, the magnitude of the second trial was used to measure the non-sensitized, non-habituated acoustic startle response.
MATERIALS AND METHODS
Animals
Male dopamine receptor D1, D2, and D3 WT and KO mice (constitutive gene deletion background mice) were used in these experiments. The D1 mice (B6.129S4-Drd1atm1Lcd/J; Drago et al., 1994) were obtained from the mutant mouse repository at the Jackson Laboratory (Bar Harbor, ME) and backcrossed onto the C57BL/6J background for 10–11 generations. The D1 cohort consisted of 47 WT and 34 KO mice. The D2 mice (B6.129S2-Drd2tm1Low/J; Kelly et al., 1998) were originally generated at the Oregon Health and Science University (OHSU, Vollum Institute, Portland, OR) and backcrossed onto the C57BL/6J background strain for 17 generations. The D2 cohort consisted of 51 WT and 36 KO mice. D3 mutant mice (B6.129S4-Drd3tm1Dac/J; Accili et al., 1996) were also obtained from The Jackson Laboratory, and were backcrossed onto the C57BL/6J background for 13 generations. The D3 cohort consisted of 39 WT and 37 KO mice. The mice were housed (n=4/cage) in a climate-controlled animal colony with a reverse 12 h/12 h light cycle (lights off at 0800 hours). Food and water were available ad libitum, except during behavioral testing. Animals were 3-6 months of age at testing, and were tested during their dark cycle. All animal testing was conducted in accordance with the “Principles of Laboratory Animal Care” NIH guidelines, as approved by the UCSD Animal Care and Use Committee.
Apparatus
Eight startle chambers (SR-LAB, San Diego Instruments, San Diego, CA) were used to measure startle reactivity. Each test chamber consisted of a sound-attenuated, lighted, and ventilated cabinet holding a clear nonrestrictive cylindrical Plexiglas stabilimeter (5 cm inner diameter). A high-frequency loudspeaker mounted inside the chamber produced all acoustic stimuli. The peak and average amplitudes of the startle response were detected by a piezoelectric accelerometer, digitized, and stored on disk. At the onset of the startling stimulus, 65 1-ms readings were recorded, and the average amplitude was used to determine the mouse startle response. A dynamic calibration system (SDI) was used to ensure comparable stabilimeter sensitivity across test chambers, and sound levels were measured in units of dBA sound pressure level (A weighting scale) (Geyer and Dulawa, 2003).
Acoustic Startle Session
The data were derived from the initial baseline characterization of the animals; the mice had not previously been exposed to startling acoustic stimuli. Acoustic startle test sessions consisted of startle trials (PULSE-ALONE) and prepulse trials (PREPULSE+PULSE). The PULSE-ALONE trial consisted of a 40-ms 120-dB pulse of broad-band white noise. PREPULSE+PULSE trials consisted of a 20-ms acoustic prepulse (either 69, 73, or 81 dB, with a 65-dB background noise), an 80 ms delay, and then a 40-ms 120-dB startle pulse (100 ms onset to onset). There was an average of 15 s (range: 8–23 s) between trials. During each inter-trial interval, the movements of the mice were recorded once to measure responding when no stimulus was present (data not shown). Each startle session began with a 10-min acclimation period to a 65-dB broad-band noise that was present continuously throughout the session. Immediately after the acclimation period, five PULSE-ALONE stimuli were presented in a first block (trials 1-5). The second block was designed to assess prepulse inhibition (unpublished data); it contained 14 PULSE-ALONE trials (trials 6, 8, 11, 13, 16, 23, 26, 28, 32, 37, 41, 45, 48, and 49) and 30 PREPULSE+PULSE trials presented in a pseudo-randomized order. The test session ended with five presentations of the PULSE-ALONE stimulus (trials 50-54).
Data Analyses
The average startle magnitude over the 65 ms record window was used for all data analysis. To assess sensitization and habituation, the startle response magnitudes for each PULSE-ALONE trial were expressed as percentage scores in relation to the magnitude of the second PULSE-ALONE trial. The presence of significant sensitization during the third trial was assessed using a one-sample t-test. The presence of significant habituation during the last trial (trial 54) was also assessed using a one-sample t-test. Differences in responses between the WT and KO mice were assessed by means of univariate repeated measures ANOVAs with the between-subject factor gene and the repeated measure PULSE-ALONE trial. Specific post hoc comparisons were done using Tukey's studentized range method. Significance was demonstrated by surpassing an alpha level of 0.05.
RESULTS
Dopamine D1 mice
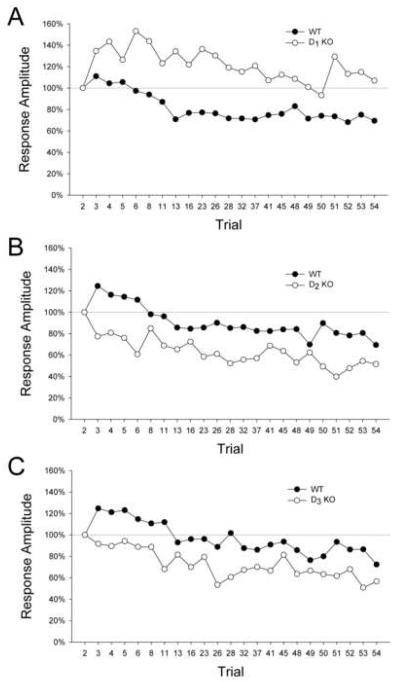
Startle magnitude values relative to the second trial are illustrated in Figure 2A. There was a significant main effect of gene (F(1,79)=21.14, p<0.0001). No significant change in the startle response occurred from the second to the third trial in the WT mice, indicating that sensitization did not occur in these animals. The D1 KO mice did display significant sensitization (mean ± S.E.M.: 134.6 ± 11.0%; t(33)=3.15, p<0.004). For WT mice, the acoustic startle response during the last startle trial (trial 54) (mean ± S.E.M .: 69.4±8.2%) was significantly reduced compared with the second trial, indicating that habituation occurred during the test session (t(46)=3.72, p=0.0005). By contrast, there was no evidence of habituation in the D1 KO mice (mean ± S.E.M.: 106.9 ± 10.4%; t(33)=0.67, n.s.).
Figure 2.
Mean startle response magnitudes (percentage scores) in relation to the second startle trial for dopamine D1, D2, and D3 receptor mutant mice. (A) D1 KO mice display exaggerated sensitization and deficient habituation compared with D1 WT mice. (B) D2 KO mice show attenuated sensitization and increased habituation compared with D2 WT mice. (C) D3 KO mice also display attenuated sensitization and increased habituation compared with D3 WT mice.
Dopamine D2 mice
There was a significant main effect of gene (F(1,74)=7.98, p<0.007). WT mice displayed significant sensitization (mean ± S.E.M.: 124.5 ± 10.5%; t(39)=2.34, p<0.03; Fig. 2B). Conversely, no significant sensitization effect was observed in D2 KO mice; in fact, these animals displayed significant habituation between the second and third trial (mean ± S.E.M.: 77.4 ± 8.9%; t(33)=−2.55, p<0.02). Significant habituation was observed for both WT mice (mean ± S.E.M.: 77.4 ± 8.9%; t(39)=−4.42, p=0.0001) and D2 KO mice (mean ± S.E.M.: 51.6 ± 7.0%; t(33)=−6.93, p<0.0001) in the last startle trial. Post hoc analysis (Tukey's test) revealed that the D2 KO mice displayed significantly more habituation than their WT littermates (p<0.05, 0.01).
Dopamine D3 mice
There was a significant main effect of gene (F(1,72)=14.68, p=0.0003). Significant sensitization was observed in the WT mice (mean ± S.E.M.: 124.7 ± 11.0%; t(38)=2.24, p<0.04; Fig. 2C) but not in the D3 KO mice (mean ± S.E.M.: 90.6 ± 8.0%; t(36)=−1.04, n.s.). Significant habituation was observed in both WT mice (mean ± S.E.M.: 72.4 ± 7.8%; t(38)=−3.52, p<0.002) and D3 KO mice (mean ± S.E.M.: 56.6 ± 6.8%; t(36)=−6.36, p<0.0001) during the last startle trial. Post hoc analysis (Tukey's test) revealed that the D3 KO mice displayed significantly more habituation than their WT littermates (p<0.05).
DISCUSSION
The goal of the present investigation was to assess the effect of genetic deletion of dopamine D1, D2, and D3 receptors on sensitization and habituation of the acoustic startle reflex. We demonstrated that loss of the D1 receptor gene produces an exaggerated sensitization effect, as well as a significant habituation deficit, similar to the abnormalities observed in patients with schizophrenia. Conversely, the loss of the D2 or D3 receptor gene produces a significant increase in the amount of habituation that occurs in response to repetitive presentation of a startling stimulus, and impairs the process of sensitization. Hence, the behavioral phenotype exhibited by D1 receptor KO mice is clearly distinct from that of the D2 and D3 receptor KO mice.
Dopamine binds to two subclasses of G protein-coupled receptors: D1-like (including D1 and D5) and D2-like (including D2, D3, and D4) (reviewed by Missale et al., 1998). The present findings indicate that the two dopamine receptor subclasses exert opposing influences on the processes of sensitization and habituation. Sensitization was enhanced in the D1 receptor KO mice and abolished in the D2 and D3 receptor KO mice. Therefore, we hypothesize that the D1 receptor exerts an inhibitory regulatory influence over sensitization whereas the two members of the D2-like family of dopamine receptors play a critical role in the generation of sensitization. We also found that habituation of the startle response was impaired in mice lacking the dopamine D1 receptor and augmented in mice lacking either D2 or D3 receptors. These findings indicate that the dopamine D1 receptor plays a role in the generation of habituation to repetitive stimuli whereas D2 and D3 receptors act to inhibit the habituation process.
Startle habituation has typically been assessed in previous investigations by comparing blocks of startle trials. For the present experiments, the startle response was analyzed using a trial-by-trial approach to test for the presence of both habituation and sensitization. A trial-by-trial analysis of our data revealed that D1, D2, and D3 KO mice display marked differences in sensitization levels compared with their WT littermates. It is important to note that a block analysis of the data would not have been able to detect these sensitization effects because they occur during the first few startle trials. We also found that D1, D2, and D3 KO mice display marked difference in habituation levels compared with their WT littermates. These findings are in clear contrast with the results of a number of previous investigations demonstrating that startle habituation is unaffected by administration of either agonists (Geyer et al., 1978; Swerdlow et al., 2002; Giakoumaki et al., 2007) or antagonists (Kumari et al., 1998; Liechti et al., 2001; Klamer et al., 2004; Oranje et al., 2004) of dopamine receptors. The fact that dopaminergic mechanisms modulate sensitization and habituation simultaneously provides one potential explanation for those earlier negative findings. The data from the dopamine receptor KO mice indicate that the dopaminergic system regulates sensitization and habituation in an antithetical manner—for example, in the D2 receptor KO mice sensitization was reduced whereas habituation was augmented. Due to the nature of the block analysis technique, the presence of sensitization will yield an overestimation of the magnitude of non-habituated startle; therefore, when a block analysis is performed there is a tendency for changes in sensitization to be offset by antithetical effects on habituation. Such an effect may explain why many studies of dopaminergic agents failed to detect effects on startle habituation. Thus, our data provide strong support for calculating habituation using a trial-by-trial approach that provides independent measures of habituation and sensitization. Indeed, a similar approach was successfully employed by Meincke et al. (2004) to detect alterations in sensitization and habituation in patients with schizophrenia.
It has been proposed that schizophrenia involves a subcortical/cortical imbalance of dopaminergic transmission, with a relative hyperdopaminergic state in the striatum and a hypodopaminergic state in cortex (Abi-Dargham and Moore, 2003; Abi-Dargham, 2004; Goldman-Rakic et al., 2004). The cortical dopaminergic deficit is associated with a reduction in D1 receptor signaling, an effect postulated to contribute to the cognitive deficits associated with schizophrenia. There are reports indicating that D1 KO mice display behavioral abnormalities that parallel some of the functional deficits associated with schizophrenia. For example, D1 KO mice display spatial learning and memory deficits (El-Ghundi et al., 1999), impairments that are analogous to the spatial working memory deficit observed in schizophrenia (Piskulic et al., 2007). Dopamine D1 KO mice also show reduced sensitivity to food-induced reinforcement (El-Ghundi et al., 2003), and impaired response initiation (Smith et al., 1998). Likewise, schizophrenia patients often display a significant motivational deficit (Schmand et al., 1994). The present results demonstrate that mice lacking the dopamine D1 receptor display substantial sensitization that was not observed in WT mice, and little or no habituation was observed in those animals. Thus, deletion of the D1 receptor gene produces a behavioral phenotype that mirrors the enhanced sensitization and impaired habituation that have been observed in schizophrenia patients (Geyer and Braff, 1982; Bolino et al., 1992, 1994; Parwani et al., 2000; Taiminen et al., 2000; Ludewig et al., 2003; Meincke et al., 2004).
One important implication of our findings with D1 KO mice is that dysfunction of the dopaminergic system in schizophrenia could potentially provoke startle sensitization and habituation abnormalities as a consequence of reduced activation of D1 receptors by endogenous dopamine. It has been suggested that agonists at dopamine D1 receptors might have therapeutic effects on the cognitive and negative symptoms of the disorder that are not treated adequately by existing antipsychotic drugs (Castner et al., 2000; Goldman-Rakic et al., 2004). Typical antipsychotic drugs that primarily block dopamine D2 receptors also appear to be ineffective in ameliorating abnormalities in startle habituation or prepulse inhibition in patients with schizophrenia (Duncan et al., 2003; Geyer, 2006). The present findings prompt the speculation that appropriate startle habituation paradigms in mice might assist in identifying effects of dopamine D1 receptor agonists that have potential efficacy in the treatment of cognitive and negative symptoms of schizophrenia.
Acknowledgements
These studies were supported by the National Institute on Drug Abuse (DA02925), the National Institute of Mental Health (MH061326, MH071916), and the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center. M.A. Geyer holds an equity interest in San Diego Instruments. We thank Mahalah R. Buell, James M. Doherty and Virginia L. Masten for technical assistance. We would also like to thank Dr. Malcolm J. Low for supplying the genetically modified mouse strains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proceedings of the National Academy of Sciences of the USA. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrist B, Gershon S. Clinical response to several dopamine agonists in schizophrenic and nonschizophrenic subjects. Advances in Biochemical Psychopharmacology. 1977;16:677–680. [PubMed] [Google Scholar]
- Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. International Journal of Neuropsychopharmacology. 2004;7(Suppl 1):S1–S5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9:404–416. doi: 10.1177/1073858403252674. [DOI] [PubMed] [Google Scholar]
- Bolino F, Di Michele V, Di Cicco L, Manna V, Daneluzzo E, Casacchia M. Sensorimotor gating and habituation evoked by electro-cutaneous stimulation in schizophrenia. Biological Psychiatry. 1994;36:670–679. doi: 10.1016/0006-3223(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Bolino F, Manna V, Di Cicco L, Di Michele V, Daneluzzo E, Rossi A, Casacchia M. Startle reflex habituation in functional psychoses: a controlled study. Neuroscience Letters. 1992;145:126–128. doi: 10.1016/0304-3940(92)90002-o. [DOI] [PubMed] [Google Scholar]
- Braff DL. Attention, habituation and information processing in psychiatric disorders. In: Michels B, Cavenar JO, Brodie HK, Cooper AM, Guze SB, Judd LL, Klerman GL, Solnit AJ, editors. Psychiatry. Vol. 3. Lippincott; Philadelphia: 1985. pp. 1–12. [Google Scholar]
- Braff DL, Geyer MA. Acute and chronic LSD effects on rat startle: data supporting an LSD--rat model of schizophrenia. Biological Psychiatry. 1980;15:909–916. [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: human and animal model studies. Archives of General Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Archives of General Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. American Journal of Psychiatry. 1999;156:596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. American Journal of Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Murphy GM, Faustman WO. Limbic/mesolimbic connections and the pathogenesis of schizophrenia. Biological Psychiatry. 1993;30:383–400. doi: 10.1016/0006-3223(91)90295-w. [DOI] [PubMed] [Google Scholar]
- Daly SA, Waddington JL. Two directions of dopamine D1/D2 receptor interaction in studies of behavioral regulation: a finding to four new, selective dopamine D1 receptor antagonists. European Journal of Pharmacology. 1992;213:251–258. doi: 10.1016/0014-2999(92)90689-2. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. Journal of Neuroscience. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Heninger GR. Comparision of response plasticity between the eyeblink and vertex potential in humans. Electroencephalography and Clinical Neurophysiology. 1972;33:283–293. doi: 10.1016/0013-4694(72)90155-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Svensson TH, Aghajanian GK. Effects of d- and l-amphetamine on habituation and sensitization of the acoustic startle response in rats. Psychopharmacologia. 1975;43:1–11. doi: 10.1007/BF00437607. [DOI] [PubMed] [Google Scholar]
- Dodge R, Louttit CM. Modification of the pattern of the guinea pig's reflex response to noise. Journal of Comparative Psychology. 1926;60:267–285. [Google Scholar]
- Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, et al. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proceedings of the National Academy of Sciences of the USA. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JK, Jackson DM. Role of D1 and D2 dopamine receptors in mediating locomotor activity elicited from the nucleus accumbens in rats. Brain Research. 1989;487:267–277. doi: 10.1016/0006-8993(89)90831-7. [DOI] [PubMed] [Google Scholar]
- Duncan EJ, Szilagyi S, Efferen TR, Schwartz MP, Parwani A, Chakravorty S, Madonick SH, Kunzova A, Harmon JW, Angrist B, Gonzenbach S, Rotrosen JP. Effect of treatment status on prepulse inhibition of acoustic startle in schizophrenia. Psychopharmacology. 2003;167:63–71. doi: 10.1007/s00213-002-1372-z. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, Fletcher PJ, Drago J, Sibley DR, O'Dowd BF, George SR. Spatial learning deficit in dopamine D1 receptor knockout mice. European Journal of Pharmacology. 1999;383:95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, O'Dowd BF, Erclik M, George SR. Attenuation of sucrose reinforcement in dopamine D1 receptor deficient mice. European Journal of Neuroscience. 2003;17:851–862. doi: 10.1046/j.1460-9568.2003.02496.x. [DOI] [PubMed] [Google Scholar]
- Fleshler M. Adequate acoustic stimulus for the startle reflex in the rat. Journal of Comparative Physiology and Psychology. 1965;60:200–207. doi: 10.1037/h0022318. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Are cross-species measures of sensorimotor gating useful for the discovery of procognitive cotreatments for schizophrenia? Dialogues in Clinical Neuroscience. 2006;8:9–16. doi: 10.31887/DCNS.2006.8.1/mgeyer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Braff DL. Habituation of the blink reflex in normals and schizophrenic patients. Psychophysiology. 1982;19:1–6. doi: 10.1111/j.1469-8986.1982.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophrenia Bulletin. 1987;13:643–668. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Dulawa SC. Assessment of murine startle reactivity, prepulse inhibition, and habituation. In: Crawley JN, Skolnick P, editors. Current Protocols in Neuroscience. John Wiley & Sons; New York: 2003. pp. 8.17.1–8.17.15. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven; New York: 1995. pp. 787–798. [Google Scholar]
- Geyer MA, Moghaddam B. Animal models relevant to schizophrenia disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams & Wilkins; Philadelphia: 2002. pp. 689–701. [Google Scholar]
- Geyer MA, Petersen LR, Rose GJ, Horwitt DD, Light RK, Adams LM, Zook JA, Hawkins RL, Mandell AJ. The effects of lysergic acid diethylamide and mescaline-derived hallucinogens on sensory-integrative function: tactile startle. Journal of Pharmacology and Experimental Therapeutics. 1978;207:837–847. [PubMed] [Google Scholar]
- Geyer MA, Segal DS, Greenberg BD. Increased startle responding in rats treated with phencyclidine. Neurobehavioral Toxicology and Teratology. 1984;6:161–164. [PubMed] [Google Scholar]
- Giakoumaki SG, Roussos P, Frangou S, Bitsios P. Disruption of prepulse inhibition of the startle reflex by the preferential D3 agonist ropinirole in healthy males. Psychopharmacology. 2007;194:289–295. doi: 10.1007/s00213-007-0843-7. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TS, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia insights for cognitive dysfunction. Psychopharmacology. 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousel MK, Davis JM, Sekerke HJ. Provocation of schizophrenic symptoms by intravenous administration of methylphenidate. Archives of Geneneral Psychiatry. 1973;28:185–191. doi: 10.1001/archpsyc.1973.01750320023004. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Bergman J. Effects of dopamine D1 ligands on eye blinking in monkeys: efficacy, antagonism, and D1/D2 interactions. Journal of Pharmacology and Experimental Therapeutics. 1994;311:1008–1015. doi: 10.1124/jpet.104.071092. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, El-Ghundi M, Fletcher PJ, O'Dowd BF. Modification of dopamine D1 receptor knockout phenotype in mice lacking both dopamine D1 and D3 receptors. European Journal of Pharmacology. 2000;399:171–181. doi: 10.1016/s0014-2999(00)00347-2. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. Journal of Neuroscience. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamer D, Pålsson E, Revesz A, Engel JA, Svensson L. Habituation of acoustic startle is disrupted by psychotomimetic drugs: differential dependence on dopaminergic and nitric oxide modulatory mechanisms. Psychopharmacology. 2004;176:440–450. doi: 10.1007/s00213-004-1901-z. [DOI] [PubMed] [Google Scholar]
- Kokkinidis L. Sensitization to amphetamine and tolerance to cocaine and phencyclidine stimulation in mice. Pharmacology Biochemistry and Behavior. 1986;25:1175–1180. doi: 10.1016/0091-3057(86)90107-3. [DOI] [PubMed] [Google Scholar]
- Kumari V, Mulligan OF, Cotter PA, Poon L, Toone BK, Checkley SA, Gray JA. Effects of single oral administrations of haloperidol and d-amphetamine on prepulse inhibition of the acoustic startle reflex in healthy male volunteers. Behavioral Pharmacology. 1998;9:567–576. doi: 10.1097/00008877-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Mathew VM, Sharma T. Prepulse inhibition of the startle response in men with schizophrenia: effects of age of onset of illness, symptoms, and medication. Archives of General Psychiatry. 2000;57:609–614. doi: 10.1001/archpsyc.57.6.609. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Prepulse inhibition of the startle response in risperidone-treated patients: comparison with typical antipsychotics. Schizophrenia Research. 2002;55:139–146. doi: 10.1016/s0920-9964(01)00276-6. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Geyer MA, Hell D, Vollenweider FX. Effects of MDMA (ecstasy) on prepulse inhibition and habituation of startle in humans after pretreatment with citalopram, haloperidol, or ketanserin. Neuropsychopharmacology. 2001;24:240–252. doi: 10.1016/S0893-133X(00)00199-8. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Vollenweider FX. Stability of the acoustic startle reflex, prepulse inhibition, and habituation in schizophrenia. Schizophrenia Research. 2002;55:129–137. doi: 10.1016/s0920-9964(01)00198-0. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biological Psychiatry. 2003;54:121–128. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. British Journal of Medical Psychology. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Meincke U, Light GA, Geyer MA, Braff DL, Gouzoulis-Mayfrank E. Sensitization and habituation of the acoustic startle reflex in patients with schizophrenia. Psychiatry Research. 2004;126:51–61. doi: 10.1016/j.psychres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological Reviews. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Oranje B, Kahn RS, Kemner C, Verbaten MN. Modulating sensorimotor gating in healthy volunteers: the effects of desipramine and haloperidol. Psychiatry Research. 2004;127:195–205. doi: 10.1016/j.psychres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, Sanfilipo M, Chappell PB, Chakravorty S, Gonzenbach S, Ko GN, Rotrosen JP. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biological Psychiatry. 2000;47:662–669. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Peng RY, Mansbach RS, Braff DL, Geyer MA. A D2 dopamine receptor agonist disrupts sensorimotor gating in rats: Implications for dopaminergic abnormalities in schizophrenia. Neuropsychopharmacology. 1990;3:211–218. [PubMed] [Google Scholar]
- Perry W, Feifel D, Minassian A, Bhattacharjie I, Braff DL. Information processing deficits in acutely psychotic schizophrenia patients medicated and unmedicated at the time of admission. American Journal of Psychiatry. 2002;159:1375–1381. doi: 10.1176/appi.ajp.159.8.1375. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Olver JS, Norman TR, Maruff P. Behavioural studies of spatial working memory dysfunction in schizophrenia: a quantitative literature review. Psychiatry Research. 2007;150:111–121. doi: 10.1016/j.psychres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Schmand B, Kuipers T, Van der Gaag M, Bosveld J, Bulthuis F, Jellema M. Cognitive disorders and negative symptoms as correlates of motivational deficits in psychotic patients. Psychological Medicine. 1994;24:869–884. doi: 10.1017/s0033291700028968. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- Silver LM. Mouse Genetics: Concepts and Applications. Oxford University Press; New York: 1995. [Google Scholar]
- Smith DR, Striplin CD, Geller AM, Mailman RB, Drago J, Lawler CP, Gallagher M. Behavioural assessment of mice lacking D1A dopamine receptors. Neuroscience. 1998;86:135–146. doi: 10.1016/s0306-4522(97)00608-8. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Amphetamine psychosis: a “model” schizophrenia mediated by catecholamines. American Journal of Psychiatry. 1973;130:61–67. doi: 10.1176/ajp.130.1.61. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Stephany N, Shoemaker JM, Ross L, Wasserman LC, Talledo J, Auerbach PP. Effects of amantadine and bromocriptine on startle and sensorimotor gating: parametric studies and cross-species comparisons. Psychopharmacology. 2002;164:82–92. doi: 10.1007/s00213-002-1172-5. [DOI] [PubMed] [Google Scholar]
- Szabo I, Kolta P. Transitory increase of the acoustic startle reaction during habituation. Acta Physiologica Academiae Scientiarum Hungaricae. 1967;31:51–56. [PubMed] [Google Scholar]
- Taiminen T, Jääskeläinen S, Ilonen T, Meyer H, Karlsson H, Lauerma H, Leinone n KM, Wallenius E, Kaljonen A, Salokangas RK. Habituation of the blink reflex in first-episode schizophrenia, psychotic depression and non-psychotic depression. Schizophrenia Research. 2000;44:69–79. doi: 10.1016/s0920-9964(99)00140-1. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Research Bulletin. 2001;56:495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- Wang JH, Short J, Ledent C, Lawrence AJ, van den Buuse M. Reduced startle habituation and prepulse inhibition in mice lacking the adenosine A2A receptor. Behavioral Brain Research. 2003;143:201–207. doi: 10.1016/s0166-4328(03)00036-6. [DOI] [PubMed] [Google Scholar]