Abstract
Adolescence is a critical phase of active brain development often characterized by the initiation of marijuana (Cannabis sativa) use. Limited information is known regarding the endogenous cannabinoid system of the adolescent brain as well as related neurotransmitters that appear sensitive to cannabis exposure. We recently observed that adult rats pre-exposed to Δ-9-tetrahydrocannabinol (THC) during adolescence self-administered higher amounts of heroin and had selective impairments of the enkephalin opioid system within the nucleus accumbens (NAc) implicated in reward-related behavior. To explore the ontogeny of the cannabinoid and opioid neuronal systems in association with adolescence THC exposure, rats were examined at different adolescent stages during an intermittent THC paradigm (1.5 mg/kg i.p. every third day) from postnatal days (PNDs) 28–49. Rat brains were examined 24 hours after injection at PND 29 (early adolescence), PND 38 (mid adolescence) and PND 50 (late adolescence) and analyzed for endocannabinoids (anandamide and 2-arachidonoylglycerol), Met-enkephalin, cannabinoid CB1 receptors and µ opioid receptors (µOR) in the NAc, caudate-putamen and prefrontal cortex (PFC). Of the markers studied, the endocannabinoid levels had the most robust alterations throughout adolescence and were specific to the PFC and NAc. Normal correlations between anandamide and 2-arachidonoylglycerol concentrations in the NAc (positive) and PFC (negative) were reversed by THC. Other significant THC-induced effects were confined to the NAc — increased anandamide, decreased Met-enkephalin and decreased µORs. These findings emphasize the dynamic nature of the mesocorticolimbic endocannabinoid system during adolescence and the selective mesocorticolimbic disturbance as a consequence of adolescent cannabis exposure.
Keywords: anandamide, 2-arachidonoylglycerol, mu opioid receptor, enkephalin, ontogeny, nucleus accumbens
Introduction
It is now well accepted that adolescence is an important period of active neural development (Rice and Barone, 2000, Charmandari et al., 2003) that may be particularly vulnerable to external insults and internal (e.g., hormonal and emotional) stress. Of growing concern is the fact that initiation of drug experimentation normally begins during adolescence (SAMHSA, 2007). Several clinical studies have linked repeated early cannabis exposure with the development of schizophrenia (Arseneault et al., 2002, van Os et al., 2002, Fergusson et al., 2003, Green et al., 2004, Veen et al., 2004) and an increased risk of other illicit drug use (Yamaguchi and Kandel, 1984, Fergusson and Horwood, 2000, Lynskey et al., 2003, Agrawal et al., 2004). While epidemiological studies have tried to evaluate underlying factors that may predispose individuals to use cannabis as well as other illicit drugs, e.g. genetic predisposition, peer-pressure, drug availability and risk-taking behavior (e.g. Hall and Lynskey, 2005), experimental animal studies have helped to provide insights into direct neurobiological alterations in the brain induced by cannabis. These alterations may contribute to the disturbance of reward neural pathways that influence the progression to future drug abuse. We recently observed in an adolescent rat model (Ellgren et al., 2007) that animals exposed to delta-9-tetrahydrocannabinol (THC; the main psychoactive ingredient of cannabis) at an early age resulted in higher intravenous heroin self-administration in adulthood. Moreover, molecular studies revealed specific alterations in the enkephalin opioid system within brain areas implicated in reward-related behavior, e.g. increased expression of proenkephalin (PENK) mRNA in the nucleus accumbens (NAc) shell and increased µ opioid receptor (µOR) activity in the ventral tegmental area. The time course of THC effects on the opioid system as well on the endogenous cannabinoids is however unknown. The aim of the present study was to explore potential neurochemical alterations during adolescent brain development in association with THC exposure that could account for the opioid reward-related disturbances observed previously in adult rats with adolescent THC use. To date, most developmental studies of the cannabinoid (Berrendero et al., 1999, Fernandez-Ruiz et al., 2000, Perez-Rosado et al., 2000, Ade and Lovinger, 2007) and opioid (Xia and Haddad, 1991, Brana et al., 1995, Georges et al., 1998) systems have focused on the embryonic and early postnatal stages. As such, another important aim of the current investigation was to assess the ontogeny of the cannabinoid and opioid systems in reward-related brain areas in the adolescent rat brain. The levels of cannabinoid receptor type 1 (CB1), mu opioid receptor (µOR), endocannabinoids (anandamide and 2-arachidonoylglycerol; 2-AG) and Met-enkephalin were analyzed in subregions of the striatum — NAc shell, NAc core, and caudate-putamen — of THC or vehicle exposed rats at ages corresponding to early (postnatal day; PND; 29), mid (PND 38) and late (PND 50) adolescence. Given the importance of the prefrontal cortex (PFC) in cognitive function and its protracted development until late adolescence/early adulthood, this cortical area was also examined
Methods
Animals
Male Long-Evan rats (21 days old) were obtained from M&B Taconic, New York, USA. They were group-housed in a temperature-controlled environment on a reversed 12-h light/dark cycle (lights off at 11 a.m.) with ad libitum access to food and water. The rats were allowed to acclimate in their new environment and were handled daily for one week before the start of the experiment. All animal experiments were performed in accordance with the guidelines of The Swedish National Board for Laboratory Animals under a protocol approved by the Ethical Committee of Northern Stockholm, Sweden.
Drugs
THC (10 mg/ml in ethanol solution; Sigma-Aldrich, Sweden) was evaporated under nitrogen gas, dissolved in 0.9% NaCl with 0.3% Tween 80 to a final concentration of 0.75 mg/ml THC and administered intra-peritoneally (i.p.) at a volume of 2 ml/kg.
Adolescent cannabis exposure
Using the treatment paradigm previously described (Ellgren et al., 2007), young rats were exposed to THC (1.5 mg/kg i.p.) or vehicle (0.9% NaCl with 0.3% Tween 80) during PND 28 to 49. The age span chosen extends beyond the prototypic adolescent period (days 28–42), which starts around 10 days before puberty and ends a few days after (Spear, 2000), to reach late adolescence on the border to young adulthood (Andersen, 2003). The animals were given one injection every third day, for a total of eight injections; this paradigm was used in order to mimic the intermittent cannabis use normally seen in teenagers. The rats were sacrificed 24 hours after the first (PND 29, early adolescence), fourth (PND 38, mid adolescence) and eighth (PND 50, late adolescence) injections respectively. Five animals/group were used for each time point studied. The PFC, caudate-putamen, NAc core, and NAc shell were collected by manual dissection on dry-ice according to the atlas of Paxinos and Watson (1997) and was immediately stored in −70°C. Tissue from the left hemisphere was used in the analysis of endogenous cannabinoids, whereas tissue from the right hemisphere was used for analysis of receptor (CB1 and µOR) and Met-enkephalin peptide levels.
Liquid chromatography- mass spectrometry (LC-MS) analysis of endogenous cannabinoids
Anandamide and 2-AG were extracted from the brain tissues according to Kingsley and Marnett (2003). In brief, the brain tissue was homogenized in ethylacetate:hexane (9:1; 40 ml per gram of tissue) containing 500 pmol of each internal standard. Lipid extracts were purified via solid phase extraction (SPE) and the eluate were analyzed by LC-MS (Giuffrida et al., 2000, Degn et al., 2007) using a Hewlett Packard 1100 Series HPLC/MS system equipped with a Phenomenex HyperClone ODS column. Synthetic standards of anandamide and 2-AG, 2H8-AEA and 2H8-2-AG were obtained from Cayman Chemical (Ann Arbor, MI, USA). Quantitative analyses were performed by positive electrospray detecting diagnostic ions (protonated molecular ions [M + H]+ and sodium adducts of molecular ions [M + Na]+) in selected ion monitoring (SIM) mode. Data analysis was performed using HP Chemstation software.
Fluorescent immunosorbent assay of CB1 and µOR density
Cell membranes were prepared for detection of CB1 and µOR density. In brief, the dissected tissue was homogenized in 50mM Tris-HCl (pH 7.4) + 10 % sucrose and 1X protease and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO, USA). Following a 10-min centrifugation (20,000 g at 4°C), the pellet was resuspended in 50mM Tris-HCl (pH 7.4) with protease and phosphatase inhibitors. After 30 min incubation on ice, the samples were centrifuged (20,000 g at 4°C for 20 min) and resuspended in 50mM Tris-HCl (pH 7.4) with protease and phosphatase inhibitors. Three µg membrane proteins were plated on a high binding 96 well ELISA plates (Fisher scientific research, NJ, USA) and dried overnight at room temperature. The following day, the membranes were washed with phosphate buffered saline (PBS) and blocked with 3 % normal goat serum in PBS for 1 hour at room temperature. Primary antibody solutions (the µOR and CB1 antibodies were directed against the activated N terminus conformation state of the receptor, [Gupta et al., 2006, Heimann et al., 2007]; 1:500 dilution) were added to the wells followed by incubation at 4°C overnight. The plates were then washed three times with PBS before a 1-hour incubation at 37°C with secondary antibody solution (fluorescence-linked goat anti-rabbit IgG; IRDye, Li-Cor biosciences, Nebraska, USA), diluted 1:2000. The plates were again washed three times with PBS before the fluorescence (corrected integrated intensity; I.I.) at 700 or 800 nm emission wavelength was determined for each well using the Odyssey Infrared Imaging System (Li-Cor biosciences).
Radioimmunoassay detecting immunoreactive (ir-) Met-enkephalin
Ir-Met-enkephalin was analyzed in the supernatant collected from the membrane purification. Approximately 10–30 µl of sample supernatant was analyzed for ir-Met-enkephalin using the Met-enkephalin radioimmunoassay (RIA). For this, the samples were incubated with a 1:2000 dilution of Met-enkephalin antiserum (Bachem Bioscience Inc, Philadelphia, USA) in a RIA buffer (10 mM Tris-Cl buffer pH 7.5 containing 0.1% gelatin; Bio-Rad, California, USA; 0.1% bovine serum albumin; protease-free; Sigma, St. Louis, USA; 0.1% Triton X-100 and 0.02% sodium azide). On the following day, 125I-Met-enkephalin (~10,000 cpm/tube; Bachem Bioscience Inc.) was added and incubated overnight at 4°C. To terminate the reaction, 100 µl of goat anti-rabbit globulin and 100 µl of normal rabbit serum were added in RIA buffer (without gelatin). The antigen-antibody complex was separated from the unbound radioligand by centrifugation and the radioactivity in the pellet was measured in a gamma counter (Perkin Elmer Life Sciences, Shelton, CT, USA). A standard curve consisting of 0–1000nM Met-enkephalin was used to calculate the amount of ir-Met-enkephalin in each sample.
Statistics
Data was analyzed with two-way analysis of variance (age × drug treatment) followed by Tukey post hoc analysis when appropriate. Pearson correlation analyses were evaluated for the levels of the endocannabinoids. Statistical significance was set as P <0.05 and trends considered for P <0.10.
Results
Endogenous cannabinoids
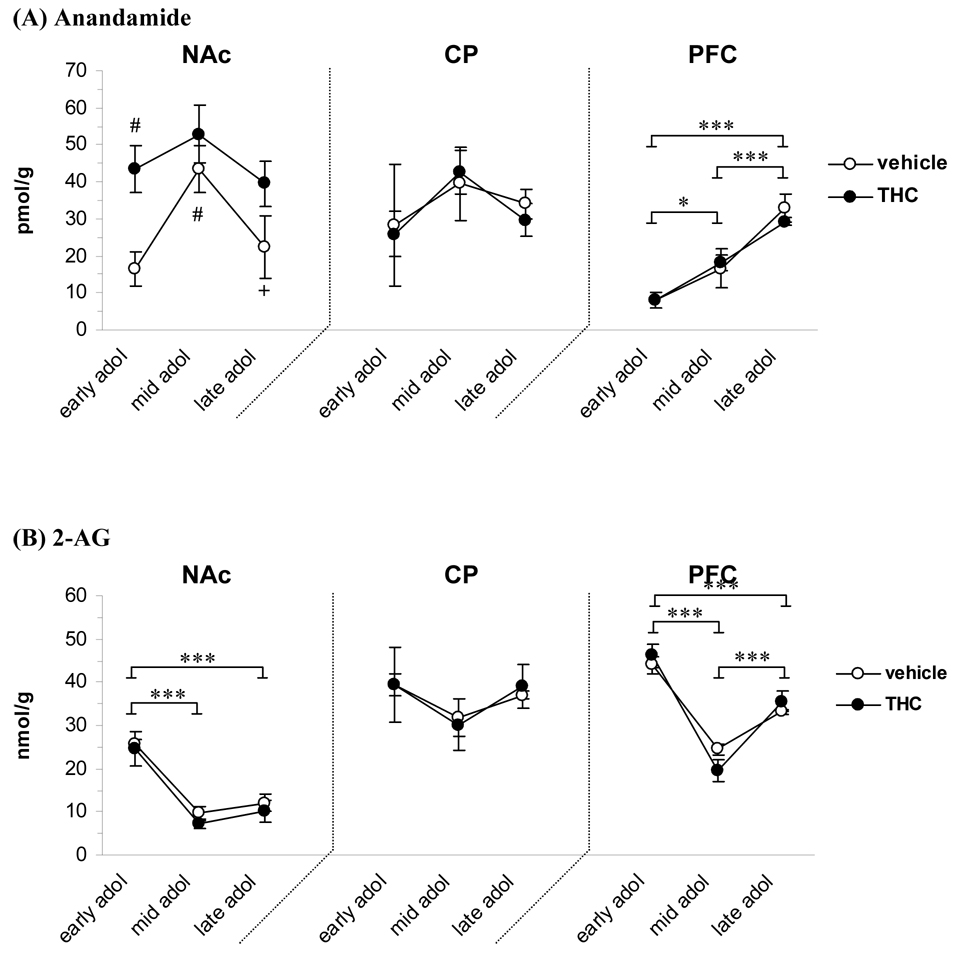
Concentrations of endogenous cannabinoids measured in the NAc, caudate-putamen and PFC during the adolescent period and the effect of THC exposure are shown in figure 1. The amount of anandamide in the NAc was found to depend on both age [F(2, 23)=4.43, P<0.05] and THC treatment [F(1, 23)=10.51, P<0.01]. Anandamide levels peaked at mid adolescence in vehicle treated animals, where it was almost three times (165%) higher than at the early adolescence stage (43.5 vs. 16.4 pmol/g, P<0.05) and almost double the amount in the late (43.5 vs. 22.4 pmol/g, P<0.05). The first injection of THC caused a strong elevation (165%) in the level of anandamide (43.5 vs. 16.4 pmol/g in the corresponding vehicle control, P<0.01) and there was still an increased trend after the full treatment period of eight injections (39.4 vs. 22.4 pmol/g; 76% increase, P=0.08). The levels of 2-AG in the NAc was dependent on age [F(2, 23)=28.45, P<0.00001] and post hoc analysis of merged treatment groups (no significant group difference) revealed that there was a significant decrease in 2-AG levels from early to mid adolescence (24.4 vs. 7.2 nmol/g, P<0.00001). The 2-AG levels were still lower in late adolescence (10.1 vs. 24.4 nmol/g in early adolescence, P<0.00001). In the caudate-putamen, anandamide and 2-AG levels were not altered by either age or treatment.
Figure 1.
Concentration of anandamide (A) and 2-AG (B) at different developmental time points during adolescence and the effect of THC exposure (1.5 mg/kg every third day). Early, mid and late adolescence groups had received 1, 4 or 8 injections of THC or vehicle, respectively. Data is shown as mean ± SEM. * significant difference between age groups, P<0.05; *** P<0.001. #, significantly different vs vehicle early adolescence, P<0.05. +, significantly different vs vehicle mid adolescence, P<0.05. (n=5). CP, caudate-putamen; NAc, nucleus accumbens; PFC, prefrontal cortex.
In the PFC, both anandamide and 2-AG levels were markedly dependent on age ([F(2, 23)=34.58, P<0.00001] and [F(2, 23)=57.93, P<0.00001], respectively). Anandamide levels progressively increased in the PFC during the course of adolescence; the levels more than doubled (118% increase) from early to mid adolescence (8.0 vs. 17.4 pmol/g, P<0.05) and increased further from mid to late adolescence (17.4 vs. 31.0 pmol/g, P<0.001). The amount of 2-AG in the PFC showed a temporal decrease during the time periods studied. The 2-AG levels decreased from early to mid adolescence (45.0 vs. 21.8 nmol/g, P<0.001), but were increased again in late adolescence (21.8 in mid vs. 34.4 nmol/g in late adolescence, P<0.001; but still lower than early adolescence, P<0.001). There were no THC treatment effects on the levels of anandamide or 2-AG in the PFC.
Based on the recent inverse relationship detected between anandamide and 2-AG synthesis in the striatum (Maccarrone et al., 2008), we evaluated correlations between the endocannabinoid levels. A strong significant negative correlation was apparent generally throughout development between anandamide and 2-AG levels in the NAc (r = −0.8005, P=0.001) in control animals. This was, however, disrupted in THC rats with a reverse correlation evident at the mid-adolescent stage (r = 0.8863, P<0.05). In contrast to the ventral striatum, no significant correlation was observed between the endocannabinoids in the caudate-putamen. Evaluation of the PFC revealed an increased correlation between anandamide and 2-AG levels throughout development with significance prominent in late adolescence (r =0.9709, P<0.01) which was reversed in THC animals (r = −0.9327, P <0.05)
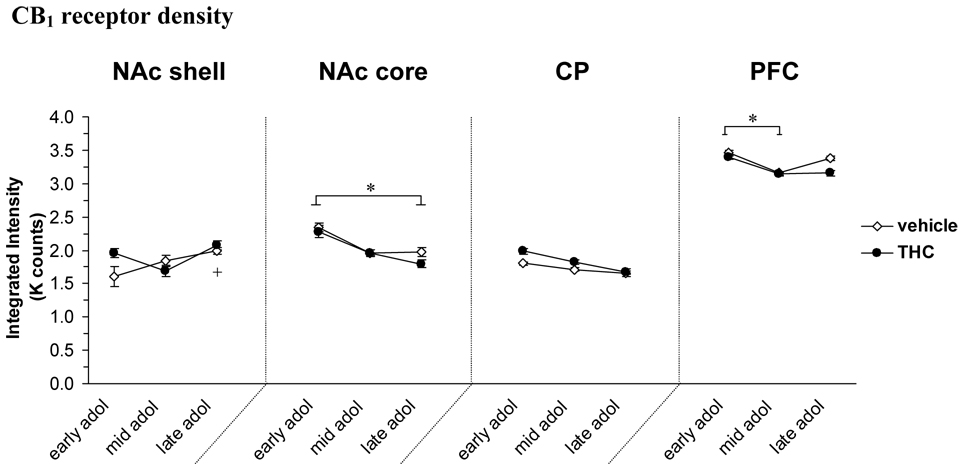
CB1 receptor density
Figure 2 shows the levels of CB1 (represented as corrected integrated intensity; I.I) in the brain regions studied. There was a significant overall effect of age [F(2, 23)=6.28, P<0.01] and an age × treatment interaction [F(2, 23)=4.01, P<0.05] in the NAc shell, with a significant increase of CB1 receptors in the vehicle treated group from early to late adolescence (24% increase, P<0.05). There was also a trend for increased CB1 levels after one injection in the THC group compared to the vehicle control (22% increase, P=0.08). In the NAc core, there was a significant effect of age [F(2, 23)=5.04, P<0.05] with a 19% decrease in activated CB1 levels from early to late adolescence (P<0.05). Also in PFC there was a significant age effect on the levels of CB1 [F(2, 23)=3.82, P<0.05]; the density of CB1 decreased 9% from early adolescence to mid adolescence (P<0.05). An age [F(2, 23)=3.35, P=0.05] and trend treatment [F(2, 23)=3.23, P=0.08] effect was also apparent in the caudate-putamen.
Figure 2.
Density of CB1 receptor (represented as corrected integrated intensity; I.I) at different developmental time points (early, mid and late) during adolescence and the effect of THC exposure. Data is shown as mean ± SEM. (n=5). *, significant difference between age groups, P<0.05. +, P<0.05 significantly difference in vehicle animals in late vs early adolescence. Abbreviations in figure 1.
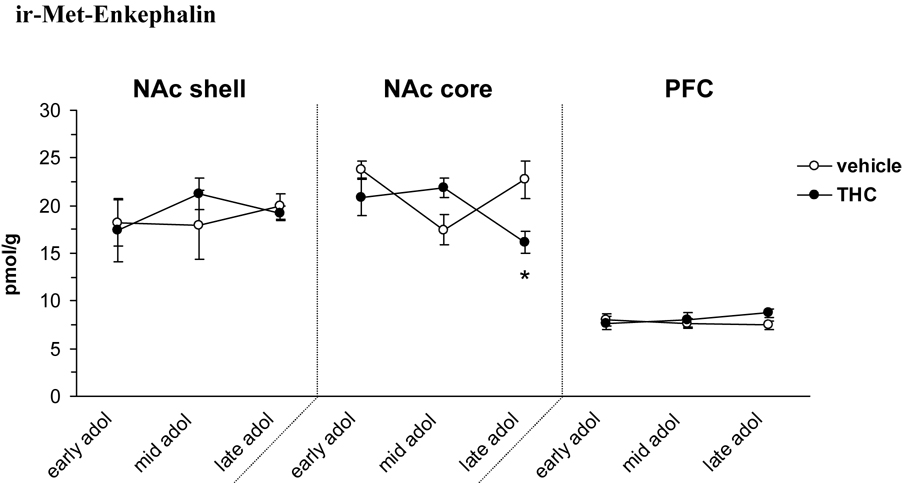
Met-enkephalin
Examination of ir-Met-enkephalin levels revealed a significant age × treatment interaction in the NAc core [F(2, 23)=6.58, P<0.01] (figure 3). The ir-Met-enkephalin concentrations in the NAc core did not vary between age groups, but the THC pretreated group had significantly decreased levels after the full treatment paradigm (16.1 vs. 22.7 pmol/g in the vehicle treated group, P<0.05). Of the other brain areas studied, the levels of ir-Met-enkephalin were not affected by age or THC treatment. It should be noted that the level of ir-Met-enkephalin was below the detection limit (50 fmol/sample) in 7 of the caudate-putamen samples, distributed between all groups studied irrespective of treatment or age.
Figure 3.
Levels of ir-Met-enkephalin at different developmental time points (early, mid and late) during adolescence and the effect of THC exposure. Data is shown as mean ± SEM. *, P<0.05 significant THC treatment effect (n=5). Abbreviations in figure 1.
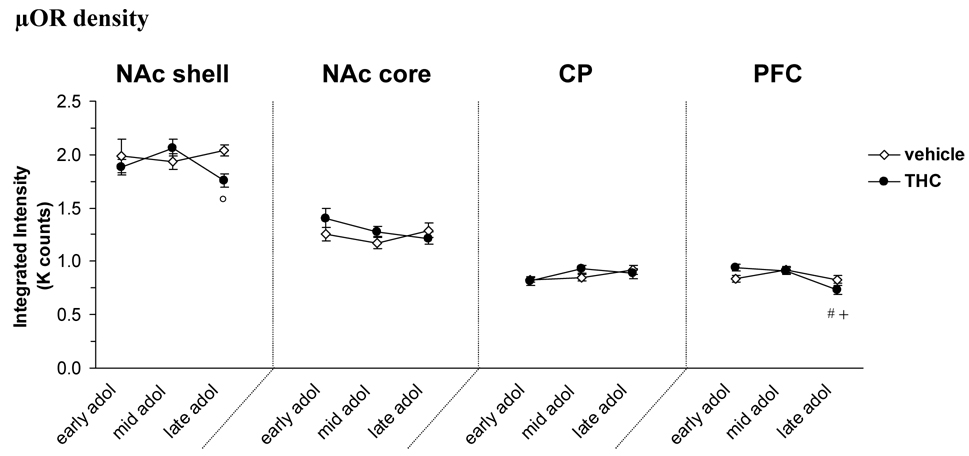
µOR density
Figure 4 shows the density of µORs in the brain regions studied. There was a significant age × treatment interaction [F(2, 23)=3.68, P<0.05] in the NAc shell and a post hoc analysis revealed significantly lower amount of µOR in the THC treated group compared to vehicle controls in late adolescence (14% decrease, P<0.05). There was no significant effect of either age or treatment in the NAc core or caudate-putamen, however, there was an age × treatment interaction on the amount of µOR detected in the PFC [F(2, 23)=4.56, P<0.05]. THC treated rats had decreased density of the µOR in late adolescence compared to both early (22% decrease, P<0.01) and mid adolescence (20% decrease, P<0.05).
Figure 4.
Density of µOR at different developmental time points (early, mid and late) during adolescence and the effect of THC exposure. Data is shown as mean ± SEM. °, P<0.05 significant treatment effect THC vs. vehicle control. #, P<0.05 significantly different vs THC early adolescence. +, P<0.05 significantly different vs THC mid adolescence. (n=5). Abbreviations in figure 1.
Discussion
The current results reveal clear developmental fluctuations throughout adolescence in endocannabinoid levels in the NAc and PFC, brain regions involved in reward, motivation, and cognition. The most profound alteration was the continuous increase in PFC anandamide levels throughout the adolescent period; concentrations were almost three times higher in late than early adolescence. However, the 2-AG concentrations were lower in the PFC in the later phases than in the beginning of the adolescent period, a finding paralleled in the NAc. These findings emphasize dynamic alterations in endocannabinoid function in mesocorticolimbic regions of the adolescent brain that are relevant to reward and, to an even greater extent, cognition and emotional learning. The strong role of endocannabinoids in cognitive functions is well established (Egerton et al., 2006). For example, blocking endocannabinoid function by the CB1 antagonist SR141617A (rimonabant) improves working memory in adult rats (Terranova et al., 1996, Lichtman, 2000). Stimulation or blockade of cannabinoid CB1 receptors also modulates emotional associative learning and memory formation in neurons of the medial PFC (Laviolette et al., 2005, Laviolette and Grace, 2006). Several investigations also implicate endocannabinoids in neurogenesis and neuroprotection, e.g., impaired adult neurogenesis was observed in CB1 knock-out mice (Jin et al., 2004). In addition to the endocannabinoids, we found CB1 receptors to vary in the PFC and NAc core during the different phases of adolescence, but the alterations were less marked than for anadamide and 2-AG. We, however, did not examine CB1 receptor function and thus cannot exclude developmentally-dependent alterations in CB1 signaling transduction.
In contrast to the endocannabinoid markers, both Met-enkephalin and µOR levels were stable across adolescence in the current developmental paradigm. These findings underscore the specific association of the endocannabinoid system with neurodevelopment, not only for the prenatal/perinatal period that has been well documented (Fernandez-Ruiz et al., 2000, Berghuis et al., 2005, Ade and Lovinger, 2007, Berghuis et al., 2007), but also during adolescence. Nevertheless, opioid neuropeptides have been shown to play an important role during perinatal neurodevelopment (Zagon et al., 1994, Wang et al., 2003) and further studies are needed to fully explore the opioid system during adolescent development.
In contrast to the strong developmental changes apparent during adolescence, THC effects were discrete on both the opioid and cannabinoid signaling systems. The lack of significant CB1 alterations by THC is not unexpected since receptor density may appear stable with alterations in signaling transduction more sensitive to changes in receptor function. Moreover, THC-induced effects on CB1 are normally observed at high dosing regimens as compared to the low THC dose used in the current study (McKinney et al., 2008). Interestingly, of the THC-induced effects seen on the cannabinoid and opioid systems, alterations were primarily evident in both the shell and core compartments of the NAc, neuronal populations associated with reward and goal-motivated behavior. No changes were detected in the caudate-putamen that is strongly linked with sensorimotor function (Alexander and Crutcher, 1990, McFarland and Haber, 2000). This indicates specific mesolimbic sensitivity within the striatum as a consequence of the THC exposure.
Of the endocannabinoids studied, anandamide appeared most sensitive to THC. There was an upward shift of anandamide concentrations in the NAc of THC-exposed rats with significance evident after the first injection, though there was still a trend towards increased levels after the full treatment paradigm. In line with the current findings, previous studies have observed increased tissue levels of anandamide in limbic forebrain areas, that contained the NAc, after chronic THC, nicotine or alcohol administration (Di Marzo et al., 2000, Gonzalez et al., 2002). It is important to note that despite the apparent modest impact of THC on the concentrations of anandamide and 2-AG there was evidence for significant disturbance in endocannabinoid transmission. Consistent with recent publication documenting an imbalance between striatal anadamide and 2-AG levels (Maccarrone et al., 2008), we detected a strong negative correlation in control animals between the endocannabinoid concentrations in the NAc which was reverse at the mid-adoloscent phase in THC-treated animals. Other studies have also reported opposite changes in anadamide and 2-AG levels (Valenti et al., 2004, Vigano et al., 2004, Bequet et al., 2007, Caille et al., 2007). These opposing effects, at least in the striatum, appear to be due in part to anadamide inhibition of 2-AG synthesis via anadamide regulation of the TRPV1 (Maccarrone et al., 2008). Although our findings in the NAc are in line with this apparent negative relationship between the endocannabinoid levels, we failed to see a similar correlation in the caudate-putamen/dorsal striatum. The striatal region was not specified by Maccarrone and colleagues (2008) and further investigations are needed to fully elucidate potential subregional differences within the striatum in regard to the relationship between anandamine and 2-AG production. The current findings also suggest regional differences between the striatum and cerebral cortex regarding the homeostatic relationship between anadamide and 2-AG, considering the opposing relationship detected between the endocannabinoids in the PFC as compared to the NAc. Although THC elevation of anandamide was only detected in the NAc, THC did influence endocannabinoid transmission in the PFC as evident by the reversal of the normal positive correlation between anadamide and 2-AG at the late developmental phase in THC-exposed animals.
Anandamide is an important regulator of synaptic plasiticity and THC-induced increase of anandamide levels in the NAc would be expected to inhibit glutamate release due to the stimulation of CB1 receptors on excitatory presynaptic terminals (Hoffman and Lupica, 2001, Wilson and Nicoll, 2002). Disturbance of NAc glutamate function is a key neurobiological feature underling drug abuse vulnerability (McFarland et al., 2004, LaLumiere and Kalivas, 2008) and thus may account in part of the enhanced opiate self-administration behavior evident in adulthood in animals with adolescent exposure to THC (Ellgren et al., 2007) or other CB1 agonists (Biscaia et al., 2008). In addition to enhanced anadamide levels and stimulation of CB1 receptors, induction of synaptic plasticity in the form of long-term depression in the striatum requires stimulation of D2 receptors (Calabresi et al., 2007). Enkephalin containing medium spiny neurons in the NAc express both dopamine D2 and CB1 receptors (Lu et al., 1998, Pickel et al., 2004) and activation of these Gi/o coupled receptors would result in a decrease in PENK gene expression, leading to lower levels of Met-enkephalin. Adolescent animals exposed to THC in our model had reduced Met-enkephalin levels in the NAc core. Previous work in our lab also demonstrated decreased PENK mRNA levels in the NAc immediately following chronic prenatal THC exposure (Spano et al., 2007). It is important to note that a microdialysis study reported increased extracellular levels of Met-enkephalin in the NAc after acute administration of THC (Valverde et al., 2001). Differences in apparent Met-enkephalin levels in response to THC between the studies could be explained by the very high dose (20 mg/kg) used by Valverde et al., (2001) as compared to the low 1.5 mg/kg used in the current experiment which might lead to different neural stimulations and mechanisms of action. Another consideration is that the current study evaluated tissue concentrations, which reflect not only released levels, but synthesis, processing, and degradation of the neuropeptide. Moreover, in contrast to the microdialysis evaluation, Met-enkephalin levels were currently examined 24 hours after the drug injection. One question of interest for the Met-enkephalin findings was the relationship to gene expression given that young adult animals with adolescent THC exposure had PENK mRNA alterations specifically in the NAc shell (Ellgren et al., 2007). THC effects apparent on Met-enkephalin at late adolescence was also restricted to the NAc, but within the NAc core. Prenatal THC-induced effects on PENK mRNA have, however, been detected in both the NAc core and shell of young adult animals (Spano et al., 2007). Additional studies are required to fully characterize subregional dissociation between mRNA and protein. Nevertheless, the findings to date emphasize NAc disturbance as a consequence to developmental THC exposure.
In addition to the Met-enkephalin findings, µOR were also decreased in the NAc, but not in caudate-putamen or PFC, as a consequence of the adolescent THC exposure. Altogether, these results continue to substantiate the specific sensitivity of mesolimbic opioid neuronal populations important for hedonic state (Kelley et al., 2002, Skoubis et al., 2005) as a consequence of early THC exposure. Such disturbances would be expected to contribute to impaired reward function.
In summary, this study emphasizes the dynamic nature of the endocannabinoid system in the adolescent brain. Active endocannabinoid neurodevelopment occurs to a very high extent from early to late adolescence with the most pronounced brain changes relevant to cognitive function. In contrast, chronic intermittent low dose THC exposure during adolescence leads to discrete alterations of both cannabinoid and opioid-related markers most evident in the NAc. The fact that cannabis-induced effects are localized to the NAc support our previous finding of increased opioid reward-related behavior after adolescent cannabis exposure (Ellgren et al., 2007) and strengthen the underlying role of mesolimbic enkephalinergic disturbance in the NAc to early cannabis use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ade KK, Lovinger DM. Anandamide regulates postnatal development of long-term synaptic plasticity in the rat dorsolateral striatum. J Neurosci. 2007;27:2403–2409. doi: 10.1523/JNEUROSCI.2916-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Neale MC, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol Med. 2004;34:1227–1237. doi: 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- Alexander G, Crutcher M. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. Bmj. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bequet F, Uzabiaga F, Desbazeille M, Ludwiczak P, Maftouh M, Picard C, Scatton B, Le Fur G. CB1 receptor-mediated control of the release of endocannabinoids (as assessed by microdialysis coupled with LC/MS) in the rat hypothalamus. Eur J Neurosci. 2007;26:3458–3464. doi: 10.1111/j.1460-9568.2007.05900.x. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, Schulte G, Ernfors P, Mackie K, Paratcha G, Hurd YL, Harkany T. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci U S A. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Biscaia M, Fernandez B, Higuera-Matas A, Miguens M, Viveros MP, Garcia-Lecumberri C, Ambrosio E. Sex-dependent effects of periadolescent exposure to the cannabinoid agonist CP-55,940 on morphine self-administration behaviour and the endogenous opioid system. Neuropharmacology. 2008;54:863–873. doi: 10.1016/j.neuropharm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Brana C, Charron G, Aubert I, Carles D, Martin-Negrier ML, Trouette H, Fournier MC, Vital C, Bloch B. Ontogeny of the striatal neurons expressing neuropeptide genes in the human fetus and neonate. J. Comp. Neurol. 1995;360:488–505. doi: 10.1002/cne.903600310. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: hormonal mediators and human development. Horm Res. 2003;59:161–179. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- Degn M, Lambertsen KL, Petersen G, Meldgaard M, Artmann A, Clausen BH, Hansen SH, Finsen B, Hansen HS, Lund TM. Changes in brain levels of N-acylethanolamines and 2-arachidonoylglycerol in focal cerebral ischemia in mice. J Neurochem. 2007;103:1907–1916. doi: 10.1111/j.1471-4159.2007.04892.x. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Berrendero F, Bisogno T, Gonzalez S, Cavaliere P, Romero J, Cebeira M, Ramos JA, Fernandez-Ruiz JJ. Enhancement of anandamide formation in the limbic forebrain and reduction of endocannabinoid contents in the striatum of delta9-tetrahydrocannabinol-tolerant rats. J Neurochem. 2000;74:1627–1635. doi: 10.1046/j.1471-4159.2000.0741627.x. [DOI] [PubMed] [Google Scholar]
- Egerton A, Allison C, Brett RR, Pratt JA. Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci Biobehav Rev. 2006;30:680–695. doi: 10.1016/j.neubiorev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–615. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Does cannabis use encourage other forms of illicit drug use? Addiction. 2000;95:505–520. doi: 10.1046/j.1360-0443.2000.9545053.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33:15–21. doi: 10.1017/s0033291702006402. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Berrendero F, Hernandez ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends Neurosci. 2000;23:14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- Georges F, Normand E, Bloch B, Le Moine C. Opioid receptor gene expression in the rat brain during ontogeny, with special reference to the mesostriatal system: an in situ hybridization study. Brain Res Dev Brain Res. 1998;109:187–199. doi: 10.1016/s0165-3806(98)00082-0. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Rodriguez de Fonseca F, Piomelli D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem. 2000;280:87–93. doi: 10.1006/abio.2000.4509. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Green AI, Tohen MF, Hamer RM, Strakowski SM, Lieberman JA, Glick I, Clark WS. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66:125–135. doi: 10.1016/j.schres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Gupta A, Decaillot FM, Gomes I, Tkalych O, Heimann AS, Ferro ES, Devi LA. Conformation state sensitive antibodies to G-protein coupled receptors. J Biol Chem. 2006 doi: 10.1074/jbc.M609254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WD, Lynskey M. Is cannabis a gateway drug? Testing hypothesis about the relationship between cannabis and the use of other illicit drugs. Drug Alcohol Rev. 2005;24:39–48. doi: 10.1080/09595230500126698. [DOI] [PubMed] [Google Scholar]
- Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, Luchessi AD, Castro LM, Giorgi R, Rioli V, Ferro ES, Devi LA. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- Jin K, Xie L, Kim SH, Parmentier-Batteur S, Sun Y, Mao XO, Childs J, Greenberg DA. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol Pharmacol. 2004;66:204–208. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kingsley PJ, Marnett LJ. Analysis of endocannabinoids by age coordination tandem mass spectrometry. Anal Biochem. 2003;314:8–15. doi: 10.1016/s0003-2697(02)00643-7. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids Potentiate Emotional Learning Plasticity in Neurons of the Medial Prefrontal Cortex through Basolateral Amygdala Inputs. J Neurosci. 2006;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH. SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol. 2000;404:175–179. doi: 10.1016/s0014-2999(00)00615-4. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, Statham DJ, Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. J.A.M.A. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agro A, Cravatt BF, Centonze D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci. 2000;20:3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, Sim-Selley LJ. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2008;324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- Perez-Rosado A, Manzanares J, Fernandez-Ruiz J, Ramos JA. Prenatal Delta(9)-tetrahydrocannabinol exposure modifies proenkephalin gene expression in the fetal rat brain: sex-dependent differences. Brain Res Dev Brain Res. 2000;120:77–81. doi: 10.1016/s0165-3806(99)00170-4. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodriguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 Suppl 3:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Rockville, MD: Office of Applied Studies; Results from the 2006 National Survey on Drug Use and Health: National Findings. 2007 NSDUH Series H-32, DHHS Publication No. SMA 07-4293.
- Skoubis PD, Lam HA, Shoblock J, Narayanan S, Maidment NT. Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur J Neurosci. 2005;21:1379–1384. doi: 10.1111/j.1460-9568.2005.03956.x. [DOI] [PubMed] [Google Scholar]
- Spano MS, Ellgren M, Wang X, Hurd YL. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol Psychiatry. 2007;61:554–563. doi: 10.1016/j.biopsych.2006.03.073. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, Soubrie P. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology (Berl) 1996;126:165–172. doi: 10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- Valenti M, Vigano D, Casico MG, Rubino T, Steardo L, Parolaro D, Di Marzo V. Differential diurnal variations of anandamide and 2-arachidonoyl-glycerol levels in rat brain. Cell Mol Life Sci. 2004;61:945–950. doi: 10.1007/s00018-003-3453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde O, Noble F, Beslot F, Dauge V, Fournie-Zaluski MC, Roques BP. Delta9-tetrahydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. Eur J Neurosci. 2001;13:1816–1824. doi: 10.1046/j.0953-816x.2001.01558.x. [DOI] [PubMed] [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156:319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- Veen ND, Selten JP, van der Tweel I, Feller WG, Hoek HW, Kahn RS. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161:501–506. doi: 10.1176/appi.ajp.161.3.501. [DOI] [PubMed] [Google Scholar]
- Vigano D, Valenti M, Cascio MG, Di Marzo V, Parolaro D, Rubino T. Changes in endocannabinoid levels in a rat model of behavioural sensitization to morphine. Eur J Neurosci. 2004;20:1849–1857. doi: 10.1111/j.1460-9568.2004.03645.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Cuzon VC, Pickel VM. Postnatal development of mu-opioid receptors in the rat caudate-putamen nucleus parallels asymmetric synapse formation. Neuroscience. 2003;118:695–708. doi: 10.1016/s0306-4522(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Xia Y, Haddad GG. Ontogeny and distribution of opioid receptors in the rat brainstem. Brain Res. 1991;549:181–193. doi: 10.1016/0006-8993(91)90457-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Kandel DB. Patterns of drug use from adolescence to young adulthood: III. Predictors of progression. Am J Public Health. 1984;74:673–681. doi: 10.2105/ajph.74.7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon IS, Isayama T, McLaughlin PJ. Preproenkephalin mRNA expression in the developing and adult rat brain. Mol. Brain Res. 1994;21:85–98. doi: 10.1016/0169-328x(94)90381-6. [DOI] [PubMed] [Google Scholar]