Abstract
We examined the frequency and course of cognitive impairment, no dementia among a group of older patients enrolled in a longitudinal study of depression. Among 230 participants, 29 with baseline dementia diagnosis were excluded from further analyses. Among the remaining 201 participants, 69 were classified with cognitive impairment, no dementia—broadly defined (34.3%) and 28 (13.9%) with cognitive impairment, no dementia—narrowly defined. At 2-year follow-up, individuals with cognitive impairment, no dementia either narrowly or broadly defined had varied outcomes including (1) improvement to normal cognition, (2) continued cognitive impairment, and (3) progression to dementia. Patients with cognitive impairment, no dementia were more likely to be assigned a later diagnosis of dementia. Our results characterize the concept of cognitive impairment, no dementia as a risk factor for dementia among older individuals with current and past depression; however, just as with the general population, the course of this condition is heterogeneous.
Keywords: depression, cognitive impairment, cognitive decline
Over the past decade, several groups of investigators have sought to classify clinical syndromes of cognitive impairment that increase an individual’s risk of developing dementia so as to more effectively initiate targeted interventions to prevent cognitive decline. The classifications most widely used are “mild cognitive impairment” or MCI1 and “cognitive impairment, no dementia” or CIND.2 The classification of CIND has use among researchers studying affective disorders, as some definitions of MCI exclude depression and other psychiatric disorders that may be contributing to the underlying cognitive impairment.3 In addition, the initial conceptualization of MCI was to characterize a prodromal stage of Alzheimer’s disease (AD),1 whereas cognitive impairment in depression may remit along with depression for some, but remain persistent for others, despite remission.4 Despite the theoretical concern that CIND may comprise a more heterogeneous group of individuals than those diagnosed with MCI, CIND has also emerged as an acceptable classification for a dementia risk category. In a recent population-based study, individuals with CIND had a 3-fold increased risk to progress to AD.5 Rates of CIND in community samples have also found, however, that individuals with a diagnosis of CIND return to normal function at approximate rates of 13% to 25%.6-8 Such improvement may reflect a number of co-occurring reversible medical conditions that can adversely affect cognition in older adults, including depression. Thus, a clinical study of CIND in the context of geriatric depression is important, particularly given that cognitive impairment during depression is a risk factor for persistent and often progressive cognitive decline.4 One challenge in examining CIND in late-life depression is that CIND might be so common in depressed patients (due to either the depression itself or conditions associated with aging) that it may not be clinically meaningful in this population. Thus, a more narrow definition of CIND that excludes identifiable causes of cognitive impairment may be helpful in the context of geriatric depression.
We sought to understand the frequency and outcomes of CIND in the Neurocognitive Outcomes of Depression in the Elderly (NCODE) study, a longitudinal study that aims to identify risks associated with development of dementia among older depressed adults.9 Given that geriatric depression itself is associated with cognitive impairment, we hypothesized that CIND among individuals with a history of late-life depression would be a risk factor for dementia over a 2- to 4-year follow-up period; however, as with community samples, we expected a range of outcomes including persistent CIND and a return to normal cognitive function. We also examined the prevalence of dementia risk associated with a more narrowly defined definition of CIND, one that excluded individuals with cognitive impairment due to a clinically identifiable condition. Here, we hypothesized that individuals with cognitive impairment not due to a specified condition would be at higher risk of incident dementia.
Methods
The Sample
The methods of the NCODE study, including a description of the sample, have been previously described.9 In brief, the NCODE sample consists of participants enrolled in the Conte Center for the Study of Depression in the Elderly, an National Institute for the Mentally Handicapped (NIMH)-supported study of depressed and nondepressed older adults (age 60 and above) at Duke University Medical Center. Some individuals were enrolled beginning in 1995 into the NIMH-supported Clinical Research Center at Duke and have subsequently agreed to continue participating in the longitudinal study associated with the Conte Center, spanning a study period from 1995 to 2006. Depressed participants met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for major depression. Study exclusions included the presence of another major axis I psychiatric disorder such as bipolar disorder, schizophrenia, schizoaffective disorder, substance abuse and dementia, and other neurological disorders such as stroke, seizure disorder, Parkinson’s disease, and multiple sclerosis. Participants with psychotic depression were included, as were those with comorbid anxiety disorders, as long as major depression was deemed by the treating geriatric psychiatrist on the study to be the primary psychiatric disorder. Participants were also excluded if they were unwilling or unable (eg, due to presence of metal in the body) to have a magnetic resonance imaging (MRI) brain scan. The sample for the current study initially consisted of 230 individuals with major depression meeting criteria for NCODE study entry who were referred to a series of Consensus Diagnostic Conferences (CDCs). We identified 29 cases who had become demented by the time of the first conference; these prevalent cases were excluded from further analyses, leaving a study cohort of 201. Figure 1 demonstrates flow of participants in the NCODE study. The study was approved by the Institutional Review Board at Duke University Medical Center, and the study procedures were explained to all participants, who then provided written informed consent to participate.
Figure 1.
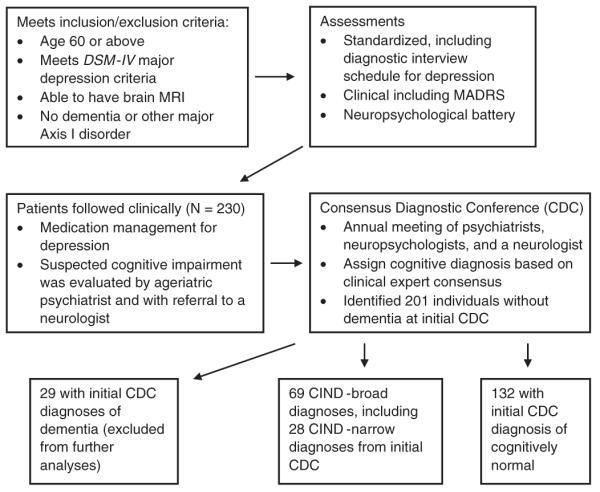
Flow of individuals in the NCODE study. NCODE = Neurocognitive Outcomes of Depression in the Elderly.
Assessments
At baseline, a study geriatric psychiatrist diagnosed depression based on clinical interview and a number of standardized clinical assessments, including the 17-item Hamilton Rating Scale for Depression,10 the Montgomery Asberg Depression Rating Scale (MADRS),11 and the Clinical Global Impression scale. A trained interviewer administered the Duke Depression Evaluation Schedule (DDES12), which assesses depression using the NIMH Diagnostic Interview Schedule,13 as well as cognitive status, physical health, and social support. Clinical assessments were repeated when clinically indicated but at least every 3 months. For the current study, the MADRS was the main depression outcome measure. All raters were trained on completion of the MADRS, and high interrater reliability (kappa > .9) was established.
Baseline Cognitive Screen
Participants were excluded if they had dementia or suspected dementia at baseline based on information available to the assigned NCODE geriatric psychiatrist, who examined the participant, reviewed medical records, and conferred with referring physicians for all participants. A Mini-Mental State Examination (MMSE14) was administered as an additional screening assessment for dementia, and most participants scored above 24 at baseline assessment. For those depressed participants with initial MMSE scores less than 25, NCODE protocol was to follow such individuals through an acute (8-week) phase of treatment to determine if cognition improved. Individuals whose MMSE scores remained below 25 were considered ambiguous for prodromal dementia and not followed longitudinally in the NCODE study. Thus, in the clinical judgment of the study geriatric psychiatrist and by established NCODE protocol, dementia was effectively excluded at or close to baseline in all depressed NCODE participants.
Clinical Follow-up of Depressed Participants
The NCODE study operates in a naturalistic treatment milieu using treatment guidelines established by the Duke Affective Disorders Program.15 Treatment modalities available included antidepressant medications, electroconvulsive therapy, and individual and group cognitive-behavioral psychotherapy. Treatment was monitored to ensure that clinical guidelines were followed appropriately. As indicated above, patients were evaluated when clinically indicated and at least every 3 months for the duration of study participation. The protocol recommends that participants receive continuation treatment for at least 1 to 2 years (some indefinitely) once they achieve remission. Each participant was thus assured to receive the most appropriate care we were able to provide.
Referral of Participants With Cognitive Impairment
Participants had the option of referral to the Memory Disorders Clinic at Duke University Medical Center when (1) they self-reported cognitive complaints, (2) family members reported cognitive concerns to the study geriatric psychiatrist, or (3) the psychiatrist had a clinical suspicion of cognitive impairment or dementia. The study sought to obtain copies of medical records from these referrals when they occurred.
Neuropsychological Battery
The neuropsychological test battery was administered to depressed participants at baseline while still symptomatic and then annually regardless of depression status. The battery was designed for efficient neurocognitive evaluation of geriatric patients and now has been successfully employed in a number of clinical and epidemiological settings.16 The battery is described fully elsewhere.9 Testing was administered by a trained psychometric technician supervised by a licensed clinical neuropsychologist.
Consensus Diagnostic Conference
Clinical diagnoses were made by a consensus panel of experts in dementia, based on a model developed in several epidemiological studies of dementia.17 The panel consisted of a core group of experts, including 3 to 4 geriatric psychiatrists, a cognitive neuroscientist, 1 to 2 neuropsychologists specializing in memory disorders, and a neurologist specializing in memory disorders. Panel members reviewed the following information for each participant presented: (1) initial and most recent clinical depression study notes, (2) neuropsychological testing profiles and provisional diagnoses for all participants who underwent testing, and (3) neurological consultations when available. The treating study psychiatrist briefly presented the case, and a neuropsychologist summarized the neuropsychological findings to the group. Discussion among the panel members would ensue until a consensus clinical diagnosis was assigned.
Panel members chose among several clinical diagnoses (see Table 2). We used published criteria for diagnoses of probable and possible AD18 and probable and possible vascular dementia.19 For any diagnoses of incident Parkinson’s dementia, Lewy body dementia, or alcoholic dementia, we decided to rely on clinical judgment. The category of dementia of undetermined etiology was used when an individual met DSM-IV criteria for dementia, but no clear diagnosis could be assigned based on the individual’s history and presentation. Thus, a diagnosis of dementia of undetermined etiology would not necessarily exclude AD or vascular dementia.
Table 2.
Most Recent Diagnostic Classifications for 69 Participants With a Consensus Diagnostic Conference (CDC) Diagnosis of Cognitive Impairment, No Dementia (CIND), Broadly Defined, and 28 Participants With CIND, Narrowly Defined, All With at Least 2 Years of Follow-up CDC Evaluations
| Number of Individuals With the Diagnosis at Most Recent CDC |
||
|---|---|---|
| Subsequent CDC Diagnosis |
CIND, broadly defined (N = 69) |
CIND, narrowly defined (N = 28) |
| Normal/no cognitive impairment | 17 | 12 |
| CIND | 13 | 7 |
| Cognitive impairment secondary to cerebrovascular disease | 3 | 1 |
| Cognitive impairment due to depression | 4 | 0 |
| Cognitive impairment due to other neuropsychiatric disorder | 6 | 1 |
| Cognitive impairment due to a medical condition | 3 | 0 |
| Cognitive impairment due to other neurological disorder | 2 | 0 |
| Cognitive impairment due to stroke | 4 | 0 |
| Cognitive impairment due to isolated memory impairment/amnestic syndrome | 1 | 0 |
| Subsyndromal Alzheimer’s disease | 6 | 2 |
| Dementia of undetermined etiology | 2 | 1 |
| Possible Vascular dementia | 0 | 0 |
| Possible Alzheimer’s disease | 6 | 4 |
| Probable Alzheimer’s disease | 2 | 0 |
We also included several categories of nondementia diagnoses selected a priori based on our previous studies (see Table 2). “Subsyndromal AD” was defined as early or prodromal stages of AD. This term is broader than “MCI,”1 in that it may include mild impairment in function or in 1 or more cognitive domains (ie, not just memory impairment) that is clinically suggestive of the early stages of AD. “Cognitive impairment secondary to cerebrovascular disease” was defined as cognitive decline consistent with progression of cerebrovascular disease in the presence of history of clinical cerebrovascular risk factors (such as hypertension, diabetes, and atrial fibrillation) but not meeting dementia criteria. Data such as focal neurological deficit or vascular changes seen on neuroimaging were considered as supportive but not necessary for this diagnosis. We assigned a diagnosis of “cognitive impairment due to another neuropsychiatric disorder” to individuals whose clinical presentation and neurpsychological profile were consistent with one of a number of clinical syndromes including frontal lobe dysfunction and late-life psychosis. “Cognitive impairment due to a medical condition” was another clinically assigned category when, in the opinion of the panel, a participant’s acute or chronic medical illness was directly affecting cognitive abilities.
A diagnosis of “CIND” was assigned to individuals with impairment on neuropsychological testing, mild or no functional impairment (as evidenced by clinician report or report of knowledgeable informant on the Dementia Severity Rating Scale), and no syndromes consistent with subsyndromal AD or subsyndromal vascular dementia. Participants were placed in categories separate from CIND, however, if the cognitive impairment was adjudicated as secondary to cerebrovascular disease, neuropsychiatric disorder, or a medical condition. In this sense, our CDC definition of CIND as a diagnosis of exclusion differed from the more inclusive definition proposed by Graham et al.2 We sought to examine both the broader, more commonly accepted definition of CIND (which we term “CIND-broad”) as well as the more narrowly defined category of cognitive impairment employed in the present study (“CIND-narrow”).
The final diagnostic category was Normal/Non-case. We have convened 5 annual CDCs at this point in the study, beginning in 2002. Given that the study has had continuous enrollment since 1995, we sought first to bring to consensus conference those cases with known or suspected cognitive impairment. Therefore, for the initial consensus conference, we targeted those individuals who met one of the following criteria: (1) the study geriatric psychiatrist suspected dementia or clinically significant cognitive decline, (2) review of neurocognitive testing by a study neuropsychologist indicated performance consistent with dementia or cognitive impairment (except for isolated cognitive syndromes), or (3) neurological consultation resulting in a diagnosis of dementia or cognitive impairment. In subsequent consensus conferences, we have reviewed both cognitively impaired individuals as well as those presumed to be cognitively normal.
Statistical Analyses
We initially examined CIND-broad, a diagnosis that included all CDC diagnoses of cognitive impairment short of dementia, including subsyndromal AD. We used bivariate statistics to examine the relationship of predictors and covariates to “CIND-broad” versus “normal” diagnoses at each participant’s first CDC. The t test, χ2, and Fisher exact test were used where appropriate and presented in Table 1. All study participants without baseline dementia at their first CDC were included in this analysis. A frequency table of cognitive outcomes (Table 2) was produced for those who had baseline CIND-broad. No inferential statistics were produced for this table because it was for descriptive purposes only. An association between diagnosis of CIND-broad and risk of later diagnosis of dementia was tested using a χ2 test, followed by a logistic regression model with dementia as the outcome and CIND-broad as a predictor. For analyses in which 1 group (noncases) had few (<5) cases of incident dementia, we also performed an exact conditional logistic regression analysis to determine significance of the CIND-broad variable.
Table 1.
Characteristics of Depressed Patients With Cognitive Impairment, No Dementia (CIND), Both Broadly and Narrowly Defined, Diagnosed at Their First Consensus Diagnostic Conference (CDC) Versus Depressed Patients Without Cognitive Impairment
| Characteristic | CIND (Broad; N = 69) |
CIND (Narrow; N = 28) |
Normal/No Cognitive Impairment (N = 132) |
Total (N = 201) |
Statistic, Degrees of Freedom, and P Value (CIND-broad) |
Statistic, Degrees of freedom, and P Value (CIND-narrow) |
|---|---|---|---|---|---|---|
| Age, M (SD) | 69.91 | 68.82 | 66.41 | 67.61 | T = -3.46, 108 df, | T = -1.53, 32.7 df, |
| (7.42) | (7.95) | (5.49) | (6.42) | P < .008 | P = .136 | |
| Sex, N (%) | χ2 = 2.43, 1 df, | χ2 = 0.448, 1 df, | ||||
| Female | 45 | 17 | 71 | 116 | P = .119 | P = .503 |
| (65.22) | (60.71) | (53.79) | (57.71) | |||
| Male | 24 | 11 | 61 | 85 | ||
| (34.78) | (39.29) | (46.21) | (42.29) | |||
| Race, N (%) | χ2 = 3.67, 1 df, | Fisher exact test, | ||||
| White | 57 | 23 | 121 | 178 | P = .055 | P = .161 |
| (82.61) | (82.14) | (91.67) | (88.56) | |||
| Other | 12 | 5 (17.86) | 11 | 23 | ||
| (17.39) | (8.33) | (11.44) | ||||
| Years of education, | 13.71 | 13.71 | 14.61 | 14.30 | T = 2.15, 111 df, | T = 1.41, 33.4 df, |
| M (SD) | (3.02) | (3.17) | (2.33) | (2.61) | P = .034 | P = .168 |
| MADRS at CDC, | 12.04 | 9.61 | 6.70 | 8.54 | T = -4.71, 113 df, | T = -2.16, 158 df, |
| M (SD) | (8.19) | (6.60) | (6.44) | (7.52) | P < .0001 | P = .0326 |
| Days to first CDC, | 1521.68 | 1516.61 | 1494.80 | 1504.03 | T = -0.17, 199 df, | T = -0.10, 158 df, |
| M (SD) | (1059.29) | (1099.65) | (1026.90) | (1035.54) | P < .862 | P = .920 |
Note: MADRS = Montgomery Asberg Depression Rating Scale.
We next examined the prevalence and outcomes of CIND-narrow, including the risk of incident dementia among these individuals compared with those clinically classified as normal. Here, individuals with subsyndromal AD (included in the prior analyses) were excluded from the CIND-narrow category. We performed analyses similar to those we used to examine CIND-broad, beginning with bivariate statistics, then generating a list of outcomes (see Table 2), and finally examining the association between CIND and later dementia.
Results
Sample
Sample characteristics appear in Table 1; participants were largely women, had a mean age of 68 years, were 89% white, and had a mean education level of 14 years. For all participants, the mean MADRS score closest to the time of CDC was 8.54, and the mean time between date of study entry and date of first CDC was 1504 days, ranging from 27 to 3550 days.
CIND-broad: prevalence, outcomes, and dementia risk
We combined all CDC diagnoses of cognitive impairment short of dementia into a CIND-broad category. A total of 69 of 201 individuals were assigned a diagnosis of CIND-broad at their first CDC. Among participants with CIND-broad, the mean time between date of study entry and date of the participant’s first CDC was 1522 days (range = 27–3550 days). The mean MADRS score available closest in time to the date of the CDC was 12.04, with a range of severity including 43.48% remitted (MADRS score 0–7, n = 30); 23.19% in partial remission (MADRS score 8–15, n = 16); and 33.33% with current depression (MADRS score >16, n = 23).
Table 2 shows longitudinal outcomes of individuals who had a baseline diagnosis of CIND-broad. These outcomes reflecting most recent CDC diagnosis are quite diverse, with 17 individuals improving to noncase, 10 worsening to dementia, and 42 remaining in various categories of cognitive impairment, including 6 with subsyndromal AD.
We examined CIND-broad as a risk factor for dementia diagnosis in subsequent CDCs. To be included in this analysis, all participants with CIND-broad had to have at least 2 subsequent CDC assessments. In subsequent CDCs, 12 participants developed incident dementia: 10 of 69 with prior CIND diagnosis at CDC and 2 of 132 without prior CIND diagnosis (Fisher exact test, P < .0001). Among those with prior CIND diagnosis, dementia diagnoses included 3 with possible AD, 1 with possible vascular dementia, and 1 with dementia of undetermined etiology. Among those with no CIND at baseline, dementia diagnoses included 1 with possible AD and 1 with possible vascular dementia. Of note, both these participants had a subsequent diagnosis of CIND-broad prior to converting to dementia diagnosis. Thus, when examining all annual CDC diagnoses, each individual with incident dementia had a prior CDC diagnosis of CIND-broad.
We constructed regression models of incident dementia controlling for age and time from study to first CDC diagnosis; however, the time variable was not significant. In a final model, both diagnosis of CIND-broad (odds ratio = 7.541, confidence interval = 1.511–37.639 ) P < .0138) and age (odds ratio = 1.104, confidence interval = 1.007–1.211, P < .0353) were associated with increased risk of incident dementia. Because of the small number of individuals with incident dementia among noncases, we performed an exact conditional logistic regression analysis and found that CIND-broad was significantly associated with dementia risk (P = .0137).
CIND-narrow: prevalence, outcomes, and dementia risk
A total of 28 of 201 individuals were assigned a diagnosis of CIND-narrow at their first CDC. Among participants with a diagnosis of CIND-narrow, the mean time between date of study entry and date of the participant’s first CDC was 1516.61 days (range = 33–3438 days). The mean MADRS score available closest in time to the date of the CDC was 9.61, with a range of severity including 53.57% remitted (MADRS score = 0–7, n = 15); 21.43% in partial remission (MADRS score 8–15, n = 6); and 25.00% with current depression (MADRS score >16, n = 7).
Table 2 shows longitudinal outcomes of individuals who had a baseline diagnosis of CIND-narrow. Based on their most recent CDC diagnosis, 12 individuals demonstrated cognitive improvement and were no longer considered cognitively impaired, 7 have continued in the CIND-narrow category, 2 were diagnosed with subsyndromal AD, 2 have other forms of cognitive impairment, and 5 were diagnosed with dementia.
We then examined CIND-narrow as a risk factor for dementia diagnosis in subsequent CDCs. In subsequent CDCs, 9 participants developed incident dementia; 5 of 28 with baseline CDC diagnosis of CIND and 2 of 132 without prior CIND diagnosis (Fisher exact test, P = .0019). In models similar to those constructed for CIND-broad, we found that both diagnosis of CIND-narrow (odds ratio = 9.766, confidence interval = 1.599–59.665, P < .0136) and age (odds ratio = 1.153, confidence interval = 1.018–1.306, P < .0256) were associated with increased risk of incident dementia. Exact conditional logistic regression analysis demonstrated that CIND-narrow was significantly associated with dementia risk (P = .0323). Of note, one of the participants with dementia who was non-CIND at baseline carried a diagnosis of CIND-narrow in the interval between baseline diagnosis and final dementia diagnosis.
Discussion
In this study, we found that the commonly used and broadly inclusive definition of CIND2 occurs frequently among older individuals with current and past depression, with a prevalence of 34.3%. A more narrow and exclusive version of CIND occurs in 13.9% of individuals with current and past depression. In this group, CIND-broad had a variable longitudinal course, with nearly one quarter of CIND-broad individuals reverting to a cognitively normal category at their most recent CDC, about 15% progressing to dementia, and over half continuing with various classifications of cognitive impairment short of dementia. The narrow definition of CIND also had a heterogeneous longitudinal course, but the diagnosis was similarly unstable but with much less variability than the broad definition. At most recent diagnosis, nearly half of participants had reverted to noncase, one quarter remained in the CIND-narrow category, and one quarter had dementia or subsyndromal AD. Only 2 participants were classified in other categories of cognitive impairment. Compared with individuals who did not have a clinical CIND diagnosis, those classified as having CIND (broadly or narrowly defined) were at much higher risk of progression to dementia over a 2- to 4-year period.
The categories of dementia diagnoses deserve comment. One might expect AD to predominate among incident dementia cases. In our cohort, AD accounted for about most of the new cases of dementia, with dementia of undetermined etiology comprising the remainder. As noted in our definition of dementia of undetermined etiology, this diagnosis was given when the patient did not have a clear etiology of dementia but did not preclude AD. For us, this diagnosis was given particularly in early cases of dementia in which the individual technically met dementia criteria but whose course was of such short duration that a more definitive diagnosis such as possible AD could not be assigned. In our experience using this diagnosis in a study of nondepressed individuals, we found that 12 of 15 individuals with dementia of undetermined etiology had a neuropathological diagnosis of AD at autopsy.20
In terms of vascular dementia, it is somewhat surprising that we did not find vascular dementia among our most recent CDC diagnoses given the well-documented link between cerebrovascular disease and depression.21 Cerebrovascular disease did play a role in the outcomes of several of our CIND cases, 3 of whom had a course consistent with vascular cognitive impairment and 4 with poststroke cognitive impairment. One explanation may be that 2 years of follow-up is not sufficient to capture the clinical progression to vascular dementia in depressed individuals. In 1 study, for instance, 48% of individuals diagnosed with “vascular CIND” were demented after 5 years of follow-up.22 Our results are consistent with findings that “vascular CIND” is associated with depression23 and do not rule out that CIND may lie on a continuum between no cognitive impairment and vascular dementia among depressed individuals. Continued follow-up of our cases may more clearly identify progression to vascular dementia, which may have a longer time to conversion than AD.
This study adds to the literature indicating that CIND in general is a risk factor for development of dementia. In the Canadian Cohort Study of Cognitive Impairment and Related Dementias, investigators recently reported a 2-year rate of progression to dementia of 34% among individuals categorized with CIND in this population-based longitudinal cohort study.6 Cognitive impairment, no dementia was also associated with increased conversion to dementia for more than 5 years in the Canadian study of Health and Aging.24 In the Kungsholmen Project (Stockholm, Sweden), the 3-year incidence of dementia was 3, 5, and 7 times higher than normal cognition for mild (35%), moderate (43%), and severe (50%) CIND, respectively.7 In a study in Ibadan, Nigeria, 16.1% of 152 individuals classified as CIND had converted to dementia after approximately 2 years of follow-up.8 Our conversion rate of 21.4% in the context of clinically treated depression is consistent with these previous studies in nondepressed samples.
Our findings are also consistent with prior literature indicating that CIND may have a variable course, with some individuals showing considerable improvement while others continue to experience cognitive impairment short of dementia. In the Canadian Cohort Study of Cognitive Impairment and Related Dementias, 20 of 146 (13.7%) individuals with CIND recovered to not cognitively impaired (NCI) at 2 years.6 In the Kungsholmen Project, 46 of 185 (24.9%) of individuals with mild CIND were classified as cognitively normal after 3 years, and the “improvers” did not have a significantly higher risk of progressing to dementia than individuals who had never been classified as cognitively impaired.7 In the Ibadan study, among those with CIND at baseline, 25.3% reverted to no cognitive impairment and 58.6% remained CIND after about 2 years.8
It is important to note that at each CDC we examined the MADRS score from the most recent clinical assessment. We found that MADRS scores among those with CIND diagnoses were significantly higher than those without cognitive impairment (Table 1). This certainly makes sense for the CIND-broad group; a diagnosis of “cognitive impairment due to depression” would have been assigned if cognitive impairment was felt to be due primarily to depression. Yet, we also found that individuals classified as CIND-narrow also had higher MADRS scores than the noncognitively impaired group. The reasons for this difference are not as evident, but perhaps it speaks to the relationship between cognitive impairment and depressive symptoms. For example, individuals with cognitive impairment may experience more depression symptoms.
Some features of the current study present limitations to the interpretation of our results. We added the CDC to our protocol in 2002; as a result, we lost an opportunity to assign consensus clinical diagnoses soon after study entry for participants enrolled prior to 2002. Because we assigned diagnoses based on participants’ most recent cognitive performance, we may have missed CIND diagnoses for those who experienced cognitive impairment while depressed earlier in the study, particularly at study entry. The later addition of the CDC allowed us to capture prevalent dementia at the time of the first CDC; however, if we had the CDC protocol in place earlier, we would have been able to capture those predementia diagnoses (CIND and non-CIND) for participants with dementia diagnoses assigned at the first CDC. Another limitation related to this relatively wide range of time between initial study enrollment and first CDC is that those individuals recruited earliest into the study will have longer follow-up and may thus be more likely to develop cognitive decline and dementia. For this reason, we truncated our follow-up period to a maximum of 4 years.
Another limitation is the small sample size of participants with CIND in the study, which limits our ability to develop predictive models with larger numbers of covariates. Nevertheless, we were able to control for 2 important variables, age and time in the study, and in this model, CIND was independently associated with dementia. In addition, the fact that this study focuses on a population of individuals with current and past depression may limit our ability to generalize our findings to broader elderly populations.
Future studies will need to focus on the clinical course of cognitive impairment during all phases of a depressive episode and beyond. The current study included 2 definitions of CIND, and though we favor a more narrow definition of CIND, the cognitive outcomes are nonetheless heterogeneous. Subsequent studies should focus on key affective and cognitive domains that may predict variability in outcome with CIND, resulting in better understanding of risk and protective factors for cognitive outcome. For example, prominent apathy in late-life depression is associated with greater cognitive impairment25,26 and may be a stronger indicator of dementia risk than somatic or mood symptoms.27 Regarding specific cognitive deficits, individuals with prominent memory problems during a depression course may have persistent memory problems on remission,28 and these problems may represent a prodrome of AD. However, individuals with clear executive impairment may continue to show impairment after successful treatment of depression,29 which may be an indication of vascular cognitive impairment30 that is prodromal to vascular dementia. Carefully designed longitudinal studies are needed to address these important clinical issues.
Acknowledgments
This study was supported by grants P50 MH60451, R01 MH54846, and K24 MH70027 from the National Institute of Mental Health. The authors acknowledge the following individuals for their participation in the expert consensus diagnostic conferences: John L. Beyer, MD; James R. Burke, MD; Kenneth R. Gersing, MD; Brenda L. Plassman, PhD; Warren D. Taylor, MD; Mugdha E. Thakur, MD; and Kathleen A. Welsh-Bohmer, PhD. The authors also thank Ms Deidra Ansah, Mr Dean Holbert, Ms Alaina Hopler, Ms Cortnee Pierce, Ms Judy Ridley, and Ms Ayana Simon for their preparation of clinical data and Ms Julie Fleenor and Ms Karee Powers for their preparation of neuropsychological data for the consensus conferences.
References
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 3.Steffens DC, Otey E, Alexopoulos GS, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry. 2006;63:130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- 4.Bhalla RK, Butters MA, Mulsant BH, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 5.Monastero R, Palmer K, Qiu C, Winblad B, Fratiglioni L. Heterogeneity in risk factors for cognitive impairment, no dementia: population-based longitudinal study from the Kungsholmen project. Am J Geriatr Psychiatry. 2007;15:60–69. doi: 10.1097/01.JGP.0000229667.98607.34. [DOI] [PubMed] [Google Scholar]
- 6.Hsiung GY, Donald A, Grand J, et al. Outcomes of cognitively impaired not demented at 2 years in the Canadian cohort study of cognitive impairment and related dementias. Dem Geriatr Cogn Disord. 2006;22:413–420. doi: 10.1159/000095751. [DOI] [PubMed] [Google Scholar]
- 7.Palmer K, Wang HX, Backman L, Winblad B, Fratiglioni L. Differential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen project. Am J Psychiatry. 2002;159:436–442. doi: 10.1176/appi.ajp.159.3.436. [DOI] [PubMed] [Google Scholar]
- 8.Baiyewu O, Unverzagt FW, Ogunniyi A, et al. Cognitive impairment in community-dwelling older Nigerians: clinical correlates and stability of diagnosis. Eur J Neurol. 2002;9:573–580. doi: 10.1046/j.1468-1331.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 9.Steffens DC, Welsh-Bohmer KA, Burke JR, et al. Methodology and preliminary results from the Neurocognitive Outcomes of Depression in the Elderly study. J Geriatr Psychiatry Neurol. 2004;17:202–211. doi: 10.1177/0891988704269819. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:55–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 12.Landerman R, George LK, Campbell RT, Blazer DG. Alternative models of the stress buffering hypothesis. Am J Community Psychol. 1989;17:626–642. doi: 10.1007/BF00922639. [DOI] [PubMed] [Google Scholar]
- 13.Robins N, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Steffens DC, McQuoid DR, Krishnan KRR. The Duke somatic treatment algorithm for geriatric depression (STAGED) approach. Psychopharmacol Bull. 2002;36:58–68. [PubMed] [Google Scholar]
- 16.Tschanz JT, Welsh-Bohmer KA, Skoog I, et al. Dementia diagnoses from clinical and neuropsychological data compared: the Cache County study. Neurology. 2000;54:1290–1296. doi: 10.1212/wnl.54.6.1290. [DOI] [PubMed] [Google Scholar]
- 17.Breitner JCS, Welsh KA, Gau BA, et al. Alzheimer’s disease in the National Academy of Sciences-National Research Council Registry of aging twin veterans. III. Detection of cases, longitudinal results, and observations on twin concordance. Arch Neurol. 1995;52:763–771. doi: 10.1001/archneur.1995.00540320035011. [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the nincds-adrda work group under the auspices of the department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 20.Plassman BL, Khachaturian AS, Townsend JJ, et al. Comparison of clinical and neuropathologic diagnoses of Alzheimer’s disease in 3 epidemiologic samples. Alzheimer’s Demen. 2006;2:2–11. doi: 10.1016/j.jalz.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan KRR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 22.Wentzel C, Rockwood K, MacKnight C, et al. Progression of impairment in patients with vascular cognitive impairment without dementia. Neurology. 2001;28:714–716. doi: 10.1212/wnl.57.4.714. [DOI] [PubMed] [Google Scholar]
- 23.Nyenhuis DL, Gorelick PB, Geenen EJ, et al. The pattern of neuropsychological deficits in vascular cognitive impairment-no dementia (vascular CIND) Clin Neuropsychol. 2004;18:41–49. doi: 10.1080/13854040490507145. [DOI] [PubMed] [Google Scholar]
- 24.Ingles JL, Fisk JD, Merry HR, Rockwood K. Five-year outcomes for dementia defined solely by neuropsychological test performance. Neuroepidemiol. 2003;22:172–178. doi: 10.1159/000069891. [DOI] [PubMed] [Google Scholar]
- 25.Boone K, Lesser B, Miller B, et al. Cognitive functioning in a geriatric depressed population: relationship of presence and severity of depression to neuropsychological scores. Neuropsychology. 1995;9:390–398. [Google Scholar]
- 26.Feil D, Razani J, Boone K, Lesser I. Apathy and cognitive performance in older adults with depression. Int J Geriatr Psychiatry. 2003;18:479–488. doi: 10.1002/gps.869. [DOI] [PubMed] [Google Scholar]
- 27.Bartolini M, Coccia M, Luzzi S, Provinciali L, Ceravolo MG. Motivational symptoms of depression mask preclinical Alzheimer’s disease in elderly subjects. Dem Geriatr Cogn Disord. 2005;19:31–36. doi: 10.1159/000080968. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, Steffens DC. Persistent mild cognitive impairment in geriatric depression. Int Psychogeriatr. 2007;19:125–135. doi: 10.1017/S1041610206003607. [DOI] [PubMed] [Google Scholar]
- 29.Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry. 2006;14:724–733. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]


