Abstract
Adenosine triphosphate sensitive potassium (KATP) channels are thought to mediate stress response by sensing intracellular ATP concentration. Cardiomyocyte KATP channels are composed of the pore-forming Kir6.2 subunit and the regulatory sulfonylurea receptor 2 (SUR2). We studied the response to acute isoproterenol in SUR2 null mice as a model of acute adrenergic stress and found that the episodic coronary vasospasm observed at baseline in SUR2 null mice was alleviated. Similar results were observed following administration of a nitric oxide donor consistent with a vasodilatory role. Langendorff-perfused hearts were subjected to global ischemia, and hearts from SUR2 null mice exhibited significantly reduced infarct size (54±4 vs 30±3%) and improved cardiac function compared to control mice. SUR2 null mice have hypertension and develop cardiac hypertrophy. However, despite longstanding hypertension, fibrosis was absent in SUR2 null mice. SUR2 null mice were administered nifedipine to block baseline coronary vasospasm, and hearts from nifedipine-treated SUR2 null mice exhibited increased infarct size compared to untreated SUR2 null mice (42±3 % vs 54±3%). We conclude that conventional sarcolemmal cardiomyocyte KATP channels containing full length SUR2 are not required for mediating the response to acute cardiovascular stress.
Keywords: Sulfonylurea receptor, KATP channel, vasospasm, myocardial infarction, cardiac stress
INTRODUCTION
Found in nearly all tissues and cell types, adenosine triphosphate sensitive potassium channels (KATP) serve as important sensors of cellular energetics [1, 2]. KATP channels sense intracellular concentrations of ATP and ADP and thereby regulate membrane potential [3]. KATP channels are protein complexes composed of four Kir6.x (Kir6.1 or Kir6.2) and four sulfonylurea receptor (SUR1 or SUR2) proteins. In addition to the pore-forming Kir6.x and regulatory SUR subunits, multiple accessory proteins including adenylate kinase, creatine kinase, lactate dehydrogenase, and glyceraldehydes-3-phosphate dehydrogenase among others have been reported [4–6]. Distinct combinations of KATP subunits generate tissue and cell type specific KATP channels with unique regulatory properties [1]. In addition to varying subunit constitution, alternative spicing occurs at 3′ end of the SUR2 gene results in two additional variants, SUR2A and SUR2B, that have differing carboxy-termini [7–9]. Cardiomyocyte KATP channels are composed of the Kir6.2 and SUR2A subunits [10, 11], while KATP channels in the vascular smooth muscle are composed of the Kir6.1 and SUR2B subunits [1, 9, 12].
Cardiovascular KATP channels have important protective roles particularly during periods of increased stress [2]. Mice generated to lack the gene encoding Kir 6.2 (KCNJ11) are viable and lack KATP channel activity in cardiomyocytes. Kir6.2 null animals were shown to have an impaired cardiac response to both acute and chronic stress [13–16]. Kir6.2 null mice were unable to maintain an elevated cardiac output following isoproterenol challenge and when subjected to treadmill stress tests exhibited a survival disadvantage [13]. Isoproterenol challenge in Kir6.2 null mice also induced repolarization arrhythmias leading to ventricular arrhythmias and sudden death [13]. Exposing Kir6.2 null mice to chronic stress, either by swimming or volume overload, revealed an impaired cardiac response similar to acute stress studies [14, 15]. Following four weeks of chronic exercise, Kir6.2 mice exhibited reduced survival, cardiac hypertrophy and reduced cardiac output [14]. Hypertension resulting from salt-induced volume overload also caused a similar adverse phenotype of impaired cardiac function, cardiac hypertrophy with fibrosis, and reduced survival [15]. Treatment of Kir6.2 null mice with verapamil, a calcium channel antagonist, ameliorated the adverse effects of stress suggesting that improper calcium handling by cardiomyocytes is an important contributor to cardiac pathology in stress. Finally, Kir6.2 null mice do not exhibit ischemic preconditioning, the phenomenon where brief episodes of ischemia paradoxically result in protection against later, more severe ischemic insults [17, 18]. Both Kir6.2 null and control mice exhibited similar infarct size following ischemia, although no reduction of infarct size was observed following a preconditioning stimulus in Kir6.2 null mice [17].
We now studied stress response in mice lacking SUR2, the partner protein of Kir6.2 in cardiomyocytes. SUR2 (ABCC9) mutant mice were generated by genetic disruption of the exons 12–16 that encode the first nucleotide binding fold 1 domain [19]. Full length SUR2 is normally expressed in cardiomyocytes, smooth muscle, skeletal muscle and endothelial cells. SUR2 null mice lack conventional, full length SUR2 and glibenclamide-sensitive sarcolemmal KATP channel activity as measured by whole cell patch clamp techniques in isolated cardiomyocytes and vascular smooth muscle cells [19, 20]. SUR2 null mice exhibit an unusual phenotype of repetitive coronary artery spasm seen as ST segment elevation on radiofrequency telemetric monitoring. Episodes of vascular spasm may cluster and lead to arrhythmias and sudden death [20]. Coronary vasospasm in SUR2 null mice can be reduced by administering nifedipine, a calcium channel antagonist. In addition, SUR2 null animal exhibit hypertension [20]. These cardiovascular phenotypes likely result from loss of KATP channels from multiple tissues including cardiac, skeletal, and smooth muscle. In contrast to the stress intolerance seen in Kir6.2 null mice, we found that SUR2 null mice exhibit cardioprotection against sympathetic stress at baseline. Additionally, SUR2 mice have smaller myocardial infarcts compared to controls. Treatment with nifedipine to suppress vascular spasm abrogates ischemic cardioprotection implicating coronary artery spasm as a potential mediator of cardioprotection. Importantly, cardioprotection in SUR2 null mice occurs independent of sarcolemmal cardiomyocyte KATP channels.
METHODS
Animals
SUR2 null mice were previously generated by targeted disruption of exons 12–16 encoding the nucleotide binding fold 1 as described previously [19]. Heterozygous SUR2 null mice were bred onto the FVB mouse substrain for more than 5 generations. Heterozygous mutant mice were interbred to generate homozygous null SUR2 animals. Male SUR2 null mice aged 12–18 weeks were used for experimental studies. Age-matched male wildtype FVB mice were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, Indiana, USA) as controls. Animals were housed, treated, and handled in accordance with guidelines set forth by the University of Chicago’s Institutional Animal Care and Use Committee, the Animal Welfare Act regulations, and the NIH Guide for the Care and Use of Laboratory Animals.
ECG Telemetry and Data Collection
Continuous ambulatory ECG recordings were obtained from SUR2 null and control mice using PhysioTel Implants (Model TA10EA-F20, Data Sciences International, St. Paul, Minnesota, USA) [20]. To implant monitors, isoflurane anesthesia was delivered at 3% during anesthesia induction and at 0.5% for maintenance via nose cone. Monitors were then surgically implanted subcutaneously on the dorsum and ECG leads tunneled subcutaneously into a Lead II configuration and sutured into place using 5/0 monofilament suture. Mice were allowed to recover for 24 hours before baseline ECG data was collected.
Isoproterenol dose was determined following dosage experiments using 1, 5, and 10 μg/g and examining the sustained increase in heart rate under ECG telemetry. The 5 μg/gm dose was chosen for consistently inducing tachycardia for more than 60 minutes. Baseline heart rate was established as the heart rate immediately prior to drug administration. The maximum heart rate and increase in heart rate (ΔHR) following injection were determined, and the length of time while the heart rate was greater than 1/2 ΔHR was defined as the tachycardic period. For experiments, monitors were implanted followed by a baseline recording period of 24 hours. Then, injections of isoproterenol (5 μg/gm body mass delivered by intraperitoneal injection) or diethylenetriamine/nitric oxide adduct (DETA/NO, 120 μg/gm body mass delivered by intraperitoneal injection) in phosphate buffered saline were given. ECG data was collected throughout the experiment. The incidence and duration of each episode of coronary vasospasm during the screening periods were documented and reported as seconds of ST segment elevation per hour of screened ECG data.
Langendorff Experiments
Mice were heparinized (0.5 units/gm body mass, intraperitoneal injection) 30 minutes prior to surgical explant, and then anesthetized with inhaled 3% isoflurane. Hearts were rapidly excised and dissected with the aid of a low power dissection microscope. Excess lung tissue, thymus, great vessels and fat were discarded. The left atrial appendage and part of the left atrium were removed to allow placement of a fluid-filled balloon into the left ventricle. The aorta was then cannulated with a 22 gauge cannula (Fine Science Tools, Foster City, California) placed immediately distal to the intact aortic valve and secured with 5/0 silk surgical suture. Hearts were perfused at constant flow (~3–3.5 ml/min) with Krebs-Henseleit solution (in mM: 120 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 15 glucose, 25 NaHCO3, 1.75 CaCl2, 0.05 EDTA) equilibrated with 95% O2 and 5% CO2 at 37°C using a standard Langendorff setup (Radnoti Glass Technology, Monrovia, California). Hearts were paced at 400 bpm via an epicardial pacing lead (Grass Technologies, West Warwick, Rhode Island). Left ventricular pressures were measured using a pressure-sensing catheter (Model SPR-839, Millar Instruments, Houston, Texas) connected to the inflated fluid filled balloon. Diastolic pressure was set at ~5 mmHg during baseline stabilization. Hearts were maintained at 37°C via a water-jacketed tissue-organ bath for the duration of the experiment. Baseline cardiac function was recorded for x>30 minutes followed by 40 minutes of no-flow global ischemia and 60 minutes reperfusion. Cardiac function data was collected throughout the duration of the experiment and analyzed using Chart5 software (ADInstruments, Colorado Springs, Colorado). Upon completion of reperfusion, hearts were snap-frozen in liquid nitrogen and stored at −80°C for later infarct size analysis.
Semi-frozen hearts were sectioned into 6–8 equivalent sections and stained with 1% 2,3,5-Triphenyl tetrazolium chloride (TTC, Sigma Aldrich, Cat# T8877) in PBS for 20 minutes at 37°C. Sections were fixed in 10% buffered formalin overnight at 4C, and then weighed and photographed for infarct size calculations. Photographs were blinded and the percentage infarct for each section was determined using computer software (Adobe Photoshop and ImageJ). Total infarct size was then calculated as (P1×W1)+(P2×W2)+(Pn×Wn) where P is the percentage infarct and W is the weight of each respective section. Atrial tissue was excluded from infarct size analysis.
Histology
Hearts were surgically explanted, weighed, fixed in 10% buffered formalin and stored at 4C. Hearts were paraffin-imbedded, sectioned at a thickness of 8 μM, and then stained with hematoxylin and eosin. Masson trichrome staining was conducted for visualization of collagen. Photomicrographs were taken using an Olympus BX40 microscope with an Jeonoptik ProgRes C10plus camera (Jena, Germany) utilizing the accompanying software package ProgRes CapturePro 2.1.
Reduction of Vascular Spasm by Nifedipine
Nifedipine (3 mg/kg body mass in dimethyl sulfoxide, Sigma Aldrich, Cat# N7634) was administered using Alzet micro-osmotic pumps (Model 1002, DURECT Corporation, Cupertino, California) as described previously [20]. Pumps were surgically implanted into the abdominal cavity and nifedipine was administered at a constant rate for at least 7 days (n=8). A subset of this experimental cohort (n=3) also underwent ambulatory ECG monitoring as described above to monitor the effects of nifedipine on vascular spasm. ECG data was manually screened for episodes of spontaneous ST segment elevation, and the incidence and duration of each episode recorded. Three hours of ECG recording data 12 hours after pump implantation (“baseline”) and three hours of ECG recording data 12 hours prior to the Langendorff ischemia experiments (“NIF”) were analyzed and quantified (seconds of ST segment elevation per hour of ECG data screened). Isolated hearts from SUR2 null mice then underwent Langendorff ischemia experiments, and infarct size and cardiac function data was collected as described above. Osmotic pumps were recovered at the end of the experiment to confirm proper drug delivery.
Statistical Analysis
Data is reported as mean±SEM, and statistical analysis was conducted using GraphPad Prism version 4.0 for Macintosh, GraphPad Software, San Diego California USA, www.graphpad.com). Student T-Tests (2 experimental groups) or two-way ANOVA and post-hoc Bonferroni comparison of means (x>2 experimental groups, 2 variables) were used where appropriate. Significance was set at a level of p=0.05.
Results
β-adrenergic stress reduces coronary artery vasospasm in SUR2 null mice
To examine the effects of acute β adrenergic stress, isoproterenol was injected at 5 μg/gm body mass into SUR2 null (n=5) and control (n=3) mice. Ambulatory ECG data, from a lead II configuration, was collected and analyzed both before and after the period of isoproterenol injection. We selected this dose of isoproterenol because this dose produced a sharp increase in heart rate of approximately 100 bpm, and this increase in heart rate was sustained for more than 60 minutes (Figure 1A). The duration of tachycardia after isoproterenol injection was similar in SUR2 null and control mice (160±47 vs 222±25 min, respectively, Figure 1B). Because at baseline SUR2 null mice display repeated ST segment elevation consistent with coronary artery vascular spasm, we expected that acute adrenergic stress may exacerbate this condition. Moreover, it has been shown that isoproterenol injection into mice lacking Kir6.2, the partner protein of SUR2 in cardiomyocytes, also induces adverse cardiovascular consequences including left ventricular dysfunction and death [13]. Surprisingly, in SUR2 null mice, we noted that isoproterenol injection was associated with reduction of ST segment elevation during tachycardia (75±48 versus 0 seconds of ST segment elevation per hour of ECG monitoring before and after isoproterenol injection, respectively, Figure 2C). No ST segment elevation was documented in any of the control mice before or after the isoproterenol injection. With this dose of isoproterenol, all control mice (n=3/3) exhibited ST segment depression following the isoproterenol injections. We interpret this ST segment depression as myocardial ischemia, although it may also relate to regional alteration in action potential. In contrast, no ST depression was noted in SUR2 null mice (n=0/5) during the tachycardic periods (Figure 2B). Additionally, isoproterenol injection did not have an adverse effect on survival, nor did it elicit any other ECG abnormalities in SUR2 null mice during the screened tachycardic periods in contrast to what was reported for Kir6.2 null mice [2, 13].
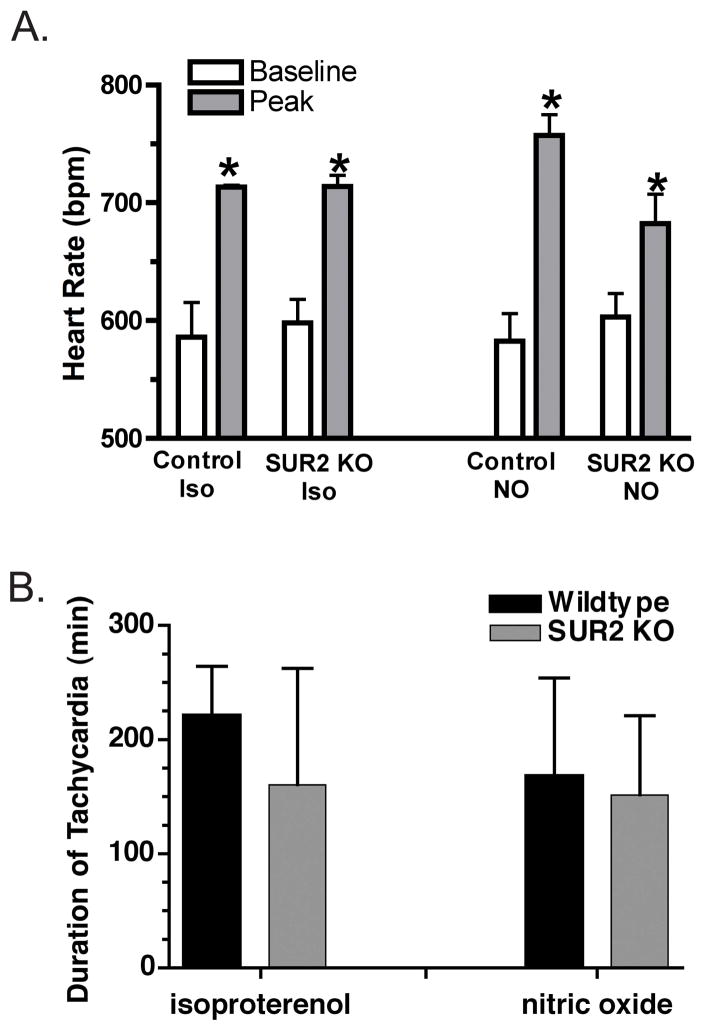
Figure 1.
Heart rate response to isoproterenol injection. A. Isoproterenol injection at 5 μg/gm body mass was used to induce acute β adrenergic stress in SUR2 null and strain matched control mice. The peak heart rate after injection was similar between mutant and controls. DETA/NO injections similarly produced an increase in heart rate. B. Average duration of tachycardia in control and SUR2 null mice following administration of isoproterenol and nitric oxide donor (DETA/NO, 120 μg/gm body mass). (*p<0.05 vs baseline)
Figure 2.
Isoproterenol decreased ST segment elevation in SUR2 mice. A. Baseline ECG recordings of control and SUR2 null mice. SUR2 null mice exhibit spontaneous episodes of coronary vasospasm evidenced by ST segment elevation on ECG. Episodes vary in length and usually resolve within 3 minutes; an example of vasospasm is shown under SUR2 KO. B. ECG recordings immediately following isoproterenol injection. Control mice (n=3/3) exhibited ST segment depression, while no ST segment depression or other ECG abnormality was observed in SUR2 null mice during the resulting tachycardic period (n=0/5). C. Quantification of ST segment elevation before and after drug administration in SUR2 null mice. While coronary vasospasm was present in all SUR2 null mice at baseline, none was observed during tachycardia.
Nitric Oxide reduces coronary artery vasospasm in SUR2 null mice
To assess the effect of vasodilatation on coronary artery vasospasm in SUR2 null mice, the nitric oxide donor DETA/NO (120 μg/g body mass) was injected and induced similar levels of tachycardia in SUR2 null (n=4) and control (n=4) mice (152±35 vs 169±43 min, respectively) [21]. The total duration of tachycardia was comparable to that for isoproterenol for both SUR2 null and control mice (Figure 1B). Coronary vasospasm that occurs episodically at baseline in SUR2 null mice was likewise alleviated during DETA/NO-induced tachycardia (15±9 versus 0 seconds of ST segment elevation per hour of ECG monitoring before and after DETA/NO injection, respectively) (Figure 2C). No electrocardiographic abnormalities were observed during the tachycardic period in SUR2 null mice. The similar results in DETA/NO and isoproterenol injected mice suggest that both agents mediate vasodilatation of the coronary arteries.
SUR2 null mice are protected from ischemic insult
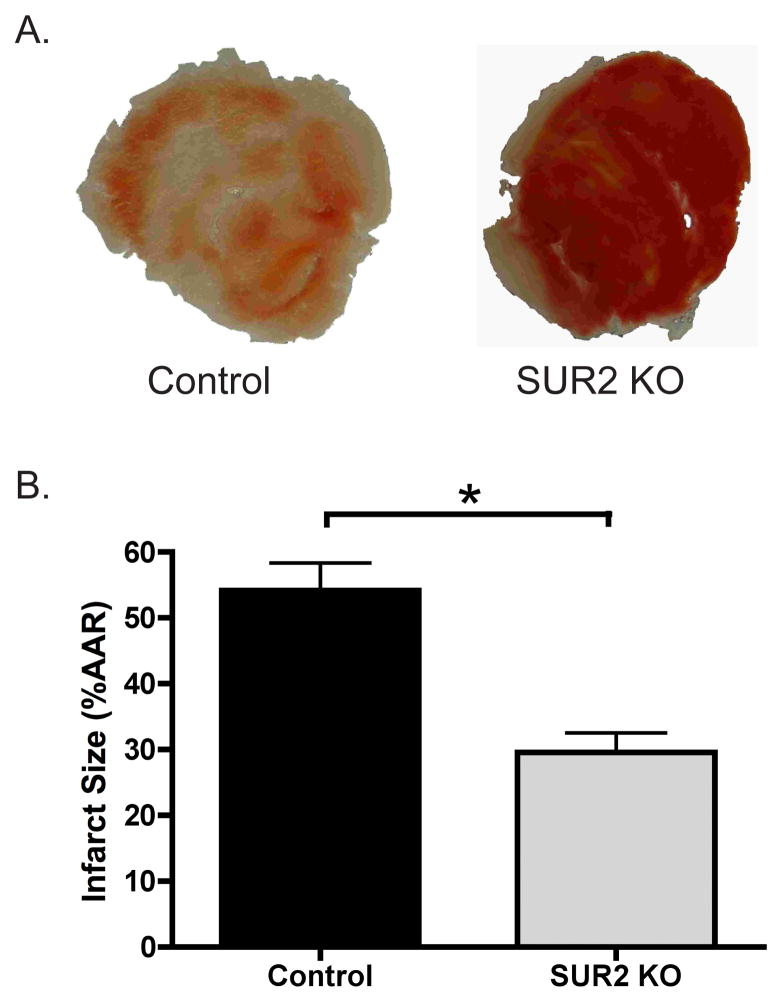
Isolated hearts from male SUR2 null and control mice were subjected to 40 minutes of “no-flow” ischemia followed by 60 minutes of reperfusion. Hearts were sectioned and stained with 1% TTC dye for measurement of infarct size. TTC interacts with enzymes remaining in viable tissue thereby turning this tissue a dark red, while infracted tissue remains a pale white. Representative heart sections from a control and SUR2 null heart are shown in Figure 3A. Infarct size in control hearts (n=10) was 54±4% of the area at risk while SUR2 null hearts (n=8) had a markedly reduced infarct size of 30±3% (p<0.001) (Figure 3B).
Figure 3.
Effect of global ischemia on SUR2 null hearts. A. Isolated hearts from control and SUR2 null mice were exposed to 40 minutes of “no-flow” ischemia via a standard Langendorff setup. Following reperfusion, hearts were sectioned and stained with 1% TTC to visualize infarct size. Representative heart sections from control and SUR2 null mice are shown. Viable tissue stains dark red (black) while infracted tissue becomes white and drained of color. B. Infarct size was quantified for control and SUR2 null hearts. SUR2 null mice exhibit significantly smaller infarcts following 40 minutes of global no flow ischemia. * p<0.001, n=10 and n=8 for control and SUR2 null, respectively.
Left ventricular pressure was measured using a pressure-sensing catheter connected to a fluid-filled balloon during the ischemia-reperfusion protocol. Baseline systolic (101±5 vs 93±3 mmHg, respectively) and diastolic (8±1 mmHg for both groups) pressures were similar between SUR2 null (n=7) and control cohorts (n=10, see Figures 4A and 4B). Likewise, baseline left ventricular developed pressure (LVDP) was similar between SUR2 null (92±6 mmHg) and control (85±3 mmHg) cohorts (See Figure 4C). SUR2 null hearts exhibited a cardioprotective response seen as preserved left ventricular function during the reperfusion period compared to controls. After 15 min of reperfusion, LVDP remained unchanged in SUR2 null hearts while dropping significantly in controls (p<0.01, 89±6 vs 61±5 mmHg, respectively, Figure 4A and Figure 4C). Throughout the reperfusion period, SUR2 null hearts exhibited greater LVDP than controls (p<0.0001 by two-way ANOVA, Figure 4C). The improved LVDP observed in SUR2 null hearts resulted from maintained systolic pressures. Systolic pressure during the reperfusion period was greater in SUR2 null than control hearts (p<0.001 by two-way ANOVA), while diastolic pressures remained similar between groups (Figure 4B). While diastolic pressure at the end of ischemic period was greater in control hearts (22±8 vs. 14±4 mmHg in SUR2 null), this difference was not significant (p=0.235).
Figure 4.
Effect of ischemia on cardiac function in SUR2 null hearts. Left ventricular pressures were measured using a pressure-sensing catheter connected to a fluid filled balloon placed inside the left ventricle. A. Representative examples of left ventricular pressure at baseline and after 15 minutes of reperfusion are shown. Hearts from SUR2 null mice exhibit improved conserved left ventricular function following ischemia versus control hearts. B. Average systolic and diastolic pressures for control and SUR2 null mice. SUR2 null hearts exhibited significantly greater systolic pressures throughout reperfusion. Diastolic pressures were similar between cohorts, though a trend of higher diastolic pressures in control hearts immediately following ischemia was observed. * p<0.001, n=10 and n=7 for control and SUR2 null, respectively. C. Left ventricular developed pressures were significantly greater in SUR2 null hearts during reperfusion compared to controls even though baseline LVDP was similar between cohorts. ** p<0.01, * p<0.05, # p<0.0001 for two-way ANOVA comparison of cohorts, n=10 and n=7 for control and SUR2 null, respectively.
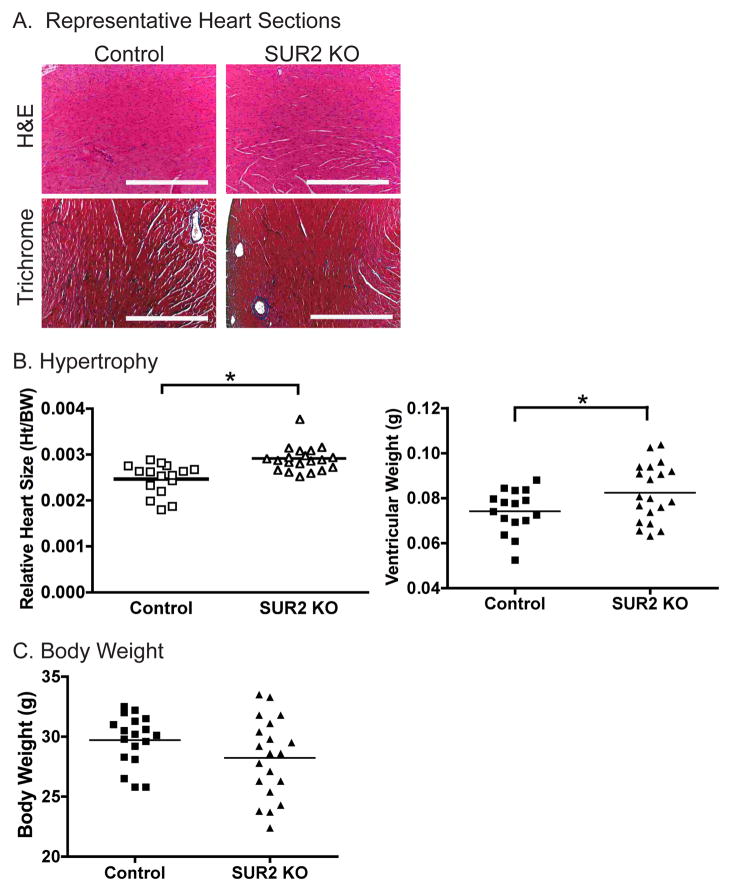
SUR2 null mice develop cardiac hypertrophy
Hearts from SUR2 null and control mice, ages 12–18 weeks, were examined for evidence of cardiac hypertrophy, fibrosis and cardiomyocyte architecture. Representative heart sections stained with hematoxylin and eosin are shown in Figure 5A documenting structurally normal hearts. Masson trichrome staining did not demonstrate enhanced fibrosis in SUR2 null mice (Figure 5A). Electron micrographs of wildtype and mutant hearts (age 18 weeks) revealed normal mitochondrial shape and content as well as normal sarcomere structure (Supplemental Figure 1). Additionally, Periodic Acid Schiff staining for glycogen content was also normal in SUR2 null hearts (Supplemental Figure 2). A cohort of SUR2 mice over 12 months of age was studied, and similarly did not display enhanced fibrosis or other significant morphological indicator of cardiac damage (data not shown).
Figure 5.
Cardiac hypertrophy in SUR2 null mice. A. Representative photomicrographs of hemotoxylin and eosin and Masson trichrome stained sections from control and SUR2 null hearts. Despite significant increases in heart size, SUR2 null hearts exhibited no fibrosis, cardiomyocyte disarray or other structural defects. (Scale bar = 0.5 mm) B. Hearts from SUR2 null mice are significantly larger than matched control hearts as measured by ventricular weight or relative heart size (ventricular weight/body weight, gm/gm). * p<0.05, n=16 and n=20 for control and SUR2 null cohorts, respectively. C. Average body weight of the control and SUR2 null cohorts were not significantly different.
Ventricular mass and relative heart size (ventricular mass/body weight) were calculated for age-matched, male SUR2 null (n=20) and control (n=16) cohorts. SUR2 null mice exhibited significantly greater ventricular mass (0.082±0.003 g) than control mice (0.074±0.002 g, p<0.05) (Figure 5B). Relative heart size was also significantly greater in SUR2 null mice compared to controls (0.0025 vs 0.0018, respectively, p<0.05). Body mass was similar between cohorts (28.2±0.7 vs 29.7±0.05 for SUR2 null and control, respectively) (Figure 5C).
Calcium channel blockade reverses cardioprotection in SUR2 null mice
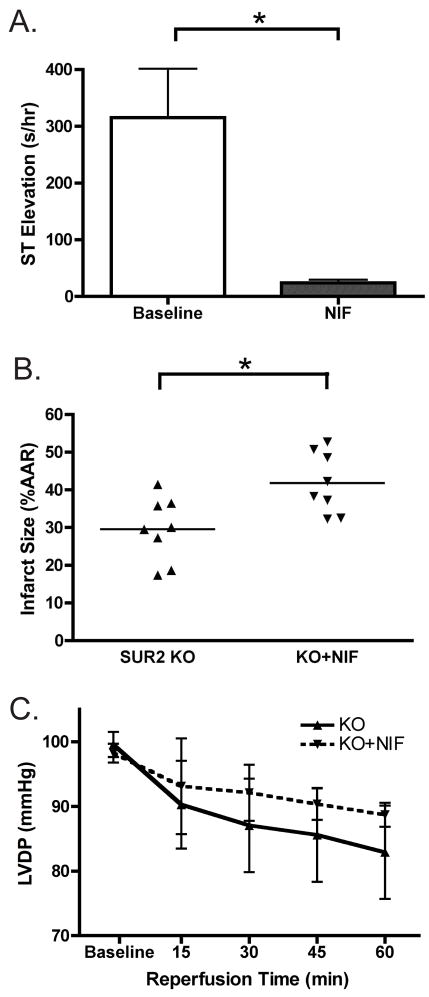
To reduce the incidence of coronary vasospasm, nifedipine was administered for at least 7 days to SUR2 null mice using implantable osmotic pumps (3 mg/kg BW, i.p., n=8). Drug administration was allowed to proceed for at least 7 days to block the cardioprotective effects of both early and late-phase preconditioning. A subset of the nifedipine cohort (n=3) was instrumented for ambulatory ECG data collection for the duration of the experiment. Three hours of ECG data approximately 12 hours after implantation of the osmotic pump were analyzed for ST segment changes (termed “baseline”). Three additional hours of ECG data was also analyzed approximately 12 hours before Langendorff ischemia experiments were completed (termed “NIF”), by which point mice had received at least seven days of chronic nifedipine administration. As seen in Figure 6A, nifedipine administration significantly reduced coronary vasospasm from 315±86 to 24±6 s/hr ST segment elevation per hour of ECG data (p<0.05, baseline vs NIF, respectively). The number of vasospasm episodes, as well as average episode duration, was also significantly reduced by nifedipine (data not shown). However, vasospasm was not completely abolished by nifedipine infusion, as a small degree of ST segment elevation was still documented after seven or more days of nifedipine administration.
Figure 6.
Reducing coronary vasospasm partially blocks cardioprotection in SUR2 mice. Nifedipine (3 mg/kg body weight) was chronically administered via intraperitoneal mini-osmotic pumps for at least 7 days. Ambulatory ECG monitoring was conducted on a subset of the cohort (n=3/8) to ensure reduction of vasospasm. A. Nifedipine significantly reduced vasospasm in SUR2 null mice. Total duration of ST segment elevation 12 hours after pump implantation (“baseline”) and after 7 days (“NIF”) was quantified. Nifedipine significantly reduced total duration of vasospasm, as well as the incidence and length of vasospasm episodes. * p<0.05, n=3. B. Hearts isolated from nifedipine treated SUR2 null mice underwent Langendorff ischemia experiments. Average infarct size was significantly greater in nifedipine-treated SUR2 null hearts compared SUR2 null animals, though infarct size was still less that control. * p<0.05, n=8 per group. C. Left ventricular developed pressure was not significantly different between nifedipine treated and untreated SUR2 null cohorts (n=8 and n=7, respectively).
Langendorff ischemia experiments were conducted on isolated hearts from SUR2 null mice (n=8) treated with nifedipine as described above. Nifedipine treated hearts displayed a significant increase in infarct size compared to hearts from untreated SUR2 null mice (42±3% vs. 30±3%, p<0.05, Figure 6B). Baseline LVDP was similar between SUR2 null mice with and without nifedipine (95±3 mmHg vs. 101±5 mmHg, respectively). During reperfusion, left ventricular function was not significantly different between treated and untreated SUR2 null hearts (Figure 6C). These data suggest that vascular spasm in SUR2 null mice may predispose to cardioprotection. Alternatively, the mechanism of cardioprotection in SUR2 null mice may rely on calcium channels.
DISCUSSION
Previous studies have implicated Kir6.2, the partner protein of SUR2 in cardiomyocytes, as a critical mediator of the acute and chronic cardiovascular stress response [1, 2, 18]. We investigated the effects of acute cardiac stress on SUR2 null mice. SUR2 null mice have a germline disruption of the ABCC9 gene and lack expression of mRNA for SUR2 [19]. This mutation results in mice lacking conventional sarcolemmal KATP channels in cardiac, smooth and skeletal muscle and produces an adverse phenotype of hypertension, coronary vasospasm, arrhythmias, and sudden cardiac death [20]. Based on the hypothesized role for KATP channels and the cardiovascular phenotype of hypertension and vascular spasm in SUR2 null phenotype, we expected that SUR2 null mice would exhibit an impaired response to cardiac stress. To our surprise, SUR2 null mice exhibit increased protection against both acute adrenergic stress and ischemia at baseline compared to control mice. During the tachycardic periods following induced adrenergic stress, coronary vasospasm, normally present at rest, was not observed. We presume that this lack of spasm relates to a general coronary vasodilation from isoproterenol infusion since alleviation of spasm was also noted with infusion of the NO donor DETA/NO. SUR2 null mice were also more resistant to ischemia than control mice, exhibiting significantly reduced infarct size and preserved cardiac function following ischemic insult. While SUR2 null mice develop cardiac hypertrophy, likely due to lifelong hypertension, no evidence of fibrosis, ventricular dysfunction or cardiac remodeling has been observed. Reducing vasospasm by nifedipine partially reduced cardioprotection suggesting that vasospasm may trigger the cardioprotective response. While KATP channels are known mediators of ischemic preconditioning, our data demonstrates that sarcolemmal sulfonylurea receptor-containing KATP channels are not required for cardioprotection.
Multiple studies support a protective role for cardiomyocyte KATP channels. Kir6.2 null mice lack KATP channels in cardiac and skeletal muscle [22]. In a series of experiments, Kir6.2 null mice exhibited significant impairment when subjected to acute cardiac stress identifying the Kir6.2-associated channel as a major stress sensor. Isoproterenol infusion induced fatal arrhythmias in Kir6.2 null animals [2, 13]. In Kir6.2 null mice given an intraperitoneal dose of isoproterenol, 2/6 mice died, 3/6 developed polymorphic ventricular tachycardia, and nearly all developed prolonged QT intervals [2, 23]. Kir6.2 null mice performed poorly on treadmill stress tests, and exhibited impaired cardiac function following isoproterenol compared to control cohorts [13]. In Langendorff-based ischemia experiments, hearts isolated from Kir6.2 null animals exhibited reduced cardiac function compared to control hearts [17]. In addition, cardiac overexpression of mutant Kir6.1 or Kir6.2 subunits resulted in increased mortality, decreased performance on treadmill stress tests, and isoproterenol induced arrhythmias in isolated myocytes [24]. Similar experiments now conducted in SUR2 null animals yielded entirely opposite results; in all experiments, SUR2 null animals better handled acute cardiac stress. Importantly, SUR2 null animals tolerated ischemia with better left ventricular developed pressure than wildtype.
Experimentally-induced hypertension in Kir6.2 null animals also resulted in an adverse phenotype. Kane and colleagues induced experimental hypertension in Kir6.2 null mice with unilateral nephrectomy, administration of mineralocorticoid, and drinking water salt supplementation for 3 weeks. Hypertensive Kir6.2 null mice developed cardiac hypertrophy with fibrosis, exhibited impaired cardiac function, had an impaired response to dobutamine and treadmill stress testing resulted in increased mortality compared to control animals [14]. In contrast, SUR2 null mice exhibit none of these negative consequences despite similar hypertensive load [20]. Similar results in Kir6.2 null mice were observed following pressure overload due to aortic banding [16]. Pressure overload in Kir6.2 null mice caused impaired left ventricular function, cardiac hypertrophy with greater fibrosis than controls, and increased mortality.
We hypothesize that cardioprotection in this SUR2 mutant arises, in part, from an unknown protective mechanism that is elicited by episodic coronary artery vascular spasm. We found that reduction of vasospasm from nifedipine treatment partially reversed cardioprotection. Nifedipine administration, itself, is not thought to be effective at reducing infarct size, although it may improve left ventricular function following ischemia [25–27]. Therefore, it is possible that nifedipine may be inhibiting cardioprotection via a cardiomyocyte specific mechanism that requires calcium. Loss of KATP channels may lead to elevated membrane potential as seen in Kir6.2 null pancreatic β-cells, thereby triggering protection via a calcium requiring mechanism [28]. Recently, it was shown mice lacking Kir6.2 and cardiomyocyte KATP channels displayed calcium accumulation in the myocardium that contributed significantly to left ventricular dysfunction after dobutamine infusion [29]. In a study conducted on isolated rabbit hearts, nifedipine was able to abolish cardioprotective effects [30]. Administration of diazoxide, a mitochondrial KATP channel opener, significantly reduced infarct size. Coadministration of nifedipine abolished the protective effects of diazoxide [30]. In a recent study by Pasdois et al., the sarcolemmal KATP channel blocker HMR 1098 induced cardioprotection in isolated rat hearts exposed to ischemia [31]. Further examination revealed elevated intracellular calcium and inhibition of the mitochondrial electron transport chain in HMR 1098 treated cardiomyocytes. More importantly, HMR 1098-induced cardioprotection was abolished by coadministration of nifedipine, suggesting that calcium handling within cardiomyocytes is directly involved with this mechanism [31].
Unique to the SUR2 model, and distinct from the Kir6.2 model, is the episodic vascular spasm that affects the coronary arteries. Therefore, it is most tempting to speculate that this spasm is the physiologic stimulus for ischemic protection. The mechanism by which repeated vascular spasm produces protection occurs independent of full length sulfonylurea receptor 2 expression since this model has been shown to lack expression of this mRNA [19]. Correspondingly, this model lacks conventional sulfonylurea-sensitive KATP activity. Therefore, it remains possible that Kir6.2 may partner with other ABC transporters to yield cardioprotection. There are also multiple inward rectifiers, Kir, and sulfonylurea receptor, SUR, subunits within the same tissue. It is unclear if cardiomyocyte Kir6.2 is able to partner with proteins other than the canonical sulfonylurea receptor to produce functional KATP channels albeit with altered channel properties. In cardiomyocytes, SUR2A is the dominant isoform, but SUR1 mRNA is also present in cardiomyocytes along with other ATP-binding cassette (ABC) superfamily proteins. While SUR2 null mice do not exhibit KATP channels with physiologic ATP/ADP, diazoxide, pinacidil, or glibenclamide sensitivities, we cannot exclude the formation of atypical KATP channels [19, 20, 32]. Regulation of KATP channel number, while not well understood, is dependent on SUR subunit. Kir6.x and other accessory proteins are produced in excess, while generation of the SUR subunit is regulated [33–35]. We generated transgenic mice expressing SUR2B, the vascular smooth muscle SUR2 subunit, driven by the vascular smooth muscle specific SM22 promoter. Despite restoration of functional KATP channels in coronary vascular smooth muscle in the background of SUR2 null mice, coronary vasospasm persisted [32]. Flagg and colleagues created transgenic mouse lines with cardiomyocyte-specific expression of SUR1 and SUR2A using the α-myosin heavy chain promoter [36]. Consistent with this, overexpression of both SUR1 and SUR2A actually reduced overall KATP channel current and channel density in isolated cardiomyocytes [36]. In a different set of experiments, nonspecific overexpression of SUR2A via the broadly expressed cytomegalovirus promoter increased channel density and provided added protection against ischemia compared to controls [33] suggesting that upregulating the conventional SUR2-KATP channel can increase protection afforded through this mechanism. Therefore, overexpression experiments may have different effects on channel density. Our data demonstrate that canonical plasma membrane-associated SUR2-KATP channels are not required for cardioprotection, but leave open the possibility that other channels, potentially sharing components of KATP channels may mediate protection.
Supplementary Material
Supplemental Figure Legend 1. Structurally normal cardiomyocytes in SUR2 null mice. Shown are high magnification (scale bar 1 μm) and higher magnification (scale bar 200 nm) images from control wildtype and SUR2 null mice. Mitochondria were of similar shape and size between control and SUR2 null cardiomyocytes. Similarly, sarcomere appearance was unchanged in SUR2 null cardiomyocytes.
Supplemental Figure Legend 2. Periodic Acid Schiff (PAS) staining was conducted on heart sections from control and SUR2 null hearts. Comparisons were conducted on heart sections from SUR2 null and control on the same microscope slide. When present, glycogen stains a maroon color. No differences in glycogen content were present (Scale bar = 100 μm).
Acknowledgments
EMM is supported by the NIH and the Burroughs Wellcome Foundation. DS was supported by Medical Scientist National Research Service Award Grant No. 5 T32 GM07281.
Abbreviations
- KATP channel
Adenosine triphosphate sensitive potassium channels
- SUR
sulfonylurea receptor
- ECG
electrocardiogram
- LVDP
left ventricular developed pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–76. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- 2.Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–43. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 4.Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2001;98:7623–8. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford RM, Ranki HJ, Botting CH, Budas GR, Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. Faseb J. 2002;16:102–4. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jovanovic S, Du Q, Crawford RM, Budas GR, Stagljar I, Jovanovic A. Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal K(ATP) channel. EMBO Rep. 2005;6:848–52. doi: 10.1038/sj.embor.7400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes. 1996;45:1439–45. doi: 10.2337/diab.45.10.1439. [DOI] [PubMed] [Google Scholar]
- 8.Chutkow WA, Makielski JC, Nelson DJ, Burant CF, Fan Z. Alternative splicing of sur2 Exon 17 regulates nucleotide sensitivity of the ATP-sensitive potassium channel. J Biol Chem. 1999;274:13656–65. doi: 10.1074/jbc.274.19.13656. [DOI] [PubMed] [Google Scholar]
- 9.Shi NQ, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. J Mol Cell Cardiol. 2005;39:51–60. doi: 10.1016/j.yjmcc.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Giblin JP, Cui Y, Clapp LH, Tinker A. Assembly limits the pharmacological complexity of ATP-sensitive potassium channels. J Biol Chem. 2002;277:13717–23. doi: 10.1074/jbc.M112209200. [DOI] [PubMed] [Google Scholar]
- 11.Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol. 2005;5:1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol. 1997;499 (Pt 3):715–20. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–83. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane GC, Behfar A, Yamada S, Perez-Terzic C, O’Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S, Terzic A. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53 (Suppl 3):S169–75. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- 15.Kane GC, Behfar A, Dyer RB, O’Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–97. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 16.Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol. 2006;577:1053–65. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–16. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol. 2003;285:H921–30. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- 19.Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, Burant CF. Disruption of Sur2-containing K(ATP) channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci U S A. 2001;98:11760–4. doi: 10.1073/pnas.201390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J Clin Invest. 2002;110:203–8. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schyvens CG, Cowden WB, Zhang Y, McKenzie KU, Whitworth JA. Hemodynamic effects of the nitric oxide donor DETA/NO in mice. Clin Exp Hypertens. 2004;26:525–35. doi: 10.1081/ceh-200031828. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marban E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–7. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- 23.Xiaoke Liu SY, Garvan Kane, Alexey E Alekseev, Denice Hodgson, Fearghas O’Cochlain, Arshad Jahangir, Mayo Clinic, Rochester, MN; Takashi Miki, Susumu Seino, Kobe Univ, Kobe, Japan; Andre Terzic, Mayo Clinic, Rochester, MN. Syndrome of Long QT and Sudden Death in the Catecholamine-Challenged, after Depolarization-Prone KATP Channel Knockout Mice. Circulation 2004; Volume 110.
- 24.Tong X, Porter LM, Liu G, Dhar-Chowdhury P, Srivastava S, Pountney DJ, Yoshida H, Artman M, Fishman GI, Yu C, Iyer R, Morley GE, Gutstein DE, Coetzee WA. Consequences of Cardiac Myocyte-Specific Ablation of KATP channels in Transgenic Mice expressing Dominant Negative Kir6 Subunits. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.00051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wajima T, Beguier B, Yaguchi M. Effects of cariporide (HOE642) on myocardial infarct size and ventricular arrhythmias in a rat ischemia/reperfusion model: comparison with other drugs. Pharmacology. 2004;70:68–73. doi: 10.1159/000074670. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto S, Matsui K, Sasabe M, Ohashi N. Effect of an orally active Na+/H+ exchange inhibitor, SMP-300, on experimental angina and myocardial infarction models in rats. J Cardiovasc Pharmacol. 2002;39:234–41. doi: 10.1097/00005344-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Kloner RA, Przyklenk K. Progress in cardioprotection: the role of calcium antagonists. Am J Cardiol. 1990;66:2H–9H. doi: 10.1016/0002-9149(90)90569-m. [DOI] [PubMed] [Google Scholar]
- 28.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–6. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gumina RJ, O’Cochlain DF, Kurtz CE, Bast P, Pucar D, Mishra P, Miki T, Seino S, Macura S, Terzic A. KATP channel knockout worsens myocardial calcium stress load in vivo and impairs recovery in stunned heart. Am J Physiol Heart Circ Physiol. 2007;292:H1706–13. doi: 10.1152/ajpheart.01305.2006. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Cone J, Liu Y. Dual roles of mitochondrial K(ATP) channels in diazoxide-mediated protection in isolated rabbit hearts. Am J Physiol Heart Circ Physiol. 2001;280:H246–55. doi: 10.1152/ajpheart.2001.280.1.H246. [DOI] [PubMed] [Google Scholar]
- 31.Pasdois P, Beauvoit B, Costa AD, Vinassa B, Tariosse L, Bonoron-Adele S, Garlid KD, Santos PD. Sarcoplasmic ATP-sensitive potassium channel blocker HMR1098 protects the ischemic heart: Implication of calcium, complex I, reactive oxygen species and mitochondrial ATP-sensitive potassium channel. J Mol Cell Cardiol. 2006 doi: 10.1016/j.yjmcc.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res. 2006;98:682–9. doi: 10.1161/01.RES.0000207498.40005.e7. [DOI] [PubMed] [Google Scholar]
- 33.Du Q, Jovanovic S, Clelland A, Sukhodub A, Budas G, Phelan K, Murray-Tait V, Malone L, Jovanovic A. Overexpression of SUR2A generates a cardiac phenotype resistant to ischemia. Faseb J. 2006;20:1131–41. doi: 10.1096/fj.05-5483com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford RM, Jovanovic S, Budas GR, Davies AM, Lad H, Wenger RH, Robertson KA, Roy DJ, Ranki HJ, Jovanovic A. Chronic mild hypoxia protects heart-derived H9c2 cells against acute hypoxia/reoxygenation by regulating expression of the SUR2A subunit of the ATP-sensitive K+ channel. J Biol Chem. 2003;278:31444–55. doi: 10.1074/jbc.M303051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranki HJ, Budas GR, Crawford RM, Davies AM, Jovanovic A. 17Beta-estradiol regulates expression of K(ATP) channels in heart-derived H9c2 cells. J Am Coll Cardiol. 2002;40:367–74. doi: 10.1016/s0735-1097(02)01947-2. [DOI] [PubMed] [Google Scholar]
- 36.Flagg TP, Remedi MS, Masia R, Gomes J, McLerie M, Lopatin AN, Nichols CG. Transgenic overexpression of SUR1 in the heart suppresses sarcolemmal K(ATP) J Mol Cell Cardiol. 2005;39:647–56. doi: 10.1016/j.yjmcc.2005.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure Legend 1. Structurally normal cardiomyocytes in SUR2 null mice. Shown are high magnification (scale bar 1 μm) and higher magnification (scale bar 200 nm) images from control wildtype and SUR2 null mice. Mitochondria were of similar shape and size between control and SUR2 null cardiomyocytes. Similarly, sarcomere appearance was unchanged in SUR2 null cardiomyocytes.
Supplemental Figure Legend 2. Periodic Acid Schiff (PAS) staining was conducted on heart sections from control and SUR2 null hearts. Comparisons were conducted on heart sections from SUR2 null and control on the same microscope slide. When present, glycogen stains a maroon color. No differences in glycogen content were present (Scale bar = 100 μm).