Abstract
Tumor necrosis factor-alpha (TNFα) induces cancer development and metastasis, which is prominently achieved by nuclear factor-kappa B (NF-κB) activation. TNFα-induced NF-κB activation enhances cellular mechanisms including proliferation, migration, and invasion. KiSS1, a key regulator of puberty, was initially discovered as a tumor metastasis suppressor. The expression of KiSS1 was lost or down-regulated in different metastatic tumors. However, it is unclear whether KiSS1 regulates TNFα-induced NF-κB activation and further tumor cell migration. In this study, we demonstrate that KiSS1 suppresses the migration of breast cancer cells by inhibiting TNFα-induced NF-κB pathway and RhoA activation. Both KiSS1 overexpression and KP10 (kisspeptin-10) stimulation inhibited TNFα-induced NF-κB activity, suppressed TNFα-induced cell migration and cell attachment to fibronectin in breast cancer cells while KP10 has little effect on cancer cell proliferation. Furthermore, KP10 inhibited TNFα-induced cell migration and RhoA GTPase activation. Therefore, our data demonstrate that KiSS1 inhibits TNFα-induced NF-κB activation via downregulation of RhoA activation and suppression of breast cancer cell migration and invasion.
Keywords: KiSS1, Gpr54, TNFα, RhoA, NF-κB
Introduction
Increasing evidence indicates that TNFα is important for cancer malignancy [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006]. The TNFα signaling cascade includes TNFα receptors, which promotes a recruitment of multiple adaptor proteins such as TRADD and TRAF [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006]. Chronically produced TNFα induces tumor cell motility and invasion via the activation of NF-κB and its target genes, resulting in cancer progression and metastasis [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006]. Abnormally and constitutively active NF-κB is implicated in cancer progression and metastasis [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006]. NF-κB consists of five subunits named RelA (p65), RelB, cRel (Rel), p50 (NF-κB1), and p52 (NF-κB2) that form homo- or heterodimers and tightly bind to their inhibitors, IκBs at the cytoplasm in the resting state [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006]. Upon diverse stimulation, IKKs (IκB kinases) phosphorylates IκBs, inducing the 26S proteasomal degradation of IκBs and liberating NF-κB to translocate into the nucleus and function as a transcriptional factor [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006]. Given the importance of TNFα induced NF-κB pathway in cancer development and metastasis, discovery of agents (peptides or chemical compounds) against TNFα-induced NF-κB pathway is crucial for cancer treatment [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006].
KiSS1, initially identified as an anti-metastatic gene, produces truncated forms of peptides named kisspeptins (KP), including KP10, KP13, KP14, and KP54 [Fernandez-Fernandez et al., 2006; Lee et al., 1996; Lee and Welch, 1997; Mead et al., 2007b; Nash et al., 2007; Nash and Welch, 2006; Ohtaki et al., 2001; Popa et al., 2008; Stafford et al., 2002]. Recent studies have provided various functions of KiSS1 in normal and disease conditions [Fernandez-Fernandez et al., 2006; Mead et al., 2007b; Nash and Welch, 2006; Popa et al., 2008]. In normal physiology, KiSS1 is implicated in the regulation of puberty, reproduction, and homeostasis [Fernandez-Fernandez et al., 2006; Mead et al., 2007b; Popa et al., 2008]. In tumorigenesis, the function of KiSS1 as one of metastasis suppressor genes was supported by both experimental and clinical data [Mead et al., 2007b; Nash and Welch, 2006]. Furthermore, KiSS1 gene products target atherosclerotic vessels, indicating its function in cardiovascular system [Mead et al., 2007a; Mead et al., 2007b]. However, a mechanism by which KiSS1 regulates cancer progression and metastasis remains yet to be resolved although the role of KiSS1 in cancer biology was first revealed and confirmed [Fernandez-Fernandez et al., 2006; Lee et al., 1996; Lee and Welch, 1997; Mead et al., 2007b; Nash et al., 2007; Nash and Welch, 2006; Ohtaki et al., 2001; Popa et al., 2008; Stafford et al., 2002]. Kisspeptins transiently stimulate the formation of focal adhesion complex and stress fiber in CHO cells overexpressing GPR54, the endogenous KiSS1 receptor [Ohtaki et al., 2001]. KiSS1 overexpression also suppresses migration and invasion in MDA-MB-435 breast cancer cells [Lee and Welch, 1997] and inhibits MMP-9 expression via downregulating NF-κB activation in HT-1080 fibrosarcoma cells [Yan et al., 2001]. Inversely, MMPs suppress the function of kisspeptins by cutting kisspeptin’s C-terminus [Takino et al., 2003].
In different cancer cells, active RhoA promotes cell migration, invasion, and metastasis [Brantley-Sieders et al., 2008; Fromigue et al., 2008; Gadea et al., 2007; Gumireddy et al., 2007; Joshi et al., 2008; Lee et al., 2008; Loberg et al., 2007; Martin et al., 2007; Sun et al., 2007; Zhu et al., 2007]. Furthermore, RhoA induces NF-kB activation, which is required for tumor progression [Benitah et al., 2003; Debidda et al., 2005; Perona et al., 1997]. However, it is still unclear whether KiSS1 regulates cell migration and invasion by regulating RhoA activation and its downstream signaling pathways. In this study, we demonstrate that KiSS1 suppresses breast cancer cell migration and invasion by suppressing RhoA-mediated TNFα-induced NF-κB activation.
Materials and methods
Cell culture and reagents
MDA-MB-231, MCF-7, and 293T cells were cultured in DMEM supplemented with 10% FBS and 1% antibiotics. C-terminal amidated Kisspeptin-10 (KP10) was prepared in distilled water with 0.005% DMSO, stored at −20°C and then diluted as needed in the cell culture medium. TNFα (kindly provided by Dr. Aggarwal) was stocked in distilled water with 0.01% DMSO.
Cell proliferation assay
Cell proliferation was monitored using CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay kit according to the manufacturer’s protocol (Promega, Madison, WI).
Flow cytometry analysis
3×106 cells were fixed with 70% ethanol, stained with propidium iodine (PI), and then subjected to flow cytometry analysis. 5000 cells were gated and PI-stained cells were counted. Experiment was performed in triplicate.
Electrophoretic mobility shift assay
Nuclear extract sampled from MDA-MB-231 cells were incubated with 32P-end-labeled 45mer double-stranded oligonucleotide (10μg of protein with 5fmol of DNA), 5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGC-3′ (the bold indicates NF-κB binding sites), for 10 min at RT. The DNA-protein complex formed was separated from free oligonucleotide on 6% native polyacrylamide gels.
Chromatin immunoprecipitation (ChIP) assay
MDA-MB-231 cells were cross-linked with formaldehyde and then nuclear extracts were sonicated. After washed three times, the chromatin was immunoprecipitated with 5μg of NF-κB p65 antibody and protein A/G beads and then heated to reverse the cross-linking. DNA was purified and analyzed by PCR using the primers specific for the NF-κB binding site of MMP-9 promoter. Primers used were as following: 5′-GACCAAGGGATGGGGGATC-3′ and 5′-CTTGACAGGCAAGTGCTGAC-3′. DNA purified from the sonicated nuclear fraction was directly analyzed by general PCR using the same primers and then used for the input control.
Western blot
30μg of total protein was resolved on SDS-PAGE, transferred to PVDF membranes and probed with specific antibodies. The blots were washed, exposed to HRP-conjugated secondary antibodies (Pierce, Rockford, IL), and detected by chemiluminescence reagents. Antibodies against pIKKα/β, IKKα, NF-κB p65, and IκBα were kindly provided by Dr. Aggarwal. Antibody against RhoA, Rac1, or Cdc42 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Rho GTPases activity assay
For detecting active RhoA, GST pull-down was performed. In brief, Rhotekin-RBD (Rho binding domain) purified from E. Coli was incubated with 20μl of 50% Glutathione-bead slurry and 500μg of whole cell lysates for 12hr at 4°C. Active RhoA binding to RhoA-RBD was detected using RhoA antibody. For detecting active Rac1 or Cdc42, protein samples mixed with GST-PAK PBD (Pak binding domain) were pulled-down.
Luciferase assay
Cells were transiently transfected with pNF-κB-luc (Stratagene, La Jolla, CA) and/or plasmids shown in figures. 5hr after transfection, fresh medium was added; cells were treated with KP10 for another 20hr, and then harvested for luciferase assay. To obtain the basal level of NF-κB activity, cells were incubated with medium containing 0.2% serum for 20hr, and then assay. Data was normalized using β-gal assays.
Chemotatic cell migration assay
Cells were cultured in the upper chamber of the Boyden Chamber precoated with gelatin, and the bottom chamber precoated with gelatin was filled with medium containing TNFα to induce chemotatic migration. To examine KiSS1 effect, different concentrations of KP10 were added in the upper chamber. 24hr after cell culture, cells attached on the bottom were stained with crystal violet (2% v/v) and counted. To verify cell counting, crystal violet was eluted and read using a microplate reader at 490mm. To visualize and confirm cell migration, scratching assay was performed. Cells were cultured on 6 well plates and scratched using a 1000μl tip, washed three times with PBS, and cultured for 24hr after treatment with KP10 and/or TNFα. Images were obtained using a Nikon invert microscope. Experiments were performed four times and data was analyzed by the two-tailed student t-test.
Cell attachment assay
Cells were trypsinized with 0.1% Trypsin/EDTA, washed with PBS, incubated in medium containing 0.01% DMSO or KP10 for 30 min. at 37°C, and then poured into fibronectin-precoated wells. After 10 min, cells were washed with PBS and stained with 0.2% crystal violet. Cells that were attached were eluted with 1% SDS and then an absorbance of elutes was counted using a spectrometer plate reader at 490nm.
Immunofluorescence
Cells were cultured on coverslips precoated with poly-L-lysine and then treated with the molecules indicated in figures. Cells were fixed with 3.7% formaldehyde for 15min and then permeablized with 0.1% Triton X-100 for 10min, and blocked with 1% bovine serum albumin (BSA) for 30 min. For p65 intracellular localization, cells were incubated with anti-p65 antibody for 1hr and then incubated with Texas red-conjugated secondary rabbit antibody for 30 min. Images were acquired using Nikon microscope.
Results
KiSS1 suppresses TNFα-induced tumor cell migration, invasion, and matrix-attachment
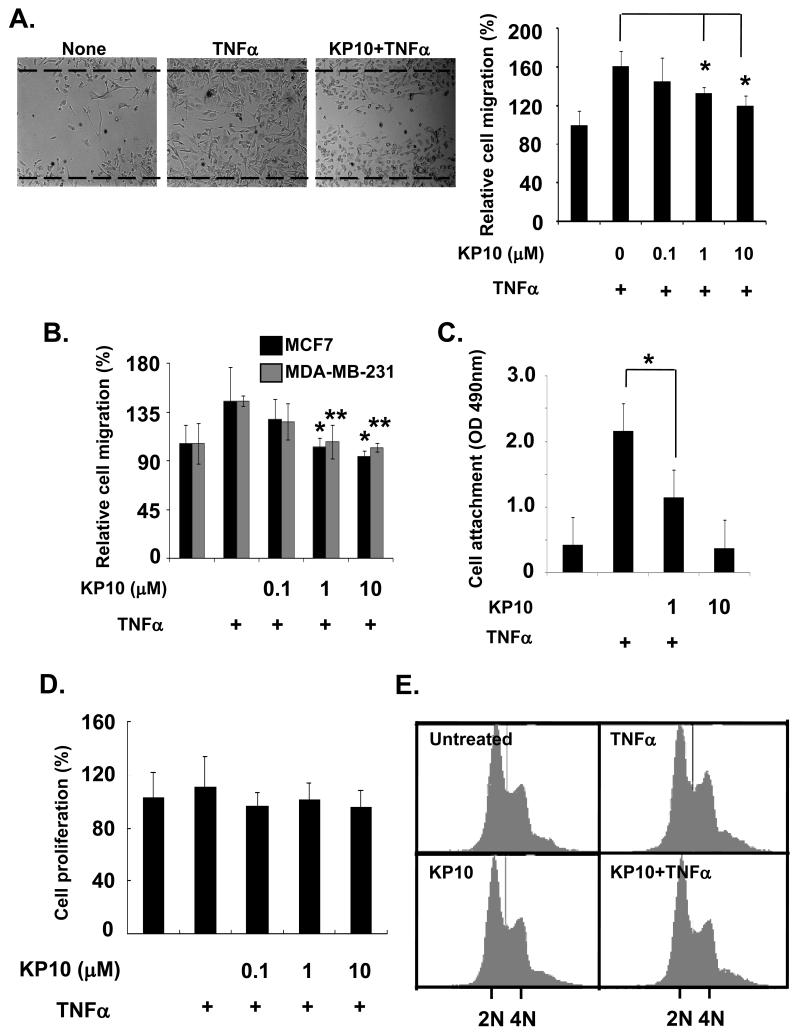
Since TNFα is highly expressed in the tumor microenvironment and induces cancer malignancy [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006], we investigate the roles of KiSS1 on breast cancer cell behavior induced by TNFα treatment. To determine how KiSS1 affects TNFα-activated tumor cell migration and invasion, we performed wound healing assays. As shown in Figure 1A, TNFα increased the migration of MDA-MB-231 cells by ~61% compared with none-treated cells (Figure 1A). Addition of KP10 at 1μM significantly inhibited TNFα-induced cell migration in MDA-MB-231 cells by 40% (Figure 1A). Similar data were obtained for the inhibition of TNFα-induced invasion in both MCF7 and MDA-MB-231 cells by KP10 using Boyden chamber assays. TNFα increased an invasiveness of both MCF7 and MDA-MB-231 cells by ~40 % (Figure 1B). However, 1μM of KP10 blocked TNFα-induced cell invasion (Figure 1B).
Figure 1.
KiSS1 inhibits breast cancer cell migration and attachment but not proliferation. (A) Cell migration was determined using scratching assays. TNFα-induced migration of MDA-MB- 231 cells was downregulated by KP10 at 1 to 10μM. Mean and standard deviations represent data from triplicate experiments (*, p<0.01 vs.TNFα alone). (B) Cell invasion was examined using Boyden chamber assays. In MCF7 and MDA-MB-231 cells, TNFα-stimulated cell invasiveness was inhibited by KP10 at 1 to 10μM. Mean and standard deviations represent data from eight independent experiments (*, p<0.01 vs. TNFα alone). (C) TNFα-induced cell attachment to fibronectin was inhibited by KP10 treatment (1μM of KP10). Mean and standard deviations represent data from triplicate experiments (*, p<0.05 vs. TNFα-treated). (D) Cell proliferation was not affected by KP10, when cells were stimulated by KP10 or TNFα for 24hr. (E) Cell cycle was analyzed using flow cytometry. KP10 and/or TNFα did not affect cell cycle at 24hr after stimulation.
To examine whether KiSS1 affects TNFα-induced cell attachment to extracelluar matrices, MDA-MB-231 cells were platted on fibronectin-coated culture dishes and then TNFα-induced cell attachment to the matrix was measured by spectrometer at 490nm. As shown in Figure 1C, incubation of 1μM of KP10 with breast cancer cells for 30 min significantly inhibited TNFα induced MDA-MB-231 cell attachment to fibronectin (Figure 1C). Together, our data indicate that KiSS1 inhibited TNFα-mediated breast cancer cell migration and matrix-attachment, suggesting a possible inhibitory function of KiSS1 against TNFα-enriched tumor microenvironment.
Since TNFα-induced NF-κB pathway regulates tumor cell proliferation and survival [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006], we further examined how KiSS1 regulated tumor cell proliferation in presence of TNFα. KP10 did not affect MDA-MB-231 (Figure 1D) or MCF7 (data not shown) cell proliferation in the presence or absence of TNFα, when we performed the cell proliferation assays. On the other hand, KiSS1 has been revealed to arrest MDA-MB-436S cells at G2/M phase [Becker et al., 2005]. However, KP10 did not affect cell cycle in the presence or absence of TNFα, when MDA-MB-231 and MCF7 cells were subjected to the flow cytometry (Figure 1E). In sum, KiSS1 affects TNFα-mediated cell motility but not proliferation.
KiSS1 blocks NF-κB transcriptional activity by inhibiting DNA binding of NF-κB
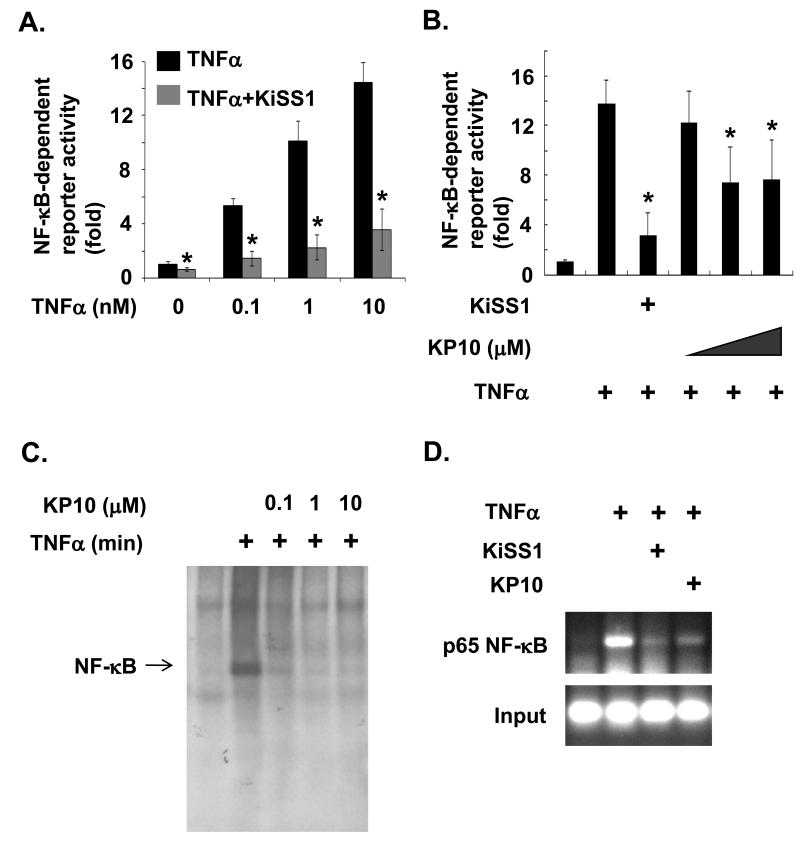
To determine whether KiSS1 inhibits TNFα-induced NF-κB activation in breast cancer cells, we first examined the transcriptional activation of NF-κB in MDA-MB-231 cells either overexpressed with KiSS1 or treated with KP10. When MDA-MB-231 cells were treated with different concentration of TNFα (0.1 to 10nM), NF-κB transcriptional activity was increased with the increase of TNFα in a dose-dependent manner (Figure 2A). Overexpression of KiSS1 gene in the cell inhibited TNFα-induced NF-κB activation even at 10nM concentration of TNFα (Figure 2A), suggesting a role of KiSS1 in TNFα-mediated NF-κB activation. Furthermore, we examine whether KP10, a 10-amino acid peptide derived from KiSS1, can inhibit TNFα-induced NF-κB transcriptional activation. As shown in figure 2B, KP10 at 1 to 10μM also decreased TNFα-induced NF-κB activation in MDA-MB-231 cells stimulated with TNFα. However, the inhibitory effect of KP10 on TNFα-induced NF-κB activation was weaker than that induced by KiSS1 overexpression (Figure 2B).
Figure 2.
Inhibition of NF-κB activation by KiSS1 and KP10. (A) KiSS1 overexpression blocks a basal and TNFα-induced NF-κB activation. Mean and standard deviations represent data from triplicate experiments (*, p<0.05 vs.TNFα-treated samples). (B) TNFα-induced NF-κB promoter activity is downregulated by KiSS1 overexpression and KP10 (at 1 to 10μM). Mean and standard deviations represent data from triplicate experiments (*, p<0.05 vs. TNFα-treated samples). (C) KiSS1 blocks TNFα-induced DNA binding activity of NF-κB in EMSA assays. Cells were treated with 1μM of KP10 for 24hr and then stimulated by 10nM of TNFα for 6hr. (D) KiSS1 inhibits TNFα-induced DNA binding activity of NF-κB on MMP-9 promoter in ChIP assays. DNA from cells stimulated with the indicated agents was cross-linked with formaldehyde, isolated, and then precipitated with anti-NF-κB p65 antibody. Immunoprecipitates were analyzed with PCR using primers specific for MMP-9 promoter region. For the input control, DNA purified from sonicated chromatins was directly subjected to the PCR using primers used.
To confirm our observation that KiSS1 and KP10 inhibit TNFα-induced NF-κB activation, we examined the DNA binding activity of NF-κB using electrophoresis gel mobility-shift assays (EMSA). As shown in figure 2C, KP10 at 0.1μM significantly inhibited TNFα induced DNA binding of NF-κB in MDA-MB-231 cells (Figure 2C). To further investigate the KiSS1-inhibitory function in TNFα-induced DNA binding of NF-κB in vivo, we examined whether KiSS1 affects DNA binding of NF-κB on MMP-9 promoter in TNFα-induced MDA-MB-231 cells using chromatin immunoprecipitation (ChIP) assays. DNAs from MDA-MB-231 cells treated with control buffer, TNFα alone, or TNFα plus KP10, or from cells overexpressing KiSS1 and treated with TNFα were extracted, precipitated with anti-NF-κB p65 antibody, and then subjected to PCR using MMP-9 promoter specific promoters in the chromatin immunoprecipitation (ChIP) assays. As shown in figure 2D, KiSS1 gene overexpression or treatment of KP10 peptide strongly suppressed TNFα—induced DNA binding of NF-κB on MMP-9 promoter (Fig. 2D, lane 3 and lane 4, respectively). Therefore, our data consistently demonstrate that KiSS1 inhibits TNFα—induced DNA binding of NF-κB.
KiSS1 blocks TNFα-induced NF-κB nuclear translocation and IκBα degradation
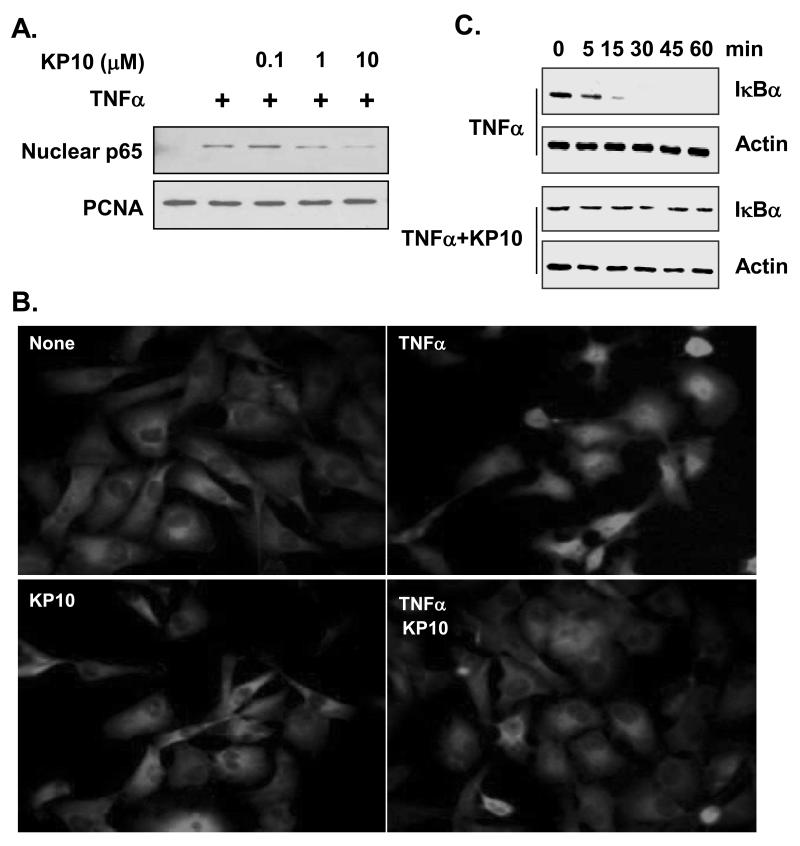
To understand the mechanism of KiSS1 inhibition of NF-κB activation, we examined whether KP10 inhibits TNFα-induced NF-κB nuclear translocation, an upstream event of NF-κB DNA binding [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006] . As shown in figure 3A, addition of TNFα induced the nuclear translocation of p65 NF-κB subunit in nuclear extracts of MDA-MB-231 cells (Fig. 3A). However, treatment of the cells by KP10 at 1 μM blocked the nuclear translocation of p65 NF-κB subunit (Figure 3A).
Figure 3.
KiSS1 inhibits NF-κB nuclear translocation by blocking IκBα degradation. (A) KP10 (1 to 10μM) inhibits nuclear translocation of p65. Nuclear fraction of cell extracts was used to examine nuclear population of p65. PCNA was used as a loading control. (B) p65 translocation was confirmed by immunofluorescence. KP10 blocked TNFα-induced p65 nuclear translocation. (C) KP10 blocked IκBα degradation that is required for the release of NF-κB. Cytosolic fraction of cell extracts was used for examination of IκBα degradation. Actin was used for a loading control.
To confirm the inhibition of NF-κB nuclear translocation by KP10, we performed immunofluorescence assays using the anti-NF-κB p65 antibody. In MDA-MB-231 cells, p65 NF-κB subunit was located at the cytosol without stimulation (Fig. 3B, left top panel, none). 1 μM KP10 alone did not alter the cytosol localization of p65 NF-κB in the resting status (Fig. 3B, left bottom panel, KP10). When the cells were stimulated with TNFα, p65 was activated and translocated into the nucleus (Fig. 3B, right top panel). Addition of 1 μM KP10 significantly blocked TNFα-induced nuclear translocation of p65 NF-κB (Fig. 3B, right bottom panel). Together, these data suggest that KP10 inhibit TNFα-induced NF-κB activation by blocking the nuclear translocation of NF-κB.
We next examined whether KP10 affects TNFα-induced IκBα degradation, as IκBα degradation liberates NF-κB from IκBα/NF-κB complex, resulting in NF-κB nuclear translocation [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006]. MDA-MB-231 cells were stimulated with TNFα in the presence or absence of KP10 for different time points, and then the cytosolic fraction was subjected for the analysis of IκBα degradation. In MDA-MB-231 cells, TNFα induced IκBα degradation at 5 min, which was completed at 15 min (Fig. 3C). Treatment of IκBα degradation KP10 inhibited TNFα-induced IκBα degradation when cytosolic IκBα was probed for 60 min (Fig. 3C).
KiSS1 blocks NF-κB transcriptional activity by inhibiting IKK phosphorylation
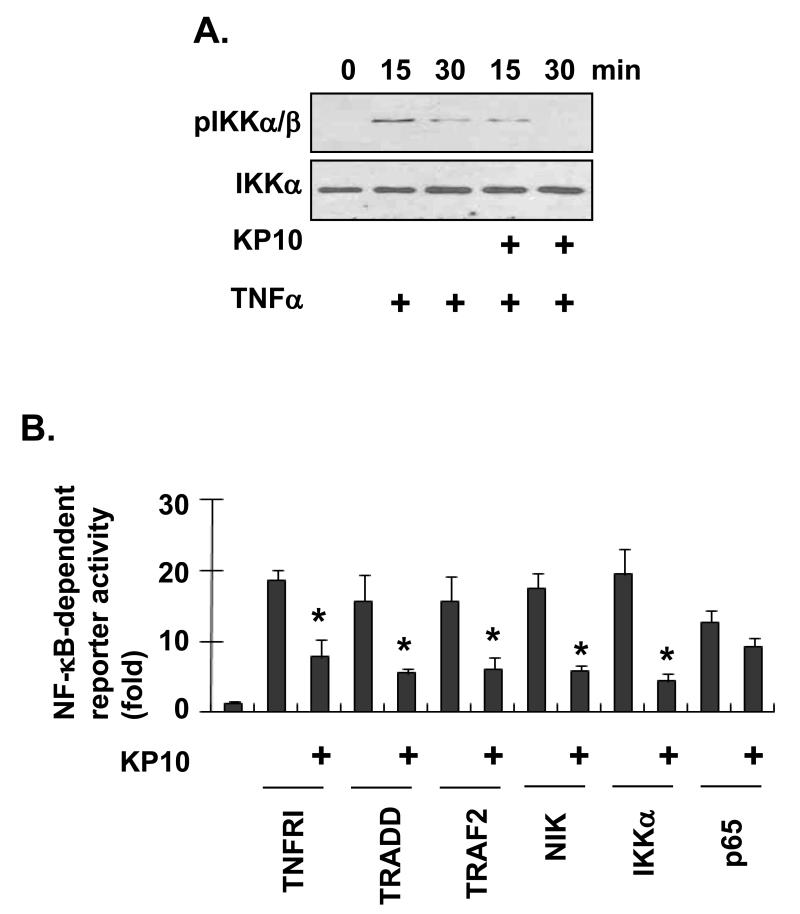
IκBα degradation and NF-κB nuclear translocation resulted from IKK phosphorylation of IκBα, which is sequentially promoted by phosphorylation of IKKs [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006]. To further understand the mechanism of KiSS1 inhibition on TNFα-induced NF-κB, we examined whether KP10 inhibits IKK phosphorylation. In MDA-MB-231 cells, TNFα induced phosphorylation of IKKα/β at 15 min, which was prolonged for 30 min (Fig. 4A). However, treatment of the cells by 1 μM KP10 suppressed TNFα-induced IKKα/β phosphorylation (Fig. 4A), suggesting that KP10 inhibit TNFα-mediated NF-κB activation by regulating IKKα/β phosphorylation.
Figure 4.
KiSS1 inhibits NF-κB transcriptional activation by inhibiting IKK phosphorylation. (A) KP10 inhibited IKK phosphorylation. Cells treated with KP10 and/or TNFα were sampled for Western blot at the times indicated. IKK phosphorylation was examined with anti-IKKα/β antibody. (B) KiSS1 downregulates NF-κB activation mediated by TNFRI, TRADD, TRAF2, NIK, or IKKα. Mean and standard deviations represent data from triplicate samples (*, p<0.05 vs. KP10-untreated).
In the TNFα-induced NF-κB pathway, TNFα receptor signaling complex mediates phosphorylation of IKKs [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006]. To investigate KP10-target molecules upstream of IKKs in TNFα-induced NF-κB pathway, MDA-MB-231 cells were transfected with NF-κB reporter gene combined with TNFRI, TRADD, TRAF2, NIK, IKKα, and p65, respectively. The cells were then stimulated with KP10 for 24hr, and subjected to the luciferase assays. As shown in figure 4B, KP10 blocked NF-κB transcriptional activation induced by molecules including TNFRI, TRADD, TRAF2, NIK, and IKKα, but not p65.
KiSS1 inhibition of RhoA activation results in the inhibition of TNFα-induced cell migration
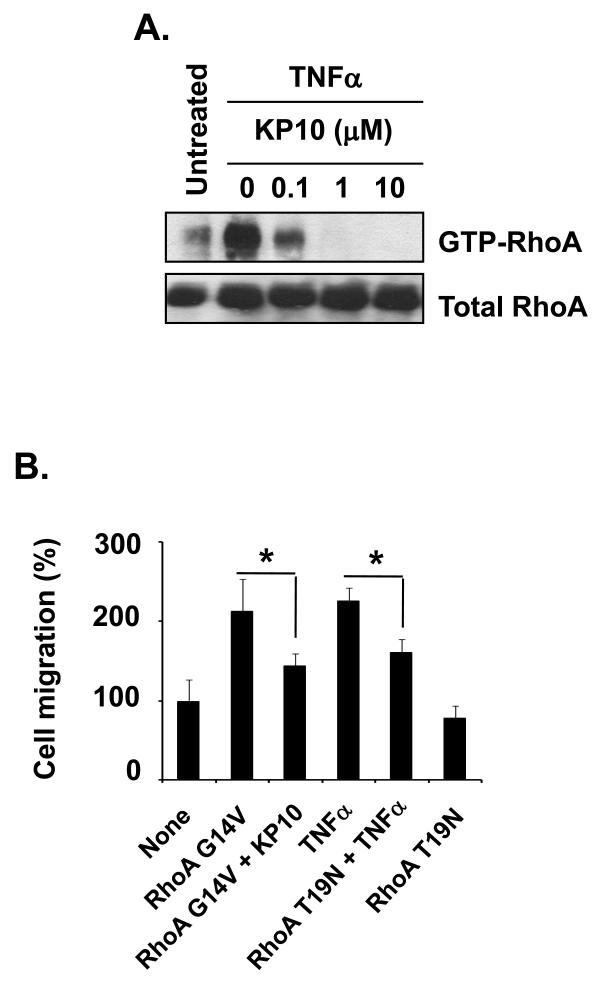
RhoA drives cell motility in terms of dynamics of Rho GTPases and is known to regulate TNFα-induced NF-κB activation [Debidda et al., 2005; Hodge et al., 2003; Perona et al., 1997; Urbinati et al., 2005]. To understand the mechanism of KiSS1 inhibition of TNFα-induced cell migration, we examined RhoA activation in MDA-MB-231 cells treated with TNFα alone or TNFα plus KP10. As shown in figure 5A, TNFα-induced RhoA activation was blocked by KP10 at 0.1 to 10 μM (Fig. 5A). Thus, our data indicate that KP10 target RhoA in TNFα-induced NF-κB activation.
Figure 5.
KiSS1 inhibited RhoA activation (GTP-bound RhoA) and RhoA-mediated cell migration. (A) Cells treated with KP10 and/or TNFα for 24hr and then subjected to the RhoA activity assays. TNFα-induced RhoA activity (GTP-bound RhoA) was inhibited by different concentrations of KP10. (B) Cells were transfected with empty vector, RhoA G14V, or RhoA T19N for 24hr, transferred to the upper chamber precoated with gelatin, and then stimulated with control peptide or KP10 for another 24hr. TNFα was added in the bottom chamber for 24hr in order for the chemotatic attractance. Mean and standard deviations represent data from triplicate experiments (*, p<0.05).
Since RhoA regulates tumor cell migration [Brantley-Sieders et al., 2008; Fromigue et al., 2008; Gadea et al., 2007; Gumireddy et al., 2007; Joshi et al., 2008; Lee et al., 2008; Loberg et al., 2007; Martin et al., 2007; Sun et al., 2007; Zhu et al., 2007] , we next examined whether KP10 inhibits RhoA-dependent migration. MDA-MB-231 cells were transfected with dominant active RhoA (RhoA G14V) and then examined cell migration in the presence or absence of KP10. Compared with cells transfected with empty vector, cells overexpressing RhoA G14V showed increased cell migration (Fig. 5B). However, KP10 treatment inhibited RhoA-enhanced cell migration (Fig. 5B). To confirm RhoA-induced cell migration and KP10 inhibition of RhoA-mediated cell migration, MDA-MB-231 cells were transfected with dominant negative RhoA (RhoA T19N), and then examined for cell migration in the presence or absence of TNFα. Cells overexpressing RhoA T19N migrated slightly slower than cells transfected with empty vector. However, TNFα-induced MDA-MB-231 cell migration was significantly inhibited by RhoA T19N (Fig. 5B, fourth and fifth bars). Thus, our data indicate that TNFα-induced cell migration requires RhoA activity and KP10 targets RhoA in cell migration.
KiSS1 inhibits TNFα-induced NF-κB activation through RhoA GTPase
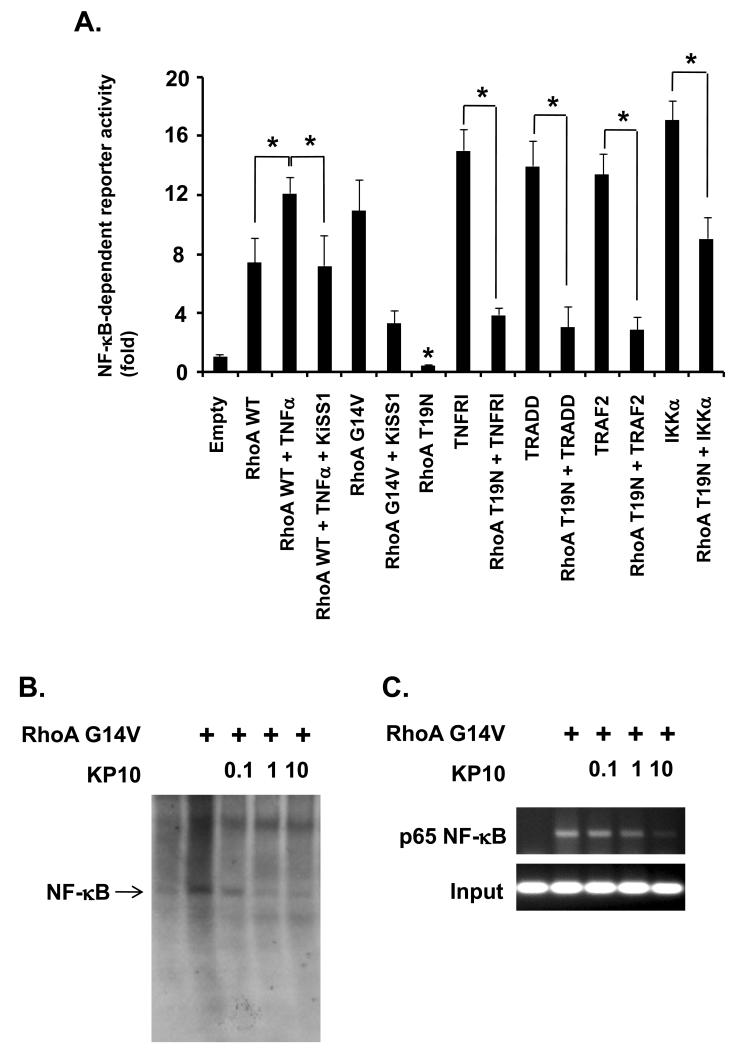
Next, we examined whether RhoA is KP10-target molecule in the KP10 inhibition of TNFα-induced NF-κB activation. To examine RhoA requirement in TNFα-induced NF-κB activation, MDA-MB-231 cells were transfected with wild type RhoA, stimulated with TNFα, and then subjected to the luciferase assays to monitor NF-κB reporter gene activity. Wild type RhoA induced NF-κB transcriptional activation, which was enhanced by TNFα (Figure 6A, left three bars). To confirm RhoA activation of NF-κB transcriptional activity, we transfected the cells with active RhoA G14V or inactive RhoA T19N, and then performed the same luciferase assays. Consistent with our experiments, RhoA G14V induced NF-κB transcriptional activity while RhoA T19N inhibited NF-κB activity (Fig. 6A, fifth and eighth bars, respectively).
Figure 6.
KiSS1 inhibited RhoA-mediated NF-κB activation. (A) NF-κB transcriptional activity was examined in cells transfected with the indicated plasmids. Luciferase assays were performed at 36hr after transfection. In the case of TNFα, cells were sampled 6hr after treatment. Mean and standard deviations represent data from triplicate experiments (*, p<0.05). (B) DNA binding activity of NF-κB was examined in the conditions as indicated in EMSA. A dominant active RhoA (Rho G14V) enhanced NF-κB binding onto DNA, but was reduced by different concentrations of KP10. (C) KiSS1 inhibits RhoA-induced DNA binding of NF-κB on MMP-9 promoter. DNA from cells stimulated with the indicatives was isolated, and then precipitated with anti- NF-κB p65 antibody. Immunoprecipitates were subjected to the PCR using primers specific for MMP-9 promoter region. For the input control, DNA purified from sonicated chromatins was directly subjected to the PCR using primers used.
To further investigate the role of RhoA in TNFα-induced NF-κB pathway, MDA-MB-231 cells were transfected with RhoA T19N combined with TNFRI, TRADD, TRAF2, and IKKα, respectively. RhoA T19N repressed NF-κB transcriptional activity induced by TNFRI, TRADD, TRAF2 (Figure 6A, tenth to twelfth bars). However, RhoA T19N partially repressed IKKα-induced NF-κB transcriptional activation (Figure 6A, ninth bar), suggesting RhoA is upstream of IKKs in TNFα-induced NF-κB activation.
We next analyzed whether KiSS1 inhibits RhoA-mediated NF-κB activation upon TNFα stimulation. Compared with NF-κB transcriptional activity in cells transfected with RhoA wild type and stimulated with TNFα, the activity in cells co-transfected with KiSS1 and RhoA wild type, and stimulated with TNFα, was significantly reduced (Figure 6A, third and fourth bars). In addition, NF-κB transcriptional activity was reduced in cells co-transfected with KiSS1 and RhoA G14V when compared to that in cells transfected with RhoA G14V alone (Figure 6A, fifth and sixth bars), suggesting KiSS1 inhibit RhoA-mediated NF-κB activation.
To confirm KiSS1 inhibition of RhoA-mediated NF-κB activation, we performed EMSA to examine DNA binding of NF-κB in MDA-MB-231 cells overexpressing RhoA G14V in the presence of different concentrations of KP10. RhoA G14V induced DNA binding of NF-κB, which was significantly inhibited by KP10 at 1μM (Figure 6B). Furthermore, we performed ChIP assays to confirm KiSS1 inhibition of RhoA-mediated DNA binding of NF-κB in vivo. In MDA-MB-231 cells overexpressing RhoA G14V, DNA binding of NF-κB on MMP-9 promoter was enhanced. However, KP10 inhibited the active RhoA-induced DNA binding of NF-κB on MMP-9 promoter in a dose-dependent manner (Figure 6C). Together, our data demonstrate that KiSS1 suppresses breast cancer cell migration and invasion by targeting RhoA and inhibiting TNFα-induced NF-κB transcriptional activation.
Discussion
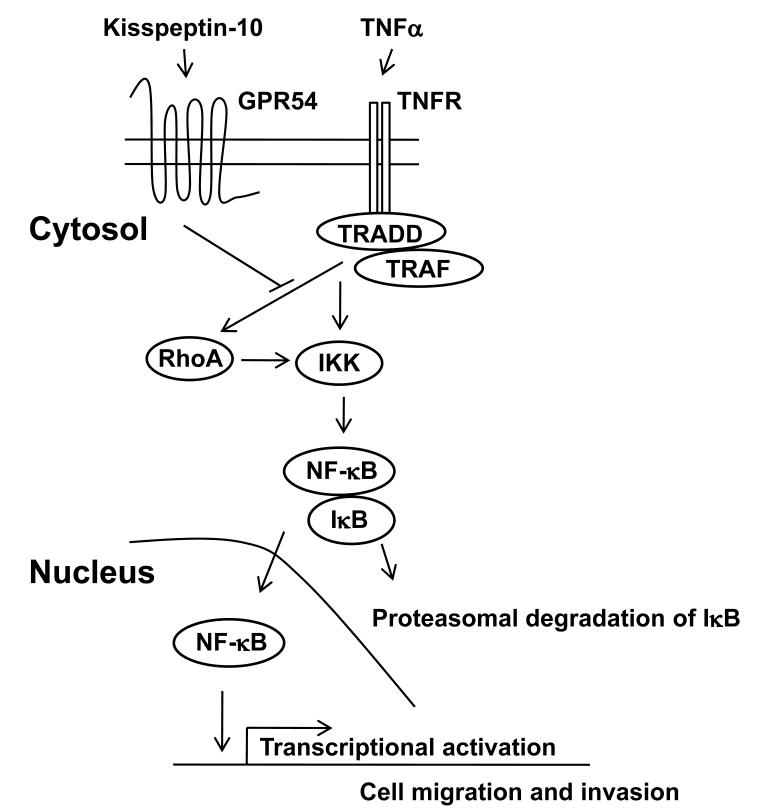
TNFα-induced NF-κB pathway is one of the well known molecular targets for cancer treatment [Balkwill, 2006; Hacker and Karin, 2006; Karin, 2006; Naugler and Karin, 2008]. In tumor microenvironment, the active NF-κB in immune cells highly produces TNFα, resulting in the NF-κB-dependent expression of MMPs in tumor cells [Deryugina and Quigley, 2006; Nyberg et al., 2008]. In this study, we demonstrate that KiSS1 inhibits breast cancer cell migration by suppressing RhoA activation and TNFαinduced NF-κB activation (Fig. 7).
Figure 7.
Schematic model for KiSS1 inhibition of TNFa-mediated NF-kB activation. TNFa-activated TNFR complex leads to the activation of NF-kB via IKK activation. In addition, TNFa-activated TNFR complex activates RhoA, which also induces IKK-mediated NF-kB activation. Upon kisspeptin-10 stimulation, KISS1 signaling via GPR54 inhibits TNFa-induced RhoA activation, which results in the suppression of TNFa-induced NF-kB activation.
We found that KiSS1 inhibits TNFα-induced breast cancer cell migration. Recently, two groups found that KiSS1 is highly expressed in breast tumors compared with normal breast tissues [Marot et al., 2007; Martin et al., 2005], although a loss of KiSS1 expression in invasive breast cancer cell lines was proven [Lee et al., 1996; Lee and Welch, 1997; Mitchell et al., 2006]. Thus, it is possible that KiSS1 has an autocrine role in non-invasive breast tumor cells and a paracrine path of kisspeptins blocks a motility of invasive breast cancer cells in TNFα-enriched tumor environment, as our data show that KiSS1 targets both MCF7 and MDA-MB-231 breast cancer cells. However, we cannot exclude another paracrine path of kisspeptins in tumor environment as suggested by Nash et al. in tumor microenvironment [Nash et al., 2007]. Our lab previously reported that KiSS1 inhibits the proliferation and migration of NIH3T3 fibroblast overexpressing GPR54 [Stafford et al., 2002]. In addition, Mead et al. found that KP10 targets atherosclerotic vessels [Mead et al., 2007a]. Therefore, the exact role of KiSS1 in tumor microenvironment needs to be elucidated in vivo.
RhoA activity is implicated in cancer cell migration, invasion, and cytoskeletal re-organization [Brantley-Sieders et al., 2008; Fromigue et al., 2008; Gadea et al., 2007; Gumireddy et al., 2007; Joshi et al., 2008; Lee et al., 2008; Loberg et al., 2007; Martin et al., 2007; Sun et al., 2007; Zhu et al., 2007] . In TNFα-stimulated cells, RhoA activation mediates NF-κB activation and further induces cell migration [Debidda et al., 2005; Hodge et al., 2003; Perona et al., 1997; Urbinati et al., 2005]. Our study demonstrates KiSS1 inhibited TNFα-induced RhoA activation and further RhoA-mediated tumor cell migration. Moreover, we also found that KiSS1 blocked RhoA-mediated NF-kB activation. However, KiSS1 alone induced stress fiber formation, which was mediated by RhoA in ACHN and Caki-1 renal carcinoma cell lines [Shoji et al., 2008]. Considering studies by Shoji et al. and us, KiSS1-targeted RhoA appears to determine cell fate [Gumireddy et al., 2007; Shoji et al., 2008]. Thus, KiSS1-mediated signaling may be pleotrophic in different biological conditions. This is consistent with recent studies that KP10 inhibits CXCL12/CXCR4-induced calcium mobilization [Navenot et al., 2005] but KP10 alone increases intracellular calcium level [Bilban et al., 2004; Brailoiu et al., 2005]. Therefore, it will be interesting to understand a functional relationship between KiSS1-induced GPR54 signaling and the TNFα-TNFR-mediated proinflammatory cytokine-mediated signaling in our future research.
Direct GPCR regulation of NF-κB pathway is also crucial for normal and disease conditions [Ye, 2001]. Intracellular signaling mediators such as PKCs and CARMAs are important for GPCR activation of NF-κB pathway [Wegener and Krappmann, 2007]. KiSS1-induced GPR54 activates Gαq/11 and PLCβ, and affects phosphorylation of PKCs and the intracellular calcium level [Jiang et al., 2005; Nash et al., 2007; Niida et al., 2006; Stafford et al., 2002]. In HT-1080 cells, KiSS1 overexpression induced IκBα expression, resulting in the inhibition of NF-κB activation [Yan et al., 2001]. Likewise, KP10 stimulation also induced NF-κB-dependent IκBα expression in MDA-MB-436S cells in the DNA microarray [Becker et al., 2005]. In ovarian cancer cells, KiSS1 increased intracellular Ca2+ level, induced NFAT-dependent expression of MCIP1 (myocyte-enriched calcineurin interacting protein 1; an inhibitor of calcineurin), and then inhibited NFAT nuclear translocation [Stathatos et al., 2005]. MCIP1-dependent negative feedback mechanism in the intracellular Ca2+-mediated signaling pathway is implicated in cancer biology and vascular biology [Arron et al., 2006; Rothermel et al., 2003; Ryeom et al., 2008; Stathatos et al., 2005; Vega et al., 2003]. However, KiSS1 inhibited PKCα, the upstream molecule of NFAT, in ovarian cancer cells [Jiang et al., 2005]. Thus, together with our study, KiSS1-induced GPR54 signaling seems to gain complexity under different biological conditions.
In conclusion, our study demonstrates that KiSS1 suppresses the activation of TNFα-RhoA-NF-κB signaling, resulting in the suppression of NF-κB-mediated gene transcription, cell migration and invasion (Fig. 7). Therefore, a loss of KiSS1 expression in invasive breast cancer cells fails to suppress TNFα-RhoA-NF-κB activation, resulting in an increase of tumor cell invasiveness [Mead et al., 2007b; Nash and Welch, 2006]. However, both direct molecular targets of KiSS1 in TNFα-stimulated tumor cells and KiSS1-target cell types in tumor microenvironment remain to be determined.
Acknowledgments
This work was supported by NIH grant 1R01CA106479 to Dr. Liu M.
Contract grant sponsor: NIH; Contract grant number: 1R01CA106479
References
- Arron JR, Winslow MM, Polleri A, Chang CP, Wu H, Gao X, Neilson JR, Chen L, Heit JJ, Kim SK, Yamasaki N, Miyakawa T, Francke U, Graef IA, Crabtree GR. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–16. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- Becker JA, Mirjolet JF, Bernard J, Burgeon E, Simons MJ, Vassart G, Parmentier M, Libert F. Activation of GPR54 promotes cell cycle arrest and apoptosis of human tumor cells through a specific transcriptional program not shared by other Gq-coupled receptors. Biochem Biophys Res Commun. 2005;326:677–86. doi: 10.1016/j.bbrc.2004.11.094. [DOI] [PubMed] [Google Scholar]
- Benitah SA, Valeron PF, Lacal JC. ROCK and nuclear factor-kappaB-dependent activation of cyclooxygenase-2 by Rho GTPases: effects on tumor growth and therapeutic consequences. Mol Biol Cell. 2003;14:3041–54. doi: 10.1091/mbc.E03-01-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, Malli R, Sharabi A, Hiden U, Graier W, Knofler M, Andreae F, Wagner O, Quaranta V, Desoye G. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–28. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Dun SL, Ohsawa M, Yin D, Yang J, Chang JK, Brailoiu E, Dun NJ. KiSS-1 expression and metastin-like immunoreactivity in the rat brain. J Comp Neurol. 2005;481:314–29. doi: 10.1002/cne.20350. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, Coffman K, Jackson D, Bruckheimer E, Muraoka-Cook RS, Chen J. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debidda M, Wang L, Zang H, Poli V, Zheng Y. A role of STAT3 in Rho GTPase-regulated cell migration and proliferation. J Biol Chem. 2005;280:17275–85. doi: 10.1074/jbc.M413187200. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol. 2006;254-255:127–32. doi: 10.1016/j.mce.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Fromigue O, Hamidouche Z, Marie PJ. Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces osteosarcoma cell invasion. J Biol Chem. 2008;283:30549–56. doi: 10.1074/jbc.M801436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea G, de Toledo M, Anguille C, Roux P. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J Cell Biol. 2007;178:23–30. doi: 10.1083/jcb.200701120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumireddy K, Sun F, Klein-Szanto AJ, Gibbins JM, Gimotty PA, Saunders AJ, Schultz PG, Huang Q. In vivo selection for metastasis promoting genes in the mouse. Proc Natl Acad Sci U S A. 2007;104:6696–701. doi: 10.1073/pnas.0701145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- Hodge JC, Bub J, Kaul S, Kajdacsy-Balla A, Lindholm PF. Requirement of RhoA activity for increased nuclear factor kappaB activity and PC-3 human prostate cancer cell invasion. Cancer Res. 2003;63:1359–64. [PubMed] [Google Scholar]
- Jiang Y, Berk M, Singh LS, Tan H, Yin L, Powell CT, Xu Y. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin Exp Metastasis. 2005;22:369–76. doi: 10.1007/s10585-005-8186-4. [DOI] [PubMed] [Google Scholar]
- Joshi B, Strugnell SS, Goetz JG, Kojic LD, Cox ME, Griffith OL, Chan SK, Jones SJ, Leung SP, Masoudi H, Leung S, Wiseman SM, Nabi IR. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 2008;68:8210–20. doi: 10.1158/0008-5472.CAN-08-0343. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–7. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–7. [PubMed] [Google Scholar]
- Lee MJ, Jeon ES, Lee JS, Cho M, Suh DS, Chang CL, Kim JH. Lysophosphatidic acid in malignant ascites stimulates migration of human mesenchymal stem cells. J Cell Biochem. 2008;104:499–510. doi: 10.1002/jcb.21641. [DOI] [PubMed] [Google Scholar]
- Loberg RD, Tantivejkul K, Craig M, Neeley CK, Pienta KJ. PAR1-mediated RhoA activation facilitates CCL2-induced chemotaxis in PC-3 cells. J Cell Biochem. 2007;101:1292–300. doi: 10.1002/jcb.21252. [DOI] [PubMed] [Google Scholar]
- Marot D, Bieche I, Aumas C, Esselin S, Bouquet C, Vacher S, Lazennec G, Perricaudet M, Kuttenn F, Lidereau R, de Roux N. High tumoral levels of Kiss1 and G-protein-coupled receptor 54 expression are correlated with poor prognosis of estrogen receptor-positive breast tumors. Endocr Relat Cancer. 2007;14:691–702. doi: 10.1677/ERC-07-0012. [DOI] [PubMed] [Google Scholar]
- Martin MJ, Tanos T, Garcia AB, Martin D, Gutkind JS, Coso OA, Marinissen MJ. The Galpha12/13 family of heterotrimeric G proteins and the small GTPase RhoA link the Kaposi sarcoma-associated herpes virus G protein-coupled receptor to heme oxygenase-1 expression and tumorigenesis. J Biol Chem. 2007;282:34510–24. doi: 10.1074/jbc.M703043200. [DOI] [PubMed] [Google Scholar]
- Martin TA, Watkins G, Jiang WG. KiSS-1 expression in human breast cancer. Clin Exp Metastasis. 2005;22:503–11. doi: 10.1007/s10585-005-4180-0. [DOI] [PubMed] [Google Scholar]
- Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor, G protein-coupled receptor 54, to atherosclerosis-prone vessels. Endocrinology. 2007a;148:140–7. doi: 10.1210/en.2006-0818. [DOI] [PubMed] [Google Scholar]
- Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins: a multifunctional peptide system with a role in reproduction, cancer and the cardiovascular system. Br J Pharmacol. 2007b;151:1143–53. doi: 10.1038/sj.bjp.0707295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DC, Abdelrahim M, Weng J, Stafford LJ, Safe S, Bar-Eli M, Liu M. Regulation of KiSS-1 metastasis suppressor gene expression in breast cancer cells by direct interaction of transcription factors activator protein-2alpha and specificity protein-1. J Biol Chem. 2006;281:51–8. doi: 10.1074/jbc.M506245200. [DOI] [PubMed] [Google Scholar]
- Nash KT, Phadke PA, Navenot JM, Hurst DR, Accavitti-Loper MA, Sztul E, Vaidya KS, Frost AR, Kappes JC, Peiper SC, Welch DR. Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy. J Natl Cancer Inst. 2007;99:309–21. doi: 10.1093/jnci/djk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash KT, Welch DR. The KISS1 metastasis suppressor: mechanistic insights and clinical utility. Front Biosci. 2006;11:647–59. doi: 10.2741/1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navenot JM, Wang Z, Chopin M, Fujii N, Peiper SC. Kisspeptin-10-induced signaling of GPR54 negatively regulates chemotactic responses mediated by CXCR4: a potential mechanism for the metastasis suppressor activity of kisspeptins. Cancer Res. 2005;65:10450–6. doi: 10.1158/0008-5472.CAN-05-1757. [DOI] [PubMed] [Google Scholar]
- Niida A, Wang Z, Tomita K, Oishi S, Tamamura H, Otaka A, Navenot JM, Broach JR, Peiper SC, Fujii N. Design and synthesis of downsized metastin (45-54) analogs with maintenance of high GPR54 agonistic activity. Bioorg Med Chem Lett. 2006;16:134–7. doi: 10.1016/j.bmcl.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Salo T, Kalluri R. Tumor microenvironment and angiogenesis. Front Biosci. 2008;13:6537–53. doi: 10.2741/3173. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–7. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal JC. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:463–75. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008;70:213–38. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- Rothermel BA, Vega RB, Williams RS. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc Med. 2003;13:15–21. doi: 10.1016/s1050-1738(02)00188-3. [DOI] [PubMed] [Google Scholar]
- Ryeom S, Baek KH, Rioth MJ, Lynch RC, Zaslavsky A, Birsner A, Yoon SS, McKeon F. Targeted deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer Cell. 2008;13:420–31. doi: 10.1016/j.ccr.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Shoji S, Tang XY, Umemura S, Itoh J, Takekoshi S, Shima M, Usui Y, Nagata Y, Uchida T, Osamura RY, Terachi T. Metastin Inhibits Migration and Invasion of Renal Cell Carcinoma with Overexpression of Metastin Receptor. Eur Urol. 2008 doi: 10.1016/j.eururo.2008.02.048. [DOI] [PubMed] [Google Scholar]
- Stafford LJ, Xia C, Ma W, Cai Y, Liu M. Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res. 2002;62:5399–404. [PubMed] [Google Scholar]
- Stathatos N, Bourdeau I, Espinosa AV, Saji M, Vasko VV, Burman KD, Stratakis CA, Ringel MD. KiSS-1/G protein-coupled receptor 54 metastasis suppressor pathway increases myocyte-enriched calcineurin interacting protein 1 expression and chronically inhibits calcineurin activity. J Clin Endocrinol Metab. 2005;90:5432–40. doi: 10.1210/jc.2005-0963. [DOI] [PubMed] [Google Scholar]
- Sun HW, Tong SL, He J, Wang Q, Zou L, Ma SJ, Tan HY, Luo JF, Wu HX. RhoA and RhoC -siRNA inhibit the proliferation and invasiveness activity of human gastric carcinoma by Rho/PI3K/Akt pathway. World J Gastroenterol. 2007;13:3517–22. doi: 10.3748/wjg.v13.i25.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takino T, Koshikawa N, Miyamori H, Tanaka M, Sasaki T, Okada Y, Seiki M, Sato H. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene. 2003;22:4617–26. doi: 10.1038/sj.onc.1206542. [DOI] [PubMed] [Google Scholar]
- Urbinati C, Bugatti A, Giacca M, Schlaepfer D, Presta M, Rusnati M. alpha(v)beta3-integrin-dependent activation of focal adhesion kinase mediates NF-kappaB activation and motogenic activity by HIV-1 Tat in endothelial cells. J Cell Sci. 2005;118:3949–58. doi: 10.1242/jcs.02518. [DOI] [PubMed] [Google Scholar]
- Vega RB, Rothermel BA, Weinheimer CJ, Kovacs A, Naseem RH, Bassel-Duby R, Williams RS, Olson EN. Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:669–74. doi: 10.1073/pnas.0237225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener E, Krappmann D. CARD-Bcl10-Malt1 signalosomes: missing link to NF-kappaB. Sci STKE. 2007;2007:pe21. doi: 10.1126/stke.3842007pe21. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang H, Boyd DD. KiSS-1 represses 92-kDa type IV collagenase expression by down-regulating NF-kappa B binding to the promoter as a consequence of Ikappa Balpha -induced block of p65/p50 nuclear translocation. J Biol Chem. 2001;276:1164–72. doi: 10.1074/jbc.M008681200. [DOI] [PubMed] [Google Scholar]
- Ye RD. Regulation of nuclear factor kappaB activation by G-protein-coupled receptors. J Leukoc Biol. 2001;70:839–48. [PubMed] [Google Scholar]
- Zhu Q, Krakowski AR, Dunham EE, Wang L, Bandyopadhyay A, Berdeaux R, Martin GS, Sun L, Luo K. Dual role of SnoN in mammalian tumorigenesis. Mol Cell Biol. 2007;27:324–39. doi: 10.1128/MCB.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]