Abstract
High hypoxia-inducible factor-2α (HIF-2α) protein levels predict poor outcome in neuroblastoma, and hypoxia dedifferentiates cultured neuroblastoma cells toward a neural crest-like phenotype. Here, we identify HIF-2α as a marker of normoxic neural crest-like neuroblastoma tumor-initiating/stem cells (TICs) isolated from patient bone marrows. Knockdown of HIF-2α reduced VEGF expression and induced partial sympathetic neuronal differentiation when these TICs were grown in vitro under stem cell-promoting conditions. Xenograft tumors of HIF-2α-silenced cells were widely necrotic, poorly vascularized, and resembled the bulk of tumor cells in clinical neuroblastomas by expressing additional sympathetic neuronal markers, whereas control tumors were immature, well-vascularized, and stroma-rich. Thus, HIF-2α maintains an undifferentiated state of neuroblastoma TICs. Because low differentiation is associated with poor outcome and angiogenesis is crucial for tumor growth, HIF-2α is an attractive target for neuroblastoma therapy.
Keywords: differentiation, tumor stroma, HIF-1, hypoxia, sympathetic nervous system
The childhood tumor neuroblastoma is a sympathetic nervous system (SNS) malignancy that can present at any location where sympathetic neuroblasts are found. Neuroblastomas vary considerably in stage of sympathetic differentiation. Patients with highly differentiated tumors (i.e., high expression of neuronal sympathetic differentiation markers) have a more favorable prognosis than those with more immature tumors (1). Whether differences in maturation stage reflect that neuroblastomas derive from precursor cells arrested at corresponding diverse differentiation stages, or whether neuroblastomas derive from stem cell-like cells with different capabilities to mature neuronally, is an open question. The recent identification in tumor sections of immature neural crest-like tumor cells virtually devoid of SNS marker expression in neuroblastoma specimens (2) might suggest that neuroblastomas derive from an immature precursor cell, a neuroblastoma stem/initiating/founder cell, and that the bulk of tumor cells are considerably more differentiated than the initiating cell.
The early regulatory steps of human SNS development are not known, but extrapolation from rodent and avian development provides a conceptual framework for human neural crest development and specification of cells destined for the sympathetic ganglionic lineage. Early induction in vertebral neural crest specification toward SNS development involves transcription factors, like Cash-1/Mash-1/Ascl1, Phox2b, Phox2a, dHAND, and Islet-1 which, in turn, induce the noradrenergic phenotype by transcribing the catecholamine-synthesizing genes TH and DBH (3).
We have demonstrated that low tumor oxygen levels—hypoxia—can lead to reduced expression of differentiation lineage-specific genes and the development of stem cell-like phenotypes, as first demonstrated in neuroblastoma (4) and later in breast cancer (5). We have also reported that high hypoxia-inducible factor-2α (HIF-2α), but not HIF-1α, protein levels in neuroblastoma and breast cancer specimens correlate with poor outcome and distal metastasis (5, 6). In neuroblastoma specimens, we further identified a small subset of tumor cells that are strongly positive for HIF-2α, express neural crest-associated genes, and lack expression of SNS markers. We postulated that these cells might be the tumor stem cells of neuroblastoma (2), but it is unclear whether HIF-2α is the cause or a consequence of the undifferentiated state.
Neuroblastoma tumor-initiating cells (TICs) were recently isolated from bone marrow metastases of patients with high-risk neuroblastoma, and when cultured as neurospheres they retained their tumor-forming properties (7). Here, we found that cultured TICs have high HIF-2α protein levels at normoxia and express neural crest and stem cell-associated genes but lack or have minute expression of SNS markers, and they are in these aspects remarkably similar to the strongly HIF-2α-positive cells in tumor specimens we reported on recently (2). Knockdown of HIF-2α or inhibition of HIF-2α synthesis in TICs resulted in enhanced ASCL1 expression and induced expression of a number of SNS differentiation marker genes. In vivo, tumors of HIF-2α knockdown cells were poorly vascularized and highly necrotic, expressed HIF-1α protein, and differentiated further toward a mature neuronal phenotype, thus resembling the bulk of cells in human neuroblastoma specimens. In contrast, tumors of control cells with high HIF-2α were well-vascularized, rich in stroma, virtually HIF-1α-negative, and retained an undifferentiated phenotype. As opposed to HIF-2α, HIF-1α was not associated with adverse clinical outcome and correlated negatively to advanced clinical stage, and thus tumor spread in human neuroblastoma. We conclude that HIF-2α maintains bone marrow-derived neuroblastoma tumor cells at a neural crest-like stage of differentiation in vitro and in vivo and has profound effects on tumor stroma and blood vessel formation in vivo.
Results
Neuroblastoma TICs Express High Levels of HIF-2α Protein at Normoxia.
As described previously, immunohistochemical staining of human neuroblastoma tissue sections identified a subset of tumor cells in well-vascularized tissue regions that expressed high levels of HIF-2α and neural crest-associated genes while lacking expression of SNS differentiation markers, such as TH (Fig. 1A) (2). In human neuroblastoma TICs isolated from high-risk patient bone marrows and grown as neurospheres (7), both HIF-1α and HIF-2α were expressed at the mRNA level in all four lines tested (Fig. 1 B and D), but only HIF-2α protein was readily detectable when cells were grown under normoxic conditions (21% O2; Fig. 1 C and E). Immunostainings revealed that HIF-2α protein was highly expressed in these cells, even at normoxia, and that they also had no or barely detectable TH protein expression (Fig. 1F). Normoxic HIF transcription and translation can be growth factor-driven, but HIF-2α expression remained high in TICs both at the mRNA (qPCR) and protein (immunostaining) levels when the culture medium did not contain EGF and bFGF (Fig. 1G and Fig. S1) and when spheres were dissociated 4 h before analysis.
Fig. 1.
Normoxic HIF-2α protein levels are high in cultured neuroblastoma TICs. (A) Neuroblastoma specimen stained by immunohistochemistry for HIF-2α and TH. *, Magnified areas as shown in the two panels to the right. Note the virtual lack of TH signal in the HIF-2α-positive cells. (B–F) HIF-2α is expressed in neuroblastoma TICs at normoxia, as determined by qPCR (B), Western blot analysis (C), and immunohistochemistry (F). HIF-1α is expressed in neuroblastoma TICs, as determined by qPCR (D), but protein is generally very low at normoxia (E). HIF-2α but not TH protein levels are high in neuroblastoma TICs, as demonstrated by immunohistochemistry (F). (G) HIF-2α is expressed in TICs at normoxia with or without EGF and bFGF in the culture medium, as shown by immunohistochemistry. In C and E, actin serves as loading control. (Scale bars: A, 50 μm; F and G, 10 μm.) The qPCR data are mean values of three independent experiments performed in triplicate. Error bars represent SEM. Immunohistochemistry and Western blot analysis data were repeated at least three times. Dividing lines in C and E indicate that two areas of the same film or two different films have been merged.
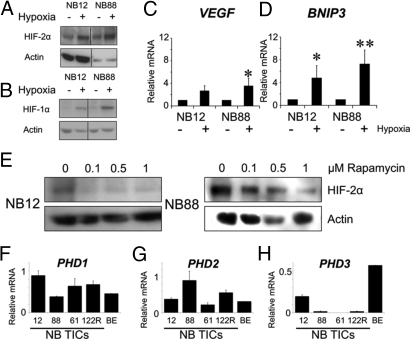
At hypoxia, HIF-2α protein levels were modestly increased (Fig. 2A) in contrast to HIF-1α protein, which was robustly induced (Fig. 2B). Accordingly, BNIP3 (HIF-1α-driven) was more substantially induced than VEGF (primarily HIF-2α-driven) at hypoxia (Fig. 2 C and D). Mechanistically, normoxic HIF-2α expression did not seem to reflect unusually high transcription, because HIF2A mRNA levels were lower in TICs than in cell lines. Therefore, we tested TICs for expression of the prolyl hydroxylases (PHD1-3) responsible for targeting HIFs for proteasomal degradation under normoxic conditions and for effects of rapamycin, because signaling through mammalian Target of Rapamycin (mTOR) is elevated in neuroblastoma TICs*, and because mTOR can increase HIF translation (8). Inhibiting mTOR in neuroblastoma TICs by rapamycin resulted in diminished HIF-2α protein levels at normoxia (Fig. 2E). Literature data suggest that PHD3 is more important than PHD1 and PHD2 for HIF-2α degradation, and that PHD3 siRNA induces HIF-2α but not HIF-1α protein at normoxia as well as hypoxia (9). Interestingly, we found that PHD1 and PHD2 expression levels in TICs were similar to those seen in neuroblastoma cell lines (Fig. 2 F and G), whereas PHD3 was expressed at very low levels in TICs compared with neuroblastoma cell lines (Fig. 2H). Together, our data suggest that normoxic HIF-2α expression in neuroblastoma TICs is due to high translation via the mTOR pathway combined with insufficient degradation due to lack of PHD3.
Fig. 2.
HIF stabilization/activation in neuroblastoma TICs in response to hypoxia. (A and B) HIF protein levels as determined by Western blot analysis in TICs grown for 72 h at 1% O2 with actin as loading control. (C and D) Expression determined by qPCR of the known hypoxia-driven genes VEGF and BNIP3 at 21% and 1% O2. *, P < 0.05; **, P < 0.01 (Student's t test, 2-sided). (E) HIF-2α protein down-regulation upon rapamycin treatment in TICs as determined by Western blot analysis. (F–H) Expression of PHDs in TICs as determined by qPCR with SK-N-BE (2)c neuroblastoma cell expression data as comparison. The qPCR data are mean values of three independent experiments performed in triplicate. Error bars represent SEM. Western blot analysis data were repeated at least three times. Dividing lines in A and B indicate that two areas of the same film have been merged.
Neuroblastoma TICs Are Neuronally Immature and Neural Crest-Like.
The bulk of cells in neuroblastoma specimens as well as cell lines typically express differentiation markers of the SNS, including TH, Neuropeptide Y (NPY), Synaptophysin (SYP), GAP43, and CHGA (10). The immature phenotype of HIF-2α-expressing cells in neuroblastoma tissue prompted us to investigate the differentiation status of TICs and, intriguingly, these neuronal markers were all undetectable or expressed at very low levels in neuroblastoma TICs (Fig. 3A). Conversely, genes expressed by neural crest and early SNS cells [ID2, NOTCH1, HES1, and Vimentin (VIM)] and the stem cell marker OCT4 were all expressed in TICs (Fig. 3B), inferring that these cells are arrested at a neural crest-like stage. Furthermore, the stem cell marker ALDH1 was expressed in HIF-2α-expressing tissue cells and a subset of TICs alike but was undetectable in neuroblastoma cell lines (Fig. S2), thus strengthening the relation between bone marrow-derived TICs and the perivascular immature neuroblastoma cells. In neural stem/progenitor cells as well as in brain tumor TICs (11–13), Notch signaling is a key factor in maintaining an undifferentiated state, and both NOTCH1 and its downstream target HES1 were expressed in TICs and HIF-2α-expressing neuroblastoma tissue cells. In keeping with this, nuclear extracts from TICs contained the activated intracellular Notch-1 (icNotch-1) domain that was undetectable in cell lines (Fig. 3C). Inhibiting Notch signaling with the γ-secretase inhibitor DAPT completely abolished the icNotch-1 signal (Fig. 3D), confirming that the Notch pathway in TICs is active and may contribute to the undifferentiated state.
Fig. 3.
Human neuroblastoma TICs are immature and express neural crest/stem cell markers. (A) Neuroblastoma TICs lack or have very low expression of sympathetic neuronal differentiation markers compared with the established neuroblastoma SK-N-BE (2)c cells, as determined by qPCR. (B) Genes associated with a neural crest/stem cell phenotype are expressed in neuroblastoma TICs. (C) The activated Notch protein (icNotch) is abundant in neuroblastoma TICs but not in SK-N-BE (2)c cells, as determined by Western blotting. (D) Inhibition of γ-secretase activity by DAPT abolishes icNotch in TICs. The qPCR data are mean values of three independent experiments performed in triplicate. Error bars represent SEM. Western blotting data were repeated at least three times.
Down-Regulation of HIF-2α in TICs Induces Early Sympathetic Differentiation.
HIF2A is transiently expressed during the development of the SNS (4, 14, 15). Despite this, there is no established role for HIF-2α in neuronal differentiation, and it is thus unclear whether HIF-2α expression in TICs and tumor specimens causes or simply reflects the immature phenotype. The link between HIF and Notch signaling (16)—that Notch is induced in hypoxic neural crest-like neuroblastoma cells (4) and the high basal Notch levels in neuroblastoma TICs demonstrated here—prompted us to investigate whether silencing of HIF-2α would alter expression of genes associated with early SNS differentiation, and specifically whether Notch signaling was directly affected. To investigate this, we generated NB12 cells stably expressing shRNA against HIF2A by retroviral transduction. Two different shRNA sequences targeting HIF-2α were used and compared to a scrambled nontargeting control shRNA (shC). One shRNA (shHIF2A) resulted in a near-complete knockdown of HIF-2α protein and mRNA, whereas the second sequence modestly affected HIF-2α protein levels despite substantial mRNA knockdown (Fig. 4 A and B, and Fig. S3A). qPCR showed that VEGF mRNA levels were significantly reduced in shHIF2A compared with shC cells (Fig. 4C), showing that HIF-2α actively transcribes hypoxia-inducible genes at normoxia in TICs.
Fig. 4.
HIF-2α knockdown induces early sympathetic differentiation in TICs. (A) HIF-2α knockdown at the protein level assessed by Western blot analysis. Dividing lines indicate that two areas of the same film have been merged. (B) HIF2A knockdown at the mRNA level assessed by qPCR. (C) Down-regulation of VEGF mRNA upon HIF-2α knockdown. (D) icNotch-1 is not affected by HIF-2α knockdown, as measured by Western blot analysis of nuclear extracts from shC and shHIF2A TICs. (E–G) Down-regulation of Notch pathway components upon HIF-2α knockdown assessed by qPCR. (H–J) Up-regulation of sympathetic neuronal differentiation markers at the mRNA level upon HIF-2α knockdown assessed by qPCR. (K) Up-regulation of chromogranin A protein by HIF-2α knockdown as shown by immunohistochemistry. (L–N) Up-regulation of sympathetic neuronal differentiation markers at the mRNA level upon rapamycin treatment in TICs, as determined by qPCR. Error bars represent SD. (O–Q) Neuronal differentiation markers are not induced by rapamycin in shHIF2A cells, as determined by qPCR. The qPCR data are mean values of three independent experiments performed in triplicate or representative data of three independent experiments performed in triplicate (L–Q). Error bars represent SEM. Immunohistochemistry and Western blot analysis data were repeated at least three times.
The shHIF2A cells had equally high levels of icNotch-1 as control cells (Fig. 4D); however, they expressed on average less than one-fifth of the Notch downstream target HES1 mRNA measured in shC cells (Fig. 4E). Similar results were obtained for HEY1, another neural crest-associated gene downstream of Notch (Fig. 4F). Furthermore, the Notch ligand JAG1 was expressed at lower levels in shHIF2A cells (Fig. 4G). In sympathetic progenitor cells, HES1 is a transcriptional repressor of the early proneural gene HASH1/ASCL1 (17) and, indeed, ASCL1 mRNA levels were significantly increased in HIF-2α knockdown cells (Fig. 4H). The shHIF2A cells additionally expressed markedly higher levels of the sympathetic neuronal markers (18, 19) ISL1 (Fig. 4I) and SCG10 (Fig. 4J). CHGA, a clinical marker of neuroblastoma and neuroendocrine tumors, was strongly up-regulated at the protein level (Fig. 4K). Effects of HIF-2α knockdown on HES1 loss and induction of ASCL1 were confirmed in cells expressing the less-efficient RS2 shRNA (Fig. S3 B and C) and in cells transiently transfected with an independent HIF-2α siRNA (Fig. S3 D–F). In addition, NB88 cells differentiated neuronally with induction of the same set of genes upon HIF-2α knockdown (Fig. S4). These data indicate that HIF-2α knockdown pushes TICs toward a sympathetic neuronal phenotype. This conclusion was further supported in an unrelated experimental system, where HIF-2α down-regulation by rapamycin (Fig. 2E) induced the same set of neuronal differentiation markers in both NB12 and NB88 TICs (Fig. 4 L–N). These markers were not further increased by rapamycin in shHIF2A cells, suggesting that the rapamycin effects on neuronal differentiation were HIF-2α-dependent (Fig. 4 O–Q). Treating shHIF2A cells with dipyridyl (DIP) stabilized the residual HIF-2α protein (Fig. S5A) and the increase in HIF-2α protein associated with increased VEGF mRNA expression, and it reverted the increase in ASCL1 levels (Fig. S5 B and C), thus further supporting a specific role for HIF-2α in regulation of differentiation.
Enhanced Neuronal Differentiation and Impaired Vascularization in HIF-2α Knockdown Tumors in Vivo.
To test effects of HIF-2α and the induced differentiation on tumor phenotype in vivo, shC and shHIF2A cells were injected s.c. in athymic mice. HIF-2α protein levels were generally high in shC and markedly lower in shHIF2A tumors (Fig. 5A), and histopathological examination revealed dramatic differences between the two tumor types. Tumor cells in control tumors were surrounded by abundant stromal structures and vasculature as measured by CD34 staining (Fig. 5 D–F). In contrast, HIF-2α knockdown tumors were overtly necrotic (Fig. 5 D and E) and contained few blood vessels (Fig. 5 D and F) and, scarcely, stromal structures (Fig. 5D). Although no apparent necrotic regions were found in shC tumors, 5–33% (average, 18.9%) of shHIF2A tumors consisted of necrotic tissue as measured morphometrically. In agreement with poor vascularization, HIF-1α protein was expressed in HIF-2α knockdown tumors but was virtually absent in control tumors (Fig. 5B). Importantly, the in vivo conditions apparently allowed HIF-2α knockdown tumors to further differentiate toward a more mature neuronal phenotype with frequent TH-positive cells, resembling the bulk of tumor cells in human neuroblastoma specimens (Fig. 5C). Similar results were obtained with xenograft tumors derived from NB88 shC and shHIF2A cells (Fig. S6).
Fig. 5.
Enhanced differentiation and reduced stromal support and vascularization in xenograft tumors derived from shHIF2A TICs. Immunohistochemistry showing HIF-2α (A), HIF-1α (B), TH (C), and CD34 (D–F) protein in shC and shHIF2A tumors. Note the high number of blood vessels in shC compared with shHIF2A tumors (D). +, Stromal regions. *, Necrotic regions. (Scale bars: A–C, E, and F, 50 μm; D, 100 μm.)
Because HIF-2α knockdown tumors expressed high levels of HIF-1α, we asked how HIF-1α protein expression associates with clinical behavior in neuroblastoma. A clinical neuroblastoma material that was previously analyzed for HIF-2α, organized in a tissue microarray, was stained for HIF-1α protein. Unlike HIF-2α (6), HIF-1α protein correlated negatively with advanced clinical stage (P = 0.02, ρ = 0.27; Fig. 6A) and did not predict outcome in neuroblastoma (Fig. 6B). We conclude that these clinical data are in agreement with our experimental tumor data; i.e., HIF-2α expression relates to undifferentiated, well-vascularized tumors, whereas tumors with reduced HIF-2α protein are neuronally differentiated, necrotic, and HIF-1α-positive.
Fig. 6.
HIF-1α correlates negatively to high stage but not to patient outcome. HIF-1α protein levels were assayed by using immunohistochemistry analyses on two tissue microarrays representing a total of 93 neuroblastoma cases. A fraction of positive cells was dichotomized into four groups as described previously (6). (A) Patient stage, as determined by the International Neuroblastoma Staging System (INSS), was divided into three groups of increasing stage (INSS 1, 2, 4s; INSS 3; and INSS 4). A weak, significant correlation was found between a high number of HIF-1α-positive cells (fraction) and low patient stage (ρ = −0.27, P = 0.02). (B) For Kaplan–Meier survival analysis HIF-1α fraction scores were pooled into two groups: low fraction (0–25% positive cells) and high fraction (26–100% positive cells). There was no significant correlation between level of HIF-1α staining fraction and patient outcome (P = 0.42, log-rank test).
Discussion
In this report, we found that neuroblastoma TICs isolated from patient bone marrow express high levels of HIF-2α protein even when oxygen supply is sufficient. Recent reports on HIF-2α regulation of stem cell-associated genes (20, 21) have led to speculations on putative roles for HIF transcription factors in stem cells and TICs (22). Knockdown of HIF-2α protein in neuroblastoma TICs resulted in a spontaneously diminished expression of neural crest-associated genes, accompanied by an increased expression of SNS neuronal lineage-specific marker genes, including ASCL1, ISL1, and SCG10. The neuronal phenotype of HIF-2α knockdown cells was further developed with induced TH expression, a hallmark of the sympathetic neuronal lineage, when cells were grown as xenograft tumors in vivo, whereas control cells remained remarkably undifferentiated in vivo. In addition, control tumors were rich in stroma and vasculature, in sharp contrast to the necrotic and hypoxic HIF-2α knockdown tumors, indicating that HIF-2α has a central role in recruiting stromal and endothelial cells to neuroblastoma tumors.
Previous and present data strongly suggest that high normoxic and hypoxic levels of HIF-2α protein in neuroblastoma cells are associated with an immature, neural crest-like phenotype (2, 4), but until now it was unclear whether HIF-2α caused or reflected the immature phenotype. Here, we present data supportive of HIF-2α as a key factor in keeping the undifferentiated state of cultured neuroblastoma TICs. Down-regulation of HIF-2α by shRNA or rapamycin resulted in increased expression of a set of genes associated with a sympathetic neuronal phenotype. However, other transcription factors, like Phox2b, Phox2a, and dHAND, and neuronal differentiation markers, like TH and GAP43, were not induced in vitro. In vivo conditions, however, allow the shHIF2A cells to further differentiate, with expression of TH and CHGA as the most notable result. We conclude that without HIF-2α, the neuroblastoma TICs give rise to tumors with a reduced number of viable cells and with a phenotype very similar to that of the bulk of cells in clinical neuroblastoma specimens, where expression of neuronal markers like TH is frequent (2).
There appear to be several layers of interplay between hypoxia/HIF and the Notch signaling pathway in regulation of differentiation, exemplified by the direct HIF interaction with the intracellular domain of Notch-1 enhancing the transcription of Notch downstream target genes (16). We report here that transcription of the Notch downstream target genes HES1 and HEY1 is markedly reduced upon HIF-2α knockdown in neuroblastoma TICs. One interpretation of these results would be that a reduction of HIF-2α protein leads to a reduction in HIF-2α–icNotch complexes available to induce transcription of Notch target genes. By such a mechanism, Notch downstream transcriptional activity could be affected even without a decrease in icNotch-1 itself, which indeed seems to be the case. The role of Notch signaling in maintaining neural precursor cells in an undifferentiated state is well-established. In cultured SH-SY5Y neuroblastoma cells, Notch inhibition results in induction of neuronal differentiation as measured by GAP43 transcription and increased neurite outgrowth (23). Furthermore, neuroblastoma cell lines transfected to overexpress icNotch-1 fail to differentiate (24).
The facts that both HIFs are expressed and HIF-2α but not HIF-1α protein expression is high in normoxic TICs show that the HIF proteins are regulated differently. As a rule, both HIF-1α and HIF-2α are proteasomally degraded after oxygen-dependent modification by three different PHDs; however, HIF-2α is particularly sensitive to degradation by PHD3-dependent hydroxylation (9). The virtual lack of PHD3 expression in neuroblastoma TICs therefore provides an attractive explanation for the high normoxic HIF-2α protein levels. As reported here, inhibiting the mTOR pathway pharmacologically results in a marked decrease of HIF-2α protein in TICs. Thus, signaling through this pathway appears to be involved in maintaining the high basal HIF-2α protein levels. It is therefore intriguing to find that the mTOR inhibitor rapamycin was one of our best candidate drugs for inhibiting neuroblastoma TIC growth in a mass screen (Zhang et al., AACR Annual Meeting 2008, abstract 1204). It may be of further interest that several recent reports have linked PI3K/Akt pathway activity specifically to brain tumor TICs and that it appears that such cells are particularly sensitive to inhibitors of these pathways (25, 26). Furthermore, it is of particular interest to note that brain tumor TICs, similarly to the situation in neuroblastoma, occupy a perivascular niche (26, 27) and promote angiogenesis by expressing high levels of VEGF (28). It was demonstrated recently that the glioma stem cell phenotype is indeed in large part regulated by high basal HIF-2α expression (29).
Another function of HIF-2α is related to the formation of blood vessels and tumor stroma. In tumors formed by the shC TICs, stroma and blood vessels are abundant, suggesting that these HIF-2α-expressing, VEGF-producing cells support neovascularization and stromal growth, mimicking the situation in neuroblastoma specimens with perivascular and stromal localization of strongly HIF-2α-positive neural crest-like cells (2). In contrast, HIF-2α knockdown cells resulted in highly necrotic and poorly vascularized tumors. Because a low-differentiation stage of neuroblastomas is an established predictor of aggressive tumor behavior, tumor angiogenesis is a prerequisite for growth of solid tumors, and the possibility that TICs rely on a vascular/stromal niche, data presented here identify HIF-2α as an attractive therapeutic target for aggressive neuroblastomas.
Materials and Methods
Detailed materials and methods may be found in SI Materials and Methods.
Cell Culture.
Human neuroblastoma SK-N-BE (2)c cells (American Type Culture Collection) were grown routinely as monolayers in Minimal Essential Medium (Sigma–Aldrich) containing 10% FCS and penicillin (100 units/mL) and streptomycin (100 μg/mL). Human neuroblastoma TICs were grown as described previously (7) in DMEM/F12 (3:1; Invitrogen), 1× B27, penicillin (100 units/mL) and streptomycin (100 μg/mL), bFGF (40 ng/mL; Peprotech), and EGF (20 ng/mL; Invitrogen). For hypoxia, cells were cultured at indicated oxygen tension in a humidified InvivO2 Hypoxia workstation 400 (Ruskinn Technology). TICs were exposed to rapamycin (0.1–1 μM; Sigma; stored at −20 °C as a 1-mM stock in DMSO) or loading control (DMSO) for 48 h.
Western Blot Analysis.
Cells were lysed in RIPA, and protein concentrations were determined by the method of Bradford. Proteins were separated by SDS/PAGE and blotted onto Hybond-C-Extra nitrocellulose membranes (Amersham). After blocking, membranes were incubated with primary antibodies at 4 °C overnight and secondary antibodies for 1 h at room temperature.
Nuclear Extracts.
For measurements of icNotch-1, nuclei were extracted as described in SI Materials and Methods, and lysates loaded on gels for Western blotting.
Quantitative Real-Time PCR.
Total RNA was extracted and washed by using the Qiashredder and RNeasy mini kits (Qiagen) according to the manufacturer's recommendations before extensive washing and DNase treatment. The cDNA synthesis was performed by using random primers and Multiscribe Reverse Transcriptase enzyme (Applied Biosystems). For normalization of expression levels, three housekeeping genes (UBC, YWHAZ, and SDHA) were used (30). Primer sequences are listed in Table S1.
Patient Material and Immunohistochemistry.
Human neuroblastoma specimens were fixed and embedded in paraffin routinely before analysis (ethics approval LU 389-98; Lund University). Human neuroblastoma TICs and cell lines were pelleted and fixed in 4% paraformaldehyde, then routinely embedded in paraffin after dehydration and several rounds of EtOH washing. A tissue microarray consisting of 93 neuroblastomas was described previously (6) and analyzed for HIF-1α expression independently by two pathologists. Immunoreactivity was detected after antigen retrieval using the Envision system and DAKO Techmate 500. Only freshly sectioned tissue was used for HIF-2α stainings.
Retrovirus Production and Transductions.
pRETRO-SUPER-shHIF-2α RS2 and RS9 were kindly provided by W. G. Kaelin, Jr. (31). rvShRNASNC expressing a scrambled negative control shRNA in a pRETRO-SUPER vector (shC) was kindly provided by G. L. Semenza (32). For retroviral transductions, cells were passaged and seeded in six-well plates in medium consisting of 75% retrovirus-containing supernatant (DMEM) and 25% F12 medium supplemented with 40 ng/mL bFGF and 20 ng/mL EGF. After 12 h, the virus-containing medium was removed. Four days after the transduction, cells were replated in selection medium, which consisted of TIC medium with the addition of 2 μg/mL puromycin or 800 ng/mL G418, then used without further cloning.
Xenograft Tumors.
Cells (3 × 106 per 200 μL of PBS) were injected s.c. on the flank of athymic mice (NMRI nu/nu strain). Female mice ages 6–8 weeks and weighing 20–25 g at arrival were used and housed in a controlled environment. All procedures were approved by the regional ethics committee for animal research (approval no. M200-06). Experiments were terminated when tumors reached a maximum size of 15-mm diameter. After mice were killed, tumors were fixed in 4% paraformaldehyde, then embedded in paraffin and immunostained as above.
Supplementary Material
Acknowledgments.
We thank Dr. W. G. Kaelin, Jr., and Dr. G. L. Semenza for kindly providing retroviral vectors. This work was supported by the Swedish Cancer Society, the Children's Cancer Foundation of Sweden, the Swedish Research Council, the Swedish Foundation for Strategic Research (SSF) Strategic Center for Translational Cancer Research–CREATE Health, Gunnar Nilsson's Cancer Foundation, and the research funds of Malmö University Hospital (to S.P.), as well as the Spanish Institute of Health Carlos III Grants Acción Transversal del Cáncer 20080143, FIS PI06/1576, and RETICS RD06/0020/0102 (to S.N. and R.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904606106/DCSupplemental.
Zhang et al., 99th Annual Meeting of the American Association for Cancer Research, April 12–16, 2008, San Diego, CA, abstr 1204.
References
- 1.Fredlund E, Ringner M, Maris JM, Pahlman S. High Myc pathway activity and low stage of neuronal differentiation associate with poor outcome in neuroblastoma. Proc Natl Acad Sci USA. 2008;105:14094–14099. doi: 10.1073/pnas.0804455105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietras A, et al. High levels of HIF-2alpha highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche. J Pathol. 2008;214:482–488. doi: 10.1002/path.2304. [DOI] [PubMed] [Google Scholar]
- 3.Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol. 2005;277:271–286. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Jögi A, et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA. 2002;99:7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helczynska K, et al. Hypoxia-inducible factor-2alpha correlates to distant recurrence and poor outcome in invasive breast cancer. Cancer Res. 2008;68:9212–9220. doi: 10.1158/0008-5472.CAN-08-1135. [DOI] [PubMed] [Google Scholar]
- 6.Holmquist-Mengelbier L, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Hansford LM, et al. Neuroblastoma cells isolated from bone marrow metastases contain a naturally enriched tumor-initiating cell. Cancer Res. 2007;67:11234–11243. doi: 10.1158/0008-5472.CAN-07-0718. [DOI] [PubMed] [Google Scholar]
- 8.Bernardi R, et al. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature. 2006;442:779–785. doi: 10.1038/nature05029. [DOI] [PubMed] [Google Scholar]
- 9.Appelhoff RJ, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 10.Fredlund E, Ovenberger M, Borg K, Pahlman S. Transcriptional adaptation of neuroblastoma cells to hypoxia. Biochem Biophys Res Commun. 2008;366:1054–1060. doi: 10.1016/j.bbrc.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 11.Fan X, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 12.Sestan N, Artavanis-Tsakonas S, Rakic P. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science. 1999;286:741–746. doi: 10.1126/science.286.5440.741. [DOI] [PubMed] [Google Scholar]
- 13.Tsarovina K, Schellenberger J, Schneider C, Rohrer H. Progenitor cell maintenance and neurogenesis in sympathetic ganglia involves Notch signaling. Mol Cell Neurosci. 2008;37:20–31. doi: 10.1016/j.mcn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson H, et al. HIF-2alpha expression in human fetal paraganglia and neuroblastoma: Relation to sympathetic differentiation, glucose deficiency, and hypoxia. Exp Cell Res. 2005;303:447–456. doi: 10.1016/j.yexcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafsson MV, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Ishibashi M, et al. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- 18.Gestblom C, et al. The basic helix-loop-helix transcription factor dHAND, a marker gene for the developing human sympathetic nervous system, is expressed in both high- and low-stage neuroblastomas. Lab Invest. 1999;79:67–79. [PubMed] [Google Scholar]
- 19.Thor S, Ericson J, Brannstrom T, Edlund T. The homeodomain LIM protein Isl-1 is expressed in subsets of neurons and endocrine cells in the adult rat. Neuron. 1991;7:881–889. doi: 10.1016/0896-6273(91)90334-v. [DOI] [PubMed] [Google Scholar]
- 20.Covello KL, et al. HIF-2alpha regulates Oct-4: Effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin CM, et al. Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells. Circ Res. 2008;102:1075–1081. doi: 10.1161/CIRCRESAHA.107.161729. [DOI] [PubMed] [Google Scholar]
- 22.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao YF, et al. Unnatural amino acid-substituted (hydroxyethyl)urea peptidomimetics inhibit gamma-secretase and promote the neuronal differentiation of neuroblastoma cells. Mol Pharmacol. 2007;71:588–601. doi: 10.1124/mol.106.024299. [DOI] [PubMed] [Google Scholar]
- 24.Grynfeld A, Pahlman S, Axelson H. Induced neuroblastoma cell differentiation, associated with transient HES-1 activity and reduced HASH-1 expression, is inhibited by Notch1. Int J Cancer. 2000;88:401–410. [PubMed] [Google Scholar]
- 25.Eyler CE, et al. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 2008;26:3027–3036. doi: 10.1634/stemcells.2007-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hambardzumyan D, et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Bao S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuyama H, et al. Expression of vascular endothelial growth factor receptor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor 1. J Biol Chem. 2006;281:15554–15563. doi: 10.1074/jbc.M602003200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.