Abstract
Purpose
The goal of this study was to investigate the role of the small guanosine triphosphatase (GTPase), Rho, in the corneal epithelial response to extracellular matrix (ECM) molecules. The avian corneal epithelial model was used to establish that Rho is required for actin reorganization and tyrosine phosphorylation of integrin-mediated signal pathway proteins.
Methods
Whole embryonic corneal epithelia were isolated without the basal lamina and either transfected with Rho-specific antisense oligonucleotides or treated with Clostridium botulinum C3 exoenzyme and then stimulated with fibronectin (FN) or collagen (COL). The epithelia were evaluated for actin reorganization and protein production including Rho protein levels and tyrosine phosphorylation with Western blot analysis.
Results
After an overnight transient transfection with anti-sense oligonucleotides, Rho protein levels were decreased more than 80%, and tyrosine phosphorylation of all integrin-mediated signal transduction proteins was decreased compared with control epithelia. Intracellular Rho distribution did not change in the presence of antisense oligonucleotides; however, the amount of immunolabeled Rho decreased. Disrupting the signaling cascade with Rho antisense also blocked FN- and COL-stimulated actin cortical mat reformation. C. botulinum C3 exoenzyme, a pharmacologic agent that specifically causes adenosine diphosphate (ADP) ribosylation and inactivation of Rho, also blocked actin reorganization and tyrosine phosphorylation. In contrast, decreasing Raf protein levels did not change FN-mediated actin reorganization or tyrosine phosphorylation.
Conclusions
Decreasing Rho protein or blocking its function inhibited ECM-stimulated actin reorganization and signal transduction, as measured by tyrosine phosphorylation.
During homeostasis, corneal epithelial cells normally migrate from the limbus to the central cornea. In addition, the corneal epithelial cells respond to wounding by migrating over the wound bed. Many factors contribute to the normal centripetal migration1 and successful closure of corneal wounds, including extracellular proteins (extracellular matrix components, growth factors, and cytokines)2,3 and intracellular signaling molecules that mediate the extracellular signals. Recently, several laboratories have identified Rho and its associated downstream kinases (Rho-associated coiled– coil containing protein kinase [ROCK-1, ROCK-2])4 as being necessary for centripetal corneal epithelial migration associated with cell cycle progression5 and migration during corneal wound healing.6
In contrast to these migration studies, the current investigation focused on the embryonic corneal epithelial actin reorganization in freshly isolated whole epithelial sheets in response to specific extracellular stimuli. Extracellular matrix (ECM)–stimulated changes in actin cytoskeleton organization have been well documented in the corneal epithelial organ culture system7–11 and in cells grown in traditional tissue culture.12–15 Corneal epithelial tissues isolated without basal lamina (−BL) responded to ECM through an actindependent mechanism. The basal cell surface flattens and the disrupted actin cortical mat (ACM) reorganizes in the presence of laminin, fibronectin (FN), or collagen (COL)7–9,16,17 with distinct configurations in the presence of different ECM molecules.10 In addition, we have shown that this tissue also reorganizes the actin cytoskeleton in response to bombesin or lysophosphatidic acid (LPA).10
The reorganization of the actin cytoskeleton is orchestrated through a signaling cascade. The epithelia has to be in contact with matrix molecules for a minimum of 15 minutes for the ACM to reform in vitro. During the first 15 minutes of stimulation of the ECM, many signaling proteins are activated.10 Focal adhesion kinase (FAK) and p190Rho-guanosine triphosphate (GTP)–activating protein (10-Rho-GAP) were tyrosine phosphorylated within 5 minutes in response to stimulation with soluble ECM. Erk-1, erk-2, and PI3 kinase were activated later in the signaling cascade (30 – 60 minutes). These phosphorylation events were necessary because a tyrosine and Src kinase inhibitor, herbimycin A, blocked actin reorganization in a dose-dependent manner.10 In summary, reorganization of actin required tyrosine phosphorylation of FAK, p190Rho-GAP, paxillin, tensin, and mitogen-activated protein (MAP) kinase and activation of PI3 kinase.10,11 Changes in the organization of the actin cytoskeleton have also been recorded in monolayer cultures of corneal epithelial cells grown on ECM substrates (laminin, FN, and COL) that initiated an increase in intracellular pH.18 According to current understanding, the cascade of events appears to be as follows: ECM proteins bind integrin molecules; FAK may be recruited to the integrin cytoplasmic domain by talin; talin binds vinculin and paxillin; FAK goes through a conformational change to bind the β1 integrin cytoplasmic tail and autophosphorylates after the integrins have clustered through a Rho-mediated mechanism19; and the phosphorylated FAK binds and activates many proteins, either directly or through adapter proteins including paxillin, talin, p85, shc, p-cas, Grb2, Sos, Src, and fyn in response to integrin activation.20 –22 In addition, there is mounting evidence that different integrin molecules stimulate alternate proteins.
The signaling molecules (FAK, Grb2, PI3 kinase, MAP kinase, Rho and RhoGAP) and their interactions have been studied extensively in many different fibroblast models.23,24 However, little is known about their interactions in a whole epithelial tissue model. It has been shown by other laboratories that Rho alternates between the GTP-bound active form (on) and the guanosine diphosphate (GDP)-bound inactive form (off) to regulate other downstream kinases. Many other proteins regulate the on and off state of Rho. The guanine-nucleotide exchange factors (GEFs) are the on signal as they add GTP to the protein. GAPs are the off signal: They remove a phosphate to deactivate the protein. Guanine-nucleotide dissociation inhibitor protein (GDI) sequesters the inactive Rho protein in the cytoplasmic pool.25,26 Therefore, in immunohistochemical localizations Rho and RhoGDI colocalize in inactivated tissue cytoplasm, whereas, activated RhoGTP is usually found at the membranes.25,26
We have established a reliable assay to determine that whole corneal epithelial sheets respond to specific ECM molecules.10,11 The present study was undertaken to determine the affect of Rho on the actin cytoskeleton in our corneal epithelial model. We used two approaches to decrease Rho activity in the corneal epithelial organ cultures. A pharmacologic agent, Clostridium botulinum C3 exoenzyme, was used to block Rho function by inducing adenosine diphosphate (ADP) ribosylation of all Rho protein.27 Because the C3 exoenzyme may not diffuse through the whole epithelial tissues, Rho was also downregulated with antisense technology.
We used Rho-specific phosphorothioate antisense oligonucleotides to decrease the expression of Rho protein and studied the effects on reorganization of the actin cytoskeleton. Antisense oligonucleotides have been shown specifically to reduce or inhibit the expression of single gene products in several cell systems.28–30
The downstream signaling events initiated by stimulation with COL and FN were also examined in tissues treated with antisense oligonucleotides and C3 exoenzyme. We found that these signaling events were altered when we decreased the expression of Rho or increased ADP ribosylation of all Rho proteins. In contrast, transient transfection with Raf-specific oligonucleotides did not block FN-stimulated tyrosine phosphorylation or actin reorganization. These experiments continue to establish the corneal epithelial model system and the groundwork for future experiments to determine the specific sequence of signaling pathways in an intact whole-tissue model.
Materials and Methods
Tissue Isolation
Fertile white leghorn chicken eggs (SPAFAS, Inc., Norwich, CT) were incubated for 8 days at 39°C. The chick embryos (Hamburger-Hamilton stages 27–34) were removed from the eggs and rinsed in Hanks’ balanced saline solution (HBSS; Gibco Laboratories, Grand Island, NY).
Whole corneas, with some surrounding sclera, were removed from the embryos with fine forceps. Corneal epithelia isolated without the basal lamina (–BL) were treated for 2 to 4 minutes at 37°C in 0.8 mg/mL trypsin and 0.8 mg/mL collagenase (Sigma, St. Louis, MO) in calcium and magnesium–free HBSS (Fig. 1A). After the enzyme treatment, the corneas were rinsed in Ham’s F12 medium (Gibco) and then HBSS to stop the action of the enzyme. A continuous sheet of corneal epithelial cells was dissected from the underlying stroma with fine tweezers (Figs. 1A, 1B) and placed basal-side down on a black polycarbonate filter (3 mm diameter, 0.4-μm pore size; Poretics, Livermore, CA).9,16
Figure 1.
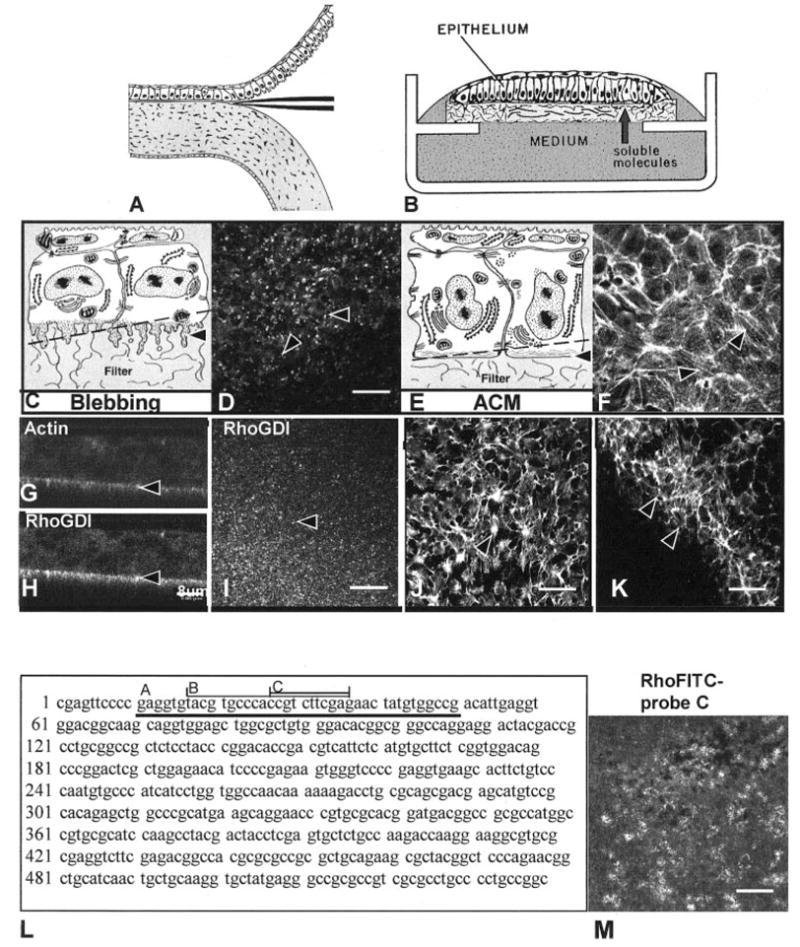
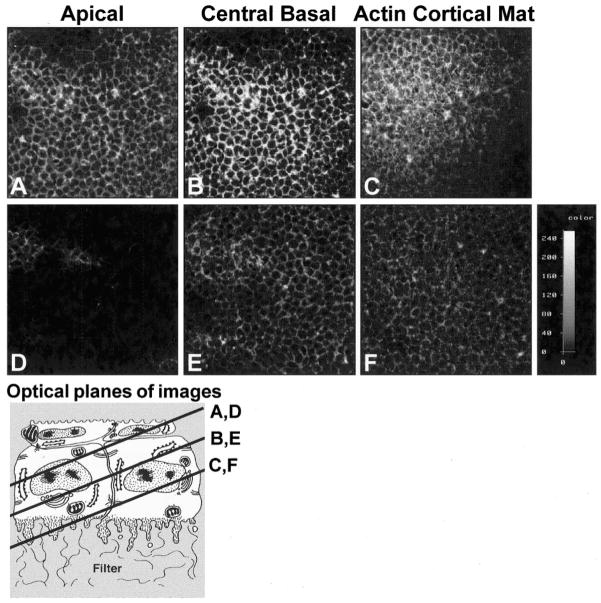
Schematic drawing of isolation of corneal epithelium (A), culture of the cells (B), and confocal images (D, F–K), human Rho A sequence (L), antisense probes (L, A–C) and confocal image of FITC-labeled antisense oligonucleotide probe (M). Epithelia were isolated as a sheet of tissue (A), placed on a polycarbonate filter basal-side down, and cultured at the air–medium interface (B). Epithelia isolated without the basal lamina extended basal cellular processes termed blebs (C, arrowhead). The blebs contain F-actin, which appeared as punctate spots in single optical sections (D, arrowheads) taken through the basal area illustrated in the schematic drawing (C, dashed line). Immunohistochemical localization of RhoGDI demonstrated that this protein was enriched in the protruding blebs from tissues isolated without the basal lamina (H, I). Tissue cultured with soluble ECM molecules reorganized the basal actin into an ACM (E, arrowhead). The bundles of F-actin viewed in a single optical section from epithelia stimulated with COL for 2 hours (F, arrowheads) appeared to align from cell to cell, across the field. The epithelium stimulated with bombesin (J) or LPA (K) reformed the ACM after 30 minutes.10 The upper right corner (K) was in the optical section through the basal cell nuclei, and the individual cell size was visible in the honeycomb pattern of the lateral cell membrane actin distribution. Corneal epithelial tissues were cultured in control media, transfected with a 40- (L-A), 20- (L-B), or 10-mer (L-C) oligonucleotide directed against the 5′ region of human Rho mRNA (5–10 ng per 20 epithelia) for 24 hours in the presence of liposomes. The largest probe (L-A) was used first, and then the shorter probes were designed. A single optical section through the basal cells demonstrates that the oligonucleotide (FITC-labeled 10-mer) was evenly transfected throughout the tissue (M). Scale bar: (D, I–K, M) 20 μm; (G, H) 8 μm.
Organ Culture
Epithelial sheets were suspended at the air–media interface by a triangular-shaped wire grid in an organ culture dish (Fig. 1B; Falcon, BD Biosciences, Franklin Lakes, NJ). The medium height barely covered the apical surface of the tissue. Cultures were incubated at 37°C in a humidified gas mixture (5% CO2 and 95% air). The control culture medium was composed of Ham’s F12 medium (Gibco), antibiotic-antimycotic (Gibco), and 50 μm ascorbic acid (Sigma). The stimulatory molecules used were 10 μg/mL LPA (Sigma), 50 μg/mL human plasma FN (BD Biosciences), and 100 μg/mL type I COL (BD Biosciences). After the stimulatory incubation (15 minutes to 2 hours), the epithelia were fixed for immunohistochemistry, stained with phalloidin, or homogenized in sample buffer for electrophoresis. In experiments with exoenzyme C3 from C. botulinum, epithelia were preincubated with 3 μg/mL exoenzyme C3 (Calbiochem, La Jolla, CA) overnight before stimulation with LPA or ECM.
Transfection Conditions
Rho antisense oligonucleotides were first purchased from Oncogene Science (Manhasset, NY) as a 40-mer. These oligos were satisfactory in pilot experiments (repeated three times), and we therefore designed a 20-mer (Fig. 1L-B, used in two experiments) and a 10-mer (Fig. 1L-A, used in all remaining experiments) complementary to the AUG start site (5′-ATGCACGGGT GGCAGAAGCT-3′; Fig. 1L-B). Control oligonucleotides included a sense sequence, a scrambled sequence, and a Raf-specific sequence (5′-GGTGCTGACC-3′) with its sense sequence. All transfection experiments were repeated at least three times for each experimental variable, the data presented are representative examples. Usually, both morphologic examination of the actin cytoskeleton and Western blot analysis were used on the same experimental groups (10 epithelia used for phalloidin staining, 20 to 25 for Western blots). Oligonucleotides were synthesized as phosphorothioates by Integrated DNA Technologies, Inc. (Coralville, IA) on an automatic synthesizer, sulfurized to the phosphorothioate form with tetraethylthiuram disulfide and purified by reversed-phase HPLC (Integrated DNA Technologies, Inc). The preparations were eluted in acetonitrile, lyophilized, reconstituted in H2O twice to remove volatile contaminants, and purified by spin-column gel filtration (Sephadex G-25; Roche Diagnostics; Indianapolis, IN). Corneal epithelia were incubated in the presence of a liposome-oligonucleotide mixture. The antisense Rho oligonucleotides were evenly distributed throughout the cytoplasm of the corneal epithelial cells detected with the 10-mer FITC-labeled probes (Fig. 1M).
Liposomes were synthesized with a stock solution of 10 μg/mL chloroform dimethyldioctadecyl-ammonium bromide and L-α-phosphatidylethanolamine dioleoyl (both from Sigma), combined and evaporated to dryness (SpeedVac; Thermo Savant, Holbrook, NY). The lipids were rehydrated with dH2O and sonicated. Liposomes were incubated with oligonucleotides at room temperature (RT), and then added to F-12 experimental culture medium at a concentration of 5 to 10 ng/20 epithelia in 1 mL medium.
Actin Labeling
The F-actin in the cells was labeled with fluorescently tagged phalloidin (Molecular Probes, Inc., Eugene, OR).16 The epithelia were incubated with FITC-phalloidin (1:20 dilution of phalloidin stock solution in 3.7% formaldehyde, 0.01% lysopalmitate; Sigma) for 30 minutes at RT, followed by three 10-minute rinses in PBS. A subset of each experiment was examined with confocal microscopy to determine whether the ACM had reorganized. These experiments were repeated numerous times; all images are representative samples from experiments. The fluorescent phallotoxins were kept as stock solutions containing 300 U/3 mL methanol (approximately 3.3 μM). A unit is defined, as the amount of material needed to stain one microscope slide of fixed cells (Molecular Probes).
Immunohistochemistry
Freshly isolated corneal epithelia were fixed as described previously,16,17 in 4% paraformaldehyde-PBS (pH 7.4) for 15 minutes at RT. The tissues were permeabilized with 0.05% Triton X-100 (Sigma) for 5 minutes, blocked with 10% normal goat serum (NGS; Gibco) or 3% BSA. Mouse monoclonal antibodies specific for Rho A (Santa Cruz Biotechnology, Santa Cruz, CA), Rho B (Hybridoma Bank, Iowa University, Iowa City, IA), and Rho-GDI (BD Transduction Laboratories, San Diego, CA) were purchased and used for immunohistochemistry. Antibodies were diluted 1:100 in 3% BSA-PBS. The epithelia were incubated overnight at RT. The primary antibody was detected and visualized with FITC or CY-5– conjugated secondary antibodies (Jackson ImmunoResearch, Philadelphia, PA). The filters were placed basal-side down in antifade mounting medium (Molecular Probes) on slides and viewed on either a confocal laser-scanning (CLSM) or a confocal (model TCS SP2) microscope (both from Leica, Deerfield, IL).
In double- and triple-label experiments, F-actin was visualized with Texas red fluorescently tagged phalloidin (Molecular Probes). Epithelia were fixed and labeled for antibody-specific proteins as described earlier and then incubated with Texas red phalloidin (1:20 dilution of phalloidin stock solution) for 30 minutes at RT.
Control Samples
Some epithelia were incubated with FITC-conjugated or Lissamine rhodamine– conjugated goat anti-rabbit IgG secondary antibodies or FITC-conjugated goat anti-mouse IgG secondary antibodies only. All secondary antibody control specimens were negative when the confocal microscope was set at the same settings as positively labeled tissue. In addition, crossover control specimens showed complete separation of two fluorophores in double-labeled tissue at the confocal settings used.
Confocal Laser Scanning Microscopy
Specimens were analyzed with the upright CLSM (Leica), equipped with an argon ion laser (488- and 514-nm excitation wavelengths), or the upright microscope (TCS SP2; Leica), equipped with one argon and two helium neon lasers (458-, 476-, 488-, 514-, 543-, and 633-nm excitation wavelengths). The continuously variable detection pinhole was set at the minimum size for optimal signal.31,32 The typical z-series was composed of optical sections in the xy optical plane. These images were en face optical sections through the vertical axis of the tissue. Specimens were also scanned in the xz plane, allowing the tissues to be visualized in cross section. The point-spread function for the confocal microscope is greater in the xz than the xy focal plane,31 causing the images to be more distorted.
Images were analyzed, enhanced, and stored. Black-and-white and color photographs were computer generated with minimal computer enhancement. These images were printed directly from image analysis software (Photoshop or PageMaker; Adobe, San Jose, CA) documents. All control specimens were collected, enhanced, and photographed with the same conditions as the positive tissue.
Western Blot Analysis
Epithelia were isolated and cultured as described (20 –25 epithelia per treatment group), placed in sample buffer with 1 mM sodium orthovanadate, boiled for 5 minutes, and resolved on 7.5% SDS-PAGE under reducing conditions.10,33 The proteins were transferred to poly-vinylidene difluoride (PVDF) membrane (Immobilon; Millipore Corp., Bedford, MA). Affinity purified anti-phosphotyrosine (PY20, anti-P-Tyr; BD-Transduction Laboratories), Raf, and Rho (Santa Cruz, CA; Transduction Laboratories, Lexington, KY) were commercially obtained. Primary antibodies were detected with horseradish peroxidase– conjugated anti-mouse or anti-rabbit IgG secondary antibodies and subsequent enhancement with chemiluminescence (Dupont NEN; Boston, MA). Protein molecular weights were determined by comparing protein bands to kaleidoscope prestained standards (Bio-Rad, Hercules, CA). Image-analysis software (Kodak 1D; Eastman Kodak, Rochester, NY) was used to perform densitometry to assess the density of either specific protein bands or all protein bands in a lane. All Western blots were repeated at least three times from three different experiments. Representative samples of Western blots are included in this manuscript.
Results
In our model system, the embryonic avian corneal epithelia are isolated from the stroma as a sheet of continuous cells (Fig. 1A). The corneal epithelium at stage 34, (embryonic day [E]8) is composed of two cell layers, with the hexagonal, flat periderm cells on top and the smaller-diameter basal cells underneath (Figs. 1C, 1E). The periderm cells range from 6 to 8 μm high and can be 25 μm wide. F-actin is found in the microvilli of the periderm cells and along the periderm-basal cell junction.16 The basal cells range 15 to 20 μm in height and 5 to 10 μm in diameter. When basal cells are isolated with the basal lamina, they have a flat basal surface. F-actin is also found along the basal lamina in an organized arrangement called the ACM (Figs. 1E, 1F).34 There are usually four basal cells underneath every periderm cell.16
F-actin (labeled with FITC-phalloidin) distribution demonstrated distinct organizational patterns when the epithelia were cultured for 2 hours in the presence of different ECM molecules or direct Rho stimulators (Fig. 1).10 Epithelia isolated −BL have large cytoplasmic extensions along the basal cell surface that contain F-actin (Figs. 1D, 1G)7,16 and RhoGDI (Figs. 1H, 1I). A single optical section taken en face (xy optical plane, at the level of the dashed line on Fig. 1C) demonstrates that the most intense actin label was localized to bright spots that represent the basal cytoplasmic blebs (Fig. 1D). This single optical section was scanned slightly tangentially to visualize the basal region of the sheet of epithelial cells. In a similar single optical section from a double-labeled tissue, the Rho-sequestering protein, RhoGDI was also prominent in the basal blebs (Fig. 1I) and colocalized with actin. In single xz optical sections, the actin (Fig. 1G) and Rho GDI (Fig 1H) profiles overlapped nearly 100% in the basal blebs (Figs. 1G, 1H, arrowheads). In contrast, epithelia cultured in the presence of type I COL had large F-actin bundles in the ACM region (Fig. 1E, dashed line) that aligned from cell to cell for 80 to 100 μm (Fig. 1F, arrowheads). The neuropeptide and Rho pathway stimulators, bombesin (Fig. 1J) and LPA (Fig. 1K) reorganized the ACM very quickly (15–30 minutes).10 The bombesin-stimulated tissue sample contained multiple actin bundles that appeared denser at cell– cell membranes (Fig. 1J, arrowhead). The LPA-treated tissue was not flat, and the optically sectioned image included the filter (lower left corner) and the nuclear region of the basal cells (upper right corner). The ACM region contained abundant actin bundles (Fig. 1K, arrowheads). In summary, different matrix molecules and different Rho pathway stimulators induced distinct actin bundle patterns in the basal ACM of the whole epithelial tissue, as shown with single optical sections taken through the basal cytoplasm. Furthermore, the presence or absence of a varied actin bundle distribution pattern was used as a morphologic and biological assay to determine the consequences of transient transfection with Rho-specific oligonucleotides and blocking Rho function with exoenzyme C3.
Corneal epithelia were transiently transfected with anti-sense oligonucleotides (Fig. 1L) complementary to the translation start site (Figs. 2C, 2D) or with control sense oligonucleotides (Figs. 2A, 2B) and stimulated with either COL (Figs. 2A, 2C, 2E, 2G) or FN (2B, 2D, 2F, 2H). Fluorescence-labeled probes were used to determine that the oligonucleotides were evenly distributed throughout the epithelia (Fig. 1M). Single confocal optical sections through basal epithelium demonstrated that the F-actin in COL- or FN-stimulated epithelium reorganized into an ACM in control tissues incubated with Rho sense oligonucleotides (Figs. 2A, 2B) similar to the tissues treated with COL alone (Fig. 1F) or FN alone (Fig. 3A). In the presence of Rho antisense, the epithelia had decreased ACM formation in the presence of COL or FN (Figs. 2C, 2D, respectively). To determine whether Rho protein had decreased, corneal epithelia (20/group) were harvested and analyzed with Western blot analysis and probed with anti-Rho antibody (Figs. 2E, 2F). The control epithelia incubated with the Rho sense oligonucleotides (Cs and FNs) had more than 80% more Rho than the antisense-treated tissue (Cas and FNas) in this sample. The same blots were reprobed with anti-phosphotyrosine antibodies to demonstrate that all phosphorylated proteins were decreased in antisense-treated tissues (Figs. 2G, 2H). In other experiments the decrease in tyrosine phosphorylation was variable (25%–90%) and correlated with the decrease in Rho protein.
Figure 2.
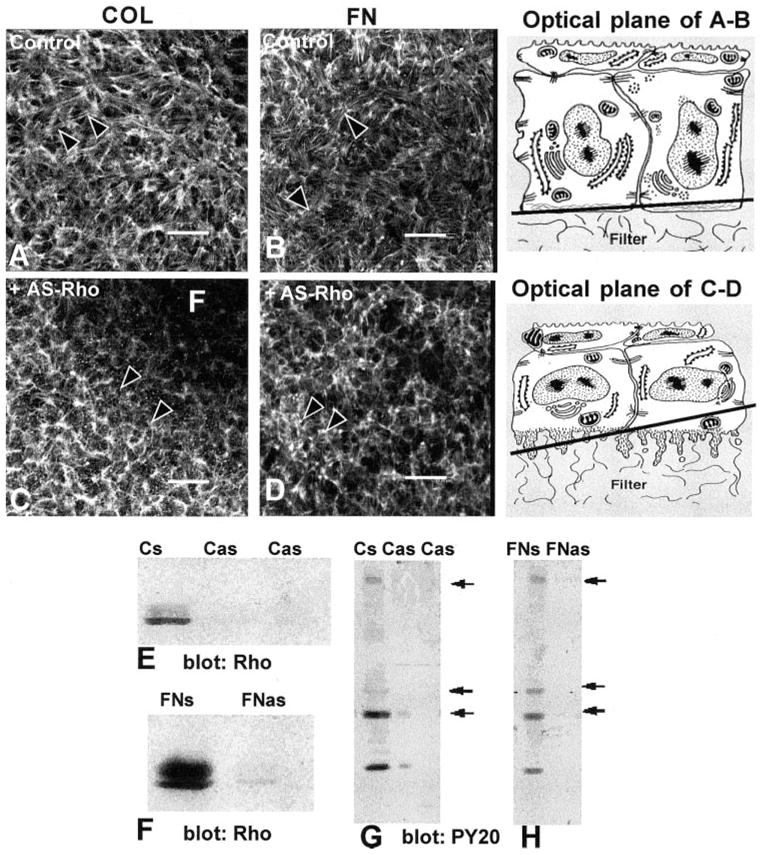
Corneal epithelia transiently transfected (24 hours) with antisense Rho oligonucleotides (10-mer, Fig. 1L-C) were stimulated with FN or COL for 2 hours and stained with fluorescent phalloidin. All experiments and procedures were repeated at least three times with similar results. Single confocal optical sections through basal cells, as illustrated in the tissue schematic, show the distribution of the F-actin in epithelia stimulated with either COL or FN in the control tissues (A, B). Areas that did not contain actin staining (dark) were optical sections through the supporting filter (F). The tissues were not flat; therefore, a single optical section may contain the filter (F) and basal cell cytoplasm (C). In tissues transiently transfected with Rho antisense (C, D), the epithelia had decreased actin in the basal compartment and did not form an ACM after stimulation with COL or FN. The optical sections were taken at the base of the epithelia, as illustrated in the schematic at various tangential planes. Epithelia harvested and analyzed with Western blot analysis were probed with anti-Rho (E, F). The sense control (FNs or Cs) had a 21-kDa protein that migrated as a doublet. In contrast, epithelia transfected with antisense Rho had 80% less Rho protein (E, F; Cas, FNas). To determine whether Rho protein affects other signal transduction proteins, the same Western blot analysis used in (E) and (F) were stripped and reprobed with anti-phosphotyrosine (G, H). In control tissues (Cs, FNs), tyrosine-phosphorylated proteins at molecular weights of approximately 190, 68, 42, and 20 kDa were detectable (arrows), whereas in the tissue treated with antisense Rho oligonucleotides (Cas, FNas), these proteins were significantly decreased. Scale bar, 20 μm.
Figure 3.
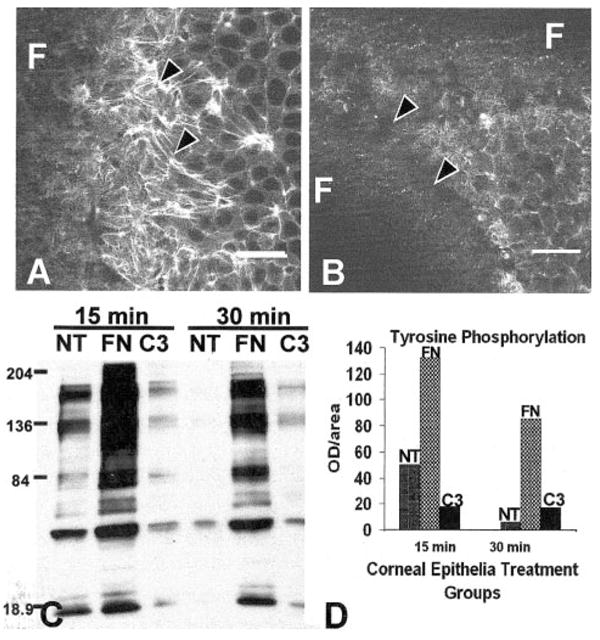
Corneal epithelia treated with exoenzyme C3 (3 μg/mL) did not reorganize the ACM (B) compared with the control (A) and decreased tyrosine phosphorylation (C, D) compared with FN-stimulated epithelia. The total optical density from this sample immunoblot using the whole lane demonstrated that C3 decreased tyrosine phosphorylation 8- to 10-fold (D). All experiments and procedures were repeated at least three times with similar results. Single confocal optical sections through basal cells, as illustrated in the tissue schematic (Fig. 2), show the distribution of the F-actin in epithelia stimulated with FN in the control tissues (A). Areas that did not contain actin staining (dark) were optical sections through the supporting filter (F). The tissues were not flat; therefore, a single optical section may contain the filter (F) and basal cell cytoplasm. Scale bar, 20 μm.
Decreasing Rho activity using the exoenzyme C3 also blocked FN-stimulated reformation of ACM (Fig. 3B) compared with control samples (Fig. 3A). In addition, FN-stimulated tyrosine phosphorylation was decreased more than 8- to 10-fold (Fig. 3D) in tissues harvested at 15 and 30 minutes (Fig. 3C), as measured by the optical density of all tyrosine-phosphorylated proteins.
In summary, either by using the pharmacologic agent C. botulinum C3 exoenzyme that blocks Rho function by inducing ADP ribosylation of all Rho protein27 or by downregulating total Rho protein by using antisense technology the ability of these cells to respond to ECM molecules was decreased by reorganization of the basal actin cytoskeleton.
The Rho antisense-treated epithelia had less Rho protein, regardless of the stimulatory ECM molecule (Figs. 2E, 2F). The decrease in Rho protein was throughout the tissue (Figs. 4D–F) compared to sense controls (Figs. 4A–C). In the control specimens, Rho was distributed in a cell membrane pattern visualized as a honeycomb pattern in single optical sections through the basal cells of the corneal epithelium (Figs. 4A–C). The transient transfection with antisense Rho did not change the distribution pattern, but did decrease the amount detected with immunohistochemistry (Figs. 4D–F, intensity wedge).
Figure 4.
Corneal epithelial cells were transfected with sense or antisense oligonucleotides directed toward Rho, immunolabeled with anti-Rho antibody, and analyzed with by CLSM, to determine intracellular distribution and transfection efficiency. The pinhole, voltage, and offset were the set at the same levels on both images. The intensity wedge indicates the range of detected light intensity from 0 (black) to 255 (white). Rho in control sense-transfected epithelia had a honeycomb distribution, similar to other membrane-associated proteins (A–C). The protein was less prominent in the periderm cells but was evenly distributed throughout the basal cells in a membrane-bound pattern (A–C). In contrast, epithelia transfected with antisense Rho had significantly less immunolabeled protein, indicating that the transfection decreased the amount of Rho protein evenly throughout the epithelium (D–F). The intensity wedge indicates the relative intensity of the pixels in each image. The schematic drawing indicates the plane of the optical sections. All images are single optical sections through representative tissues from three experiments.
As a further control for the unforeseen effects of antisense experiments, we designed oligonucleotides to decrease another signaling molecule, Raf (Fig. 5). Raf is downstream of Ras and upstream of the MAP kinase pathway that has been shown to contribute to actin reorganization and COL binding in this epithelial model.11 Decreasing Raf levels with transient transfection (Fig. 5C) did not inhibit FN-stimulated reformation of ACM, as shown in single optical sections taken through the ACM (Figs. 5A, 5B), nor did it inhibit tyrosine phosphorylation (Fig. 5D).
Figure 5.
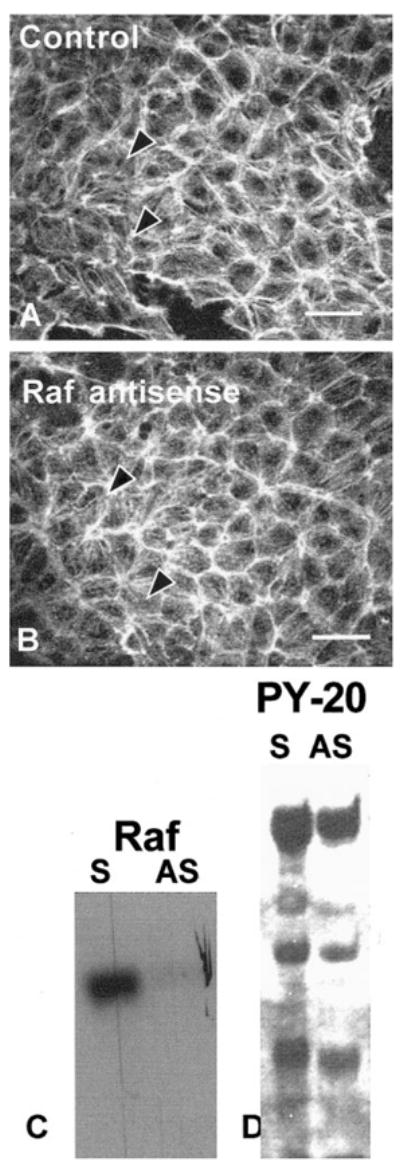
Corneal epithelia transiently transfected (24 hours) with antisense Raf oligonucleotides (10-mer directed to the 5′ region) and stimulated with FN for 2 hours had a normal actin distribution (A, B) and tyrosine phosphorylation pattern (D). All experiments were repeated at least three times with similar results. Single confocal optical sections through basal cells (as illustrated in the schematic in Fig. 2) demonstrated the F-actin distribution in epithelia stimulated with FN in the presence of Raf sense (A) or antisense (B) oligonucleotides formed an ACM. Epithelia harvested and analyzed with Western blot analysis probed with anti-Raf (C). The sense control had protein that migrated at the appropriate molecular weight. In contrast, epithelia transfected with antisense Raf had less Raf protein (C). To determine whether Raf protein affected other signal transduction proteins, the same Western blot shown in (C) was stripped and reprobed with anti-phosphotyrosine. Nearly all the phosphoproteins found in the sense control were also present in the antisense-treated tissue (D).
Discussion
This corneal epithelial organ culture model facilitates the study of ECM interactions with actin-associated proteins and other downstream signaling molecules in whole tissue rather than in cultured cells. In addition to confirming in vitro cultured cell studies, this developmental model allows the study of ECM–cell interactions in a whole-tissue model that preserves many in vivo characteristics including apical-basal polarity and cell– cell junctions. The current experiments clearly demonstrated that Rho was necessary for COL- or FN-stimulated actin reorganization; however, Raf was not mandatory. The antisense oligonucleotide decreased Rho protein levels, but did not change the distribution of the remaining Rho. This was an interesting observation, because a recent study using green fluorescent protein (GFP)–tagged plasmids for seven Rho family guanosine triphosphatases (GTPases) to analyze RhoGDI binding in live cells has shown that RhoB but not RhoA localizes to the plasma membrane in cultured Madin-Darby canine kidney (MDCK) and COS-1 cells.35 Furthermore, mutation of specific amino acids that affect either RhoGDI binding or palmitoylation also changes intracellular localization of these proteins.35 The current experiments have not identified the specific isoforms of Rho that may be present in the avian corneal epithelium. However, to date, many (at least nine) isoforms have been identified in other mammalian tissues. Multiple isoforms have been identified in other avian tissues, and, at the amino acid level, human RhoA and chick RhoA are 100% homologous.36 The variation between different forms of Rho appears to be predominately in the 3′ sequence regions, whereas the 5′ areas are highly homologous.36 RhoB may be transiently expressed in embryonic ocular tissues. A recent report demonstrates that RhoB is found in the mouse lens from E11.5 to postnatal day 18. RhoB immunohistochemistry is positive in the corneal epithelium at postnatal days 1 to 7.37 We used RT-PCR to demonstrate that RhoA, -B, and -C mRNAs can be detected in chick corneal epithelia (data not shown).
Cellular signaling initiated by integrin activation is a complex ballet of molecules interacting and stimulating surrounding proteins, lipids, and ions and resulting in cytoskeletal reorganization, modulation of differentiation, and induction of gene expression. The choreography of events in this signaling pathway remains unknown; however, both integrin ligand occupancy and receptor clustering are critical.38 Integrin receptor engagement and clustering leads to the formation of focal adhesions in cultured cells, where integrins link to intracellular cytoskeletal complexes and bundles of actin filaments. The signaling pathways activated by integrins have been identified through biochemical and morphologic analyses. Protein phosphorylation is detected in response to integrin stimulation. The current experiments demonstrated that Rho was necessary for the tyrosine phosphorylation of the signaling cascade: Decreasing either Rho protein or activity decreased the phosphorylation of signaling proteins.
In this epithelial model, we have shown that both MAP kinase and PI3 kinase are involved in reorganization of the ACM in response to COL.11 These two apparently independent events may be linked through the small GTP-binding proteins that include Rho, Rac, and Cdc42, each of which are important players in focal adhesion assembly and actin cytoskeletal formation.25 These small GTPases shuttle between the GTP-bound active form and the GDP-bound inactive form. The theory is that GAPs bind Rho and hydrolyze GTP to GDP–inactivating Rho. GEFs release GDP from Rho-GDP, enabling it to bind GTP. Several and GEFs and GAPs have been identified in many cell systems.25 In this epithelial model, p190Rho-GAP becomes tyrosine phosphorylated very quickly (2–5 minutes) in response to ECM stimulation.10 Cheng et al.39 and Hu and Settleman40 demonstrated that Src phosphorylates p190Rho-GAP. One hypothesis is that phosphorylated p190Rho-GAP binds to p120Ras-GAP41 to form a complex with an additional unidentified 14-kDa target protein40 in glutathione S-transferase (GST) fusion protein assays. We have demonstrated that an antibody specific for phosphorylated tyrosine residues does not immunoprecipitate p190Rho-GAP10 from ECM-stimulated corneal epithelial cell lysates. We hypothesize that this complex may decrease or slow down the GAP function (changing RhoGTP to RhoGDP) of these proteins, allowing RhoGTP to stay activated longer. The activated RhoGTP prolongs the interaction with downstream kinases to increase actin polymerization and contraction.
Results from several laboratories using a variety of approaches support this hypothesis. Overexpressing v-Src in chick embryo fibroblasts increases p190Rho-GAP phosphorylation and association with p120Ras-GAP but decreases actin stress fibers. If the dominant active form of Rho (Val14-RhoA) is introduced into the v-Src–transfected cells, the actin stress fibers return.42 Further evidence that p190Rho-GAP influences Rho-dependent actin bundle formation comes from microinjection experiments in which p190Rho-GAP preferentially inhibited Rho-mediated stress fiber formation in fibroblasts.43 Additional evidence to support the role of p190Rho-GAP in actin assembly was obtained from transgenic mice without functional p190Rho-GAP, which have abnormal accumulation of polymerized actin in the neural tube floor plate.44 A recent investigation showed the interesting result that RhoGTP actually transiently decreases in the first 15 minutes after FN binding in Swiss 3T3 cells.45,46 The data shown here provide evidence that without Rho, the cell does not form actin bundles. The actin bundles may act to cluster the integrins and amplify signaling by tyrosine phosphorylation.
In this system, decreasing Rho also decreased tyrosine phosphorylation drastically. The decrease in tyrosine phosphorylation correlated with the decrease in Rho protein or its activity (C3 exoenzyme experiments), indicating that Rho may have broad effects on either decreasing kinase or increasing phosphatase activity. These data may also indicate that the cells are dying, either through a regulated apoptosis pathway or necrosis. Additional experiments must be completed to determine why tyrosine phosphorylation changes in this tissue.
Several of the RhoGTP target proteins are serine-threonine kinases (referred to as ROCK) including ROCK-I (p160ROCK/ROKβ) and ROCK-II (ROKα/Rho-kinase) and have effects on actin polymerization, actin myosin contraction, and pH changes. As discussed previously, ROCK has been implicated in corneal epithelial migration.4,5 ROCK activates myosin light-chain kinase to increase actin–myosin contraction47,48 and has been found in adult corneal epithelia4,5 and the avian chick corneal epithelium.49 Another protein, p140Dia increases actin polymerization by binding to the actin-binding protein profilin and works through the PIP2 pathway.50
In conclusion, our data support the hypothesis of a possible sequence of events: First, contact with ECM causes integrin-mediated FAK phosphorylation that in turn phosphorylates the surrounding proteins, such as paxillin and Src. It is known that Src phosphorylates p190Rho-GAP and inactivates or slows its GAP function, allowing RhoGTP to stay active longer.41 Activated RhoGTP binds to downstream kinases, such as p160 ROCK and p140Dia to increase actin polymerization and contraction.47,48 The actin reorganization aids integrin clustering, allowing more ECM binding to increase FAK and other signal transduction events. The current experiments support this theory, in that decreasing Rho blocked the reformation of ACM and tyrosine phosphorylation.
Acknowledgments
The authors thank Petra Moessner, Jesus Acevedo, Tamara Field, Tracie Robinson, and Andrew Campbell for technical assistance.
Supported by National Eye Institute Grant EY08886 and the Baylor Oral Health Foundation (KKHS), who provided funds to purchase the Leica TCS SP2 microscope.
Footnotes
Commercial relationships policy: N.
References
- 1.Zieske JD. Perpetuation of stem cells in the eye. Eye. 1994;8:163 – 169. doi: 10.1038/eye.1994.41. [DOI] [PubMed] [Google Scholar]
- 2.Gipson I, Inatomi F. Extracellular matrix and growth factors in corneal wound healing. Curr Opin Ophthalmol. 1995;6:3 – 10. doi: 10.1097/00055735-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Nishida T, Tanaka T. Extracellular matrix and growth factors in corneal wounding. Curr Opin Ophthalmol. 1996;7:2 – 11. doi: 10.1097/00055735-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Sundarraj N, Kinchington PR, Wessel H, et al. A Rho-associated protein kinase: differentially distributed in limbal and corneal epithelia. Invest Ophthalmol Vis Sci. 1998;39:1266 – 1272. [PubMed] [Google Scholar]
- 5.Anderson SC, Sundarraj N. Regulation of a Rho-associated kinase expression during the corneal epithelial cell cycle. Invest Ophthalmol Vis Sci. 2001;42:933 – 940. [PubMed] [Google Scholar]
- 6.Nakamura M, Nagano T, Chikama T, Nishida T. Role of the small GTP-binding protein Rho in epithelial cell migration in the rabbit cornea. Invest Ophthalmol Vis Sci. 2001;42:941 – 947. [PubMed] [Google Scholar]
- 7.Sugrue SP, Hay ED. Response of basal epithelial cell surface and cytoskeleton to solubilized extracellular matrix molecules. J Cell Biol. 1981;91:45 – 54. doi: 10.1083/jcb.91.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugrue SP, Hay ED. The identification of extracellular matrix (ECM) binding sites on the basal surface of embryonic corneal epithelium and the effect of ECM binding on epithelial collagen production. J Cell Biol. 1986;102:1907 – 1916. doi: 10.1083/jcb.102.5.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svoboda KKH, Hay ED. Embryonic corneal epithelial interaction with exogenous laminin and basal lamina is F-actin dependent. Dev Biol. 1987;123:455 – 469. doi: 10.1016/0012-1606(87)90403-9. [DOI] [PubMed] [Google Scholar]
- 10.Svoboda KKH, Orlow DL, Chu CL, Reenstra WR. ECM stimulated actin bundle formation in embryonic corneal epithelia is tyrosine phosphorylation dependent. Anat Rec. 1999;254:348 – 359. doi: 10.1002/(sici)1097-0185(19990301)254:3<348::aid-ar5>3.0.co;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu CL, Reenstra RW, Orlow DL, Svoboda KKH. Erk and PI3 kinase are necessary for collagen binding and actin reorganization in avian corneal epithelia. Invest Ophthalmol Vis Sci. 2000;41:3374 – 3382. [PMC free article] [PubMed] [Google Scholar]
- 12.Burridge K, Fath K. Focal contacts: transmembrane links between the extracellular matrix and the cytoskeleton. Bioessays. 1989;10:104 – 108. doi: 10.1002/bies.950100403. [DOI] [PubMed] [Google Scholar]
- 13.Burridge K, Nuckolls G, Otey C, Pavalko G, Simon K, Turner C. Actin-membrane interaction in focal adhesions. Cell Diff Dev. 1990;32:337 – 342. doi: 10.1016/0922-3371(90)90048-2. [DOI] [PubMed] [Google Scholar]
- 14.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Ann Rev Cell Biol. 1988;4:487 – 525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 15.Bockholt SM, Burridge K. An examination of focal adhesion formation and tyrosine phosphorylation in fibroblasts isolated from src-, fyn-, and yes- mice. Cell Adhes Commun. 1995;3:91 – 100. doi: 10.3109/15419069509081279. [DOI] [PubMed] [Google Scholar]
- 16.Svoboda KKH. Embryonic corneal epithelial actin alters distribution in response to laminin. Invest Ophthalmol Vis Sci. 1992;33:324 – 333. [PMC free article] [PubMed] [Google Scholar]
- 17.Khoory W, Wu E, Svoboda KKH. Intracellular relationship between actin and alpha-actinin in a whole corneal epithelial tissue. J Cell Sci. 1993;106:703 – 717. doi: 10.1242/jcs.106.3.703. [DOI] [PubMed] [Google Scholar]
- 18.Wu XY, Svoboda KKH, Trinkaus-Randall V. Intracellular distribution of cytoskeletal and adhesion proteins in response to corneal substrata. Exp Eye Res. 1995;60:445 – 458. doi: 10.1016/s0014-4835(05)80101-0. [DOI] [PubMed] [Google Scholar]
- 19.Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691 – 700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 20.Petch LA, Bockholt SM, Bouton A, Parsons JT, Burridge K. Adhesion-induced tyrosine phosphorylation of the p130 Src substrate. J Cell Sci. 1995;108:1371 – 1379. doi: 10.1242/jcs.108.4.1371. [DOI] [PubMed] [Google Scholar]
- 21.Parsons JT. Integrin-mediated signalling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr Opin Cell Biol. 1996;8:146 – 152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- 22.Defilippi P, Olivo C, Venturino M, Dolce L, Silengo L, Tarone G. Actin cytoskeleton organization in response to integrin-mediated adhesion. Micro Res Tech. 1999;47:67 – 78. doi: 10.1002/(SICI)1097-0029(19991001)47:1<67::AID-JEMT7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Ridley A. What initiates actin polymerization? Genome Biol. 2000;1:102. doi: 10.1186/gb-2000-1-1-reviews102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridley A. Rho family proteins and regulation of the actin cytoskeleton. Prog Mol Subcell Biol. 1999;22:1 – 22. doi: 10.1007/978-3-642-58591-3_1. [DOI] [PubMed] [Google Scholar]
- 25.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;297:509 – 514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 26.Ridley A. Rho GTPases: integrating integrin signaling. J Cell Biol. 2000;150:107 – 109. doi: 10.1083/jcb.150.4.f107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rankin S, Morii N, Narumiya S, Rozengurt E. Botulinum C3 exoenzyme blocks the tyrosine phosphorylation of p125FAK and paxillin induced by bombesin and endothelin. FEBS Lett. 1994;354:315 – 319. doi: 10.1016/0014-5793(94)01148-6. [DOI] [PubMed] [Google Scholar]
- 28.Chow G, Nietfeld JJ, Knudson CB, Knudson W. Antisense inhibition of chondrocyte CD44 expression leading to cartilage chondrolysis. Arthritis Rheum. 1998;41:1411 – 1419. doi: 10.1002/1529-0131(199808)41:8<1411::AID-ART10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 29.Gewirtz AM, Sokol DL, Ratajczak MZ. Nucleic acid therapeutics: state of the art and future prospects. Blood. 1998;92:712 – 736. [PubMed] [Google Scholar]
- 30.Woolf TM, Jennings CG, Rebagliati M, Melton DA. The stability, toxicity and effectiveness of unmodified and phosphorothioate antisense oligonucleotides in Xenopus oocytes and embryos. Nucleic Acids Res. 1990;18:1763 – 1769. doi: 10.1093/nar/18.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawley JB, editor. Handbook of Biological Confocal Microscopy. 2. New York: Plenum; 1990. Fundamental limits in confocal microscopy; pp. 19–37. [Google Scholar]
- 32.Wilson T. The role of the pinhole in confocal imaging systems. In: Pawley JB, editor. Handbook of Biological Confocal Microscopy. 2. New York: Plenum; 1990. pp. 113–126. [Google Scholar]
- 33.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680 – 685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Hay ED, Revel JP. Fine structure of the developing Avian cornea. In: Wolsky A, Chen PS, editors. Monographs in Developmental Biology. Vol. 1. Basel, Switzerland: S. Karger; 1969. pp. 66–74. [PubMed] [Google Scholar]
- 35.Michaelson D, Silletti J, Murphy G, D’eustachio P, Rush M, Philips MR. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J Cell Biol. 2001;152:111 – 126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malosio M, Gilardelli D, Paris S, Albertinazzi C, DeCurtis I. Differential expression of distinct members of Rho family GTP-binding proteins during neuronal development: identification of Rac1B, a new neural-specific member of the family. J Neurosci. 1997;17:6717 – 6728. doi: 10.1523/JNEUROSCI.17-17-06717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maddala R, Peng Y-W, Rao PV. Selective expression of the small GTPase RhoB in the early developing mouse lens. Dev Dyn. 2001;222:534 – 537. doi: 10.1002/dvdy.1202. [DOI] [PubMed] [Google Scholar]
- 38.Dedhar S, Hannigan GE. Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Curr Opin Cell Biol. 1996;8:657 – 669. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- 39.Cheng JC, Frackelton AR, Jr, Bearer EL, et al. Changes in tyrosine-phosphorylated p190 and its association with p120 type I and p100 type II RasGAPs during myelomonocytic differentiation of human leukemic cells. Cell Growth Diff. 1995;6:139 – 148. [PMC free article] [PubMed] [Google Scholar]
- 40.Hu KQ, Settleman J. Tandem SH2 binding sites mediate the RasGAP-RhoGAP interaction: a conformational mechanism for SH3 domain regulation. EMBO J. 1997;16:473 – 483. doi: 10.1093/emboj/16.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foster R, Hu KQ, Shaywitz DA, Settleman J. p190 RhoGAP, the major RasGAP-associated protein, binds GTP directly. Mol Cell Biol. 1994;14:7173 – 7181. doi: 10.1128/mcb.14.11.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fincham VJ, Chudleigh A, Frame MC. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J Cell Sci. 1999;112:947 – 956. doi: 10.1242/jcs.112.6.947. [DOI] [PubMed] [Google Scholar]
- 43.Ridley AJ, Self AJ, Kasmi F, et al. Rho family GTPase activating proteins p190, bcr and RhoGAP show distinct specificities in vitro and in vivo. EMBO J. 1993;12:5151 – 5160. doi: 10.1002/j.1460-2075.1993.tb06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brouns MR, Matheson SF, Hu KQ, et al. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development. 2000;127:4891 – 4903. doi: 10.1242/dev.127.22.4891. [DOI] [PubMed] [Google Scholar]
- 45.Ren X-D, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113:3673 – 3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 46.Ren X-D, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578 – 585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura K, Ito M, Amano M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245 – 248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 48.Amano M, Chihara K, Kimura K, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308 – 1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 49.Svoboda KKH, Reenstra WR. Approaches to studying cellular signaling: a primer for morphologists. Anat Rec. 2002;269:123 – 139. doi: 10.1002/ar.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe N, Madaule P, Reid T, et al. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044 – 3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]