Abstract
The embryonic chicken corneal epithelium is a unique tissue that has been used as an in vitro epithelial sheet organ culture model for over 30 years (Hay and Revel [1969] Fine structure of the developing Avian cornea. Basel, Switzerland: S. Karger A.G.). This tissue was used to establish that epithelial cells could produce extracellular matrix (ECM) proteins such as collagen and proteoglycans (Dodson and Hay [1971] Exp Cell Res 65:215–220; Meier and Hay [1973] Dev Biol 35:318–331; Linsenmayer et al. [1977] Proc Natl Acad Sci U S A 74:39–43; Hendrix et al. [1982] Invest Ophthalmol Vis Sci 22:359–375). This historic model was also used to establish that ECM proteins could stimulate actin reorganization and increase collagen synthesis (Sugrue and Hay [1981] J Cell Biol 91:45–54; Sugrue and Hay [1982] Dev Biol 92:97–106; Sugrue and Hay [1986] J Cell Biol 102:1907–1916). Our laboratory has used the model to establish the signal transduction pathways involved in ECM-stimulated actin reorganization (Svoboda et al. [1999] Anat Rec 254:348–359; Chu et al. [2000] Invest Ophthalmol Vis Sci 41:3374–3382; Reenstra et al. [2002] Invest Ophthalmol Vis Sci 43:3181–3189). The goal of the current study was to investigate the role of ECM in epithelial cell survival and the role of Rho-associated kinase (p160 ROCK, ROCK-1, ROCK-2, referred to as ROCK), in ECM and lysophosphatidic acid (LPA) -mediated actin reorganization. Whole sheets of avian embryonic corneal epithelium were cultured in the presence of the ROCK inhibitor, Y27632 at 0, 0.03, 0.3, 3, or 10 μM before stimulating the cells with either collagen (COL) or LPA. Apoptosis was assessed by Caspase-3 activity assays and visualized with annexin V binding. The ROCK inhibitor blocked actin cortical mat reformation and disrupted the basal cell lateral membranes in a dose-dependent manner and increased the apoptosis marker annexin V. In addition, an in vitro caspase-3 activity assay was used to determine that caspase-3 activity was higher in epithelia treated with 10 μM Y-27632 than in those isolated without the basal lamina or epithelia stimulated with fibronectin, COL, or LPA. In conclusion, ECM molecules decreased apoptosis markers and inhibiting the ROCK pathway blocked ECM stimulated actin cortical mat reformation and increased apoptosis in embryonic corneal epithelial cells.
Keywords: corneal epithelia, apoptosis, Rho, ROCK, actin, caspase-3, annexin V, FAK and tyrosine phosphorylation
Introduction
Extracellular matrix (ECM) stimulated changes in actin cytoskeleton organization have been well documented in the avian corneal epithelial organ culture system (Sugrue and Hay, 1981, 1986; Svoboda and Hay, 1987; Svoboda et al., 1999; Chu et al., 2000) and in cells grown in traditional tissue culture (Burridge et al., 1988, 1990; Burridge and Fath, 1989; Bockholt and Burridge, 1995). Corneal epithelial tissues isolated without basal lamina responded to ECM using an actin-dependent mechanism (Fig. 1). The basal cell surface flattens and the disrupted actin cortical mat (ACM) reorganizes in the presence of laminin, fibronectin, or collagen (Sugrue and Hay, 1981, 1986; Svoboda and Hay, 1987; Svoboda, 1992; Khoory et al., 1993) with distinct configurations in the presence of different ECM molecules (Fig. 1; Svoboda et al., 1999). In addition, we have shown that this tissue also reorganizes the actin cytoskeleton in response to bombesin or lysophosphatidic acid (LPA; Svoboda et al., 1999).
Fig. 1.
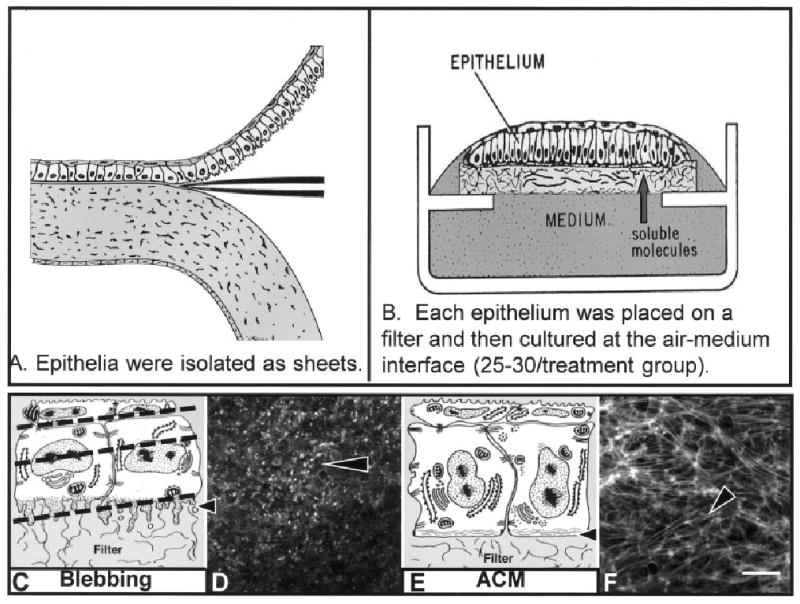
A,B: Schematic drawings of corneal epithelia isolated from the underlying stroma (A) and cultured at the air–medium interface (B). Soluble stimulating and inhibiting substances can be added to the medium for specific culture times. C: Epithelia isolated without the basal lamina extended basal cellular processes termed blebs as shown in the schematic drawing (arrowhead). D: The blebs contain F-actin (labeled with fluorescein isothiocyanate-phalloidin) that appears as punctate spots in a single confocal optical section taken through the basal area illustrated in the schematic drawing (C, lower dashed line). E: Tissue cultured with soluble extracellular matrix molecules reorganize the basal actin into an actin cortical mat (ACM) illustrated in the schematic drawing (arrowhead). F: The bundles of F-actin (phalloidin labeled) viewed in a single optical section from epithelia stimulated with collagen for 2 hr appear to align from cell to cell, across the field. Scale bar = 20 μm in F (applies to D,F).
The reorganization of the actin cytoskeleton is orchestrated through a signaling cascade. The epithelia needed to be in contact with matrix molecules for a minimum of 15 min for the ACM to reform. In the first 15 min of ECM stimulation, many signaling proteins were activated (Svoboda et al., 1999). Focal adhesion kinase (FAK) and p190Rho-GAP were tyrosine phosphorylated within 5 min in response to soluble ECM stimulation (Svoboda and Reenstra, 2002). Erk-1, erk-2, and PI3 kinase were activated later in the signaling cascade (30–60 min). These phosphorylation events were required, as a tyrosine and src kinase inhibitor, Herbimycin A blocked actin reorganization in a dose-dependent manner (Svoboda et al., 1999). In summary, actin reorganization required tyrosine phosphorylation of FAK, p190Rho-GAP, paxillin, tensin, MAP kinase, and PI3 kinase activation (Svoboda et al., 1999; Chu et al., 2000; Svoboda and Reenstra, 2002). Changes in actin cytoskeleton organization have also been recorded in monolayer cultures of corneal epithelial cells grown on ECM substrates (laminin, fibronectin, and collagen) that initiated an increase in intracellular pH (Wu et al., 1995). The current understanding of the cascade of events appears to be the following: ECM proteins bind integrin molecules; FAK may be recruited to the integrin cytoplasmic domain by talin. Talin binds vinculin and paxillin. FAK goes through a conformational change to bind the β1 integrin cytoplasmic tail and autophosphorylates after the integrins have clustered by means of a Rho-mediated mechanism (Giancotti, 1997; Svoboda and Reenstra, 2002). The phosphorylated FAK binds and activates many proteins either directly or through adapter proteins, including paxillin, talin, p85, shc, p-cas, Grb2, Sos, src, and fyn in response to integrin activation (Petch et al., 1995; Parsons, 1996; Defilippi et al., 1999). In addition, there is mounting evidence that different integrin molecules stimulate alternate proteins.
Ras homology Rho proteins are small GTPases involved in signal transduction pathways that regulate actin bundle formation in whole tissues (Reenstra et al., 2002) and actin stress fibers in cultured fibroblast cells (Hall, 1998; Ridley, 2001). The active form of the small GTPase family are GTP-bound. Rho alternates between the GTP-bound active form (on) and the GDP-bound inactive form (off) to regulate other downstream kinases. Many other proteins regulate the “on” and “off” state of Rho. The guanine-nucleotide exchange factors are the “on” signal as they add GTP to the protein. GTP-activating proteins (GAPs) are the “off” signal as they remove a phosphate to deactivate the protein. When cultured fibroblasts are treated with LPA or bombesin, new stress fibers and focal adhesions form (Ridley and Hall, 1992, 1994), corneal epithelia also respond to these stimulants by reorganizing the ACM rapidly (Svoboda et al., 1999). Activated Rho proteins stimulate several downstream kinases, including the ROCK family that facilitate actin–myosin contraction by phosphorylating the myosin light chain (Amano et al., 1997; Chihara et al., 1997) and the myosin binding subunit of myosin phosphatase (Kimura et al., 1996). Phosphorylating the myosin phosphatase deactivates the enzyme; therefore, myosin light chain stays active longer.
Recently, several laboratories have identified Rho and its associated downstream kinases (ROCK family; SundarRaj et al., 1998) as being necessary for centripetal corneal epithelial migration associated with cell cycle progression (Anderson and SundarRaj, 2001) and migration during corneal wound healing (Nakamura et al., 2001). In addition, other groups working on glaucoma had reported that human trabecular meshwork and Schlemm's canal primary cell cultures treated with Y27632 (10 μM) had altered cell shape and decreased actin stress fibers, focal adhesions, and protein phosphotyrosine staining (Rao et al., 2001). In addition, the cellular permeability increased and myosin light-chain phosphorylation decreased, indicating that the cells were stressed.
In contrast to monolayer cell culture studies, the current investigation focuses on embryonic corneal epithelial actin reorganization in freshly isolated whole epithelial sheets in response to specific extracellular stimuli. We hypothesize that the ECM or LPA induced actin reorganization contributes to further ECM binding and cell survival.
It is well established that cultured epithelial and endothelial cells require attachment to substrate for survival; however, the evidence for sheets of epithelia is not known (Meredith et al., 1993; Frisch and Francis, 1994; Re et al., 1994; Meredith and Schwartz, 1997; Nicholson, 2001). Apoptosis, also known as programmed cell death, causes susceptible cells to undergo a series of enzymatic and morphologic changes. During apoptosis, several signal transduction pathways and specific degradative enzymes become activated. The degradative enzymes are in the caspase-3 family of cysteinyl proteases, also known as ICE-like because they resemble the first member described, interleukin-1 converting enzyme (ICE). These enzymes may cleave essential structural components of the cell, including actin cytoskeletal elements, nuclear lamins, and small nucleoproteins (Whyte, 1996; De Laurenzi and Melino, 2000). These changes eventually lead to nuclear material condensing as the nuclear DNA breaks down and cellular material pinches off, forming apoptotic bodies. One effect of caspase activation is the activation of a flipase that flips phosphatidylserine (PS) from the internal to the external plasma membrane leaflet.
External membrane bound PS serves as a signal to surrounding cells, identifying the PS-positive cell as undergoing apoptosis. We visualized the “flipped out” PS with a 35-kDa physiologic protein, annexin V lipocortin (labeled with a fluorescent tag), as it bound with nanomolar affinity to membranes containing PS (Blankenberg et al., 1999, 2000). One of the caspase substrates is gelsolin, an actin-severing protein (Kothakota et al., 1997); however, many more proteins with potential caspase cleavage sites have been identified. Important to this research is that cleavage of the actin cytoskeleton is an activity of the caspase-3 family. In these experiments, we measured the amount of activated caspase-3 in an assay that depends on the cleavage of a short peptide (asparagines-glycinevaline-aspargine; DEVD) that contains the caspase-3 recognition sequence.
This research supports the hypothesis that ECM molecules or LPA stimulation facilitates survival of epithelial cells in an intact sheet. In addition, we demonstrate that disrupting actin-myosin contraction with the ROCK inhibitor, Y27632 increased apoptosis markers in embryonic corneal epithelia.
Results
In this model, the embryonic avian corneal epithelia were isolated from the stroma as a sheet of continuous cells (Fig. 1A) and cultured at the air–medium interface (Fig. 1B). The corneal epithelium at stage 34 (embryonic day 8, E8) was composed of two cell layers, with the hexagonal, flat periderm cells apical to smaller diameter basal cells (Fig. 1C,E). The periderm cells range from 6 to 8 μm in height and can be 25 μm wide. F-actin is found in the microvilli of the periderm cells and along the periderm-basal cell junction (Svoboda, 1992). The basal cells range 15–20 μm in height and 5–10 μm in diameter. When basal cells are isolated with the basal lamina, they have a flat basal surface. F-actin is also found along the basal lamina in an organized arrangement called the ACM (Fig. 1E,F; Hay and Revel, 1969). There are usually four basal cells underneath every periderm cell (Svoboda, 1992).
F-actin (labeled with fluorescein isothiocyanate [FITC]-phalloidin) distribution demonstrated distinct organizational patterns when the epithelia were cultured for 2 hr in the presence of different ECM molecules or direct Rho stimulators (Svoboda et al., 1999). In contrast, epithelia isolated without the basal lamina have long cytoplasmic extensions (blebs) along the basal cell surface that contain F-actin (Fig. 1C,D; Sugrue and Hay, 1981; Svoboda, 1992). Interestingly, the direct Rho pathway stimulator, LPA, reorganized the ACM very quickly (15 to 30 min; Svoboda et al., 1999).
The ACM failed to reorganize in a dose-dependent manner in epithelia treated with Y27632 (Figs. 2, 3). Single optical images taken through the periderm cells, central basal cells, and at the tissue–filter interface (dashed lines, Fig. 1C) were used to analyze the F-actin in treated epithelia. Images from representative epithelia demonstrated that the lowest concentration of ROCK inhibitor (0.03 μM Y27632) had no effect on ACM reformation in epithelia stimulated with COL (Fig. 2A–C) or LPA (Fig. 3A–C), similar to controls (COL or LPA only). The periderm cells and cortical actin staining in the basal cells were also not altered with the low concentrations (0.03 μM) of the ROCK inhibitor (Figs. 2, 3A–C,B′) regardless of the stimulus. However, the 0.3 μM concentration of Y27632 blocked ACM reorganization in the collagen-stimulated tissues (Fig. 2F) and decreased the actin filament bundles in the LPA stimulated tissues (Fig. 3F). In addition, the cortical actin staining was more diffuse (Figs. 2 and 3E, box and E′) in both COL- and LPA-treated tissues. The highest concentration used for morphologic analysis (3 μM) blocked actin reorganization in both COL-treated (Fig. 2I) and LPA-treated (Fig. 3I) epithelia. We also observed that, in the higher concentrations of Y27632 (3 and 10 μM), the lateral basal cell membranes were thicker and the actin was interrupted into small circular formations (Figs. 2, 3H box, and H′; 5C–8′ and C–8′). In a series of protein analysis studies, we observed that tryosine phosphorylation of all ECM stimulated proteins decreased modestly in a dose-dependent manner with increasing concentrations of Y27632 with the larger decrease in lysates from epithelia treated with 10 μM (data not shown). We also observed a modest decrease in phosphorylated focal adhesion kinase (data not shown).
Fig. 2.
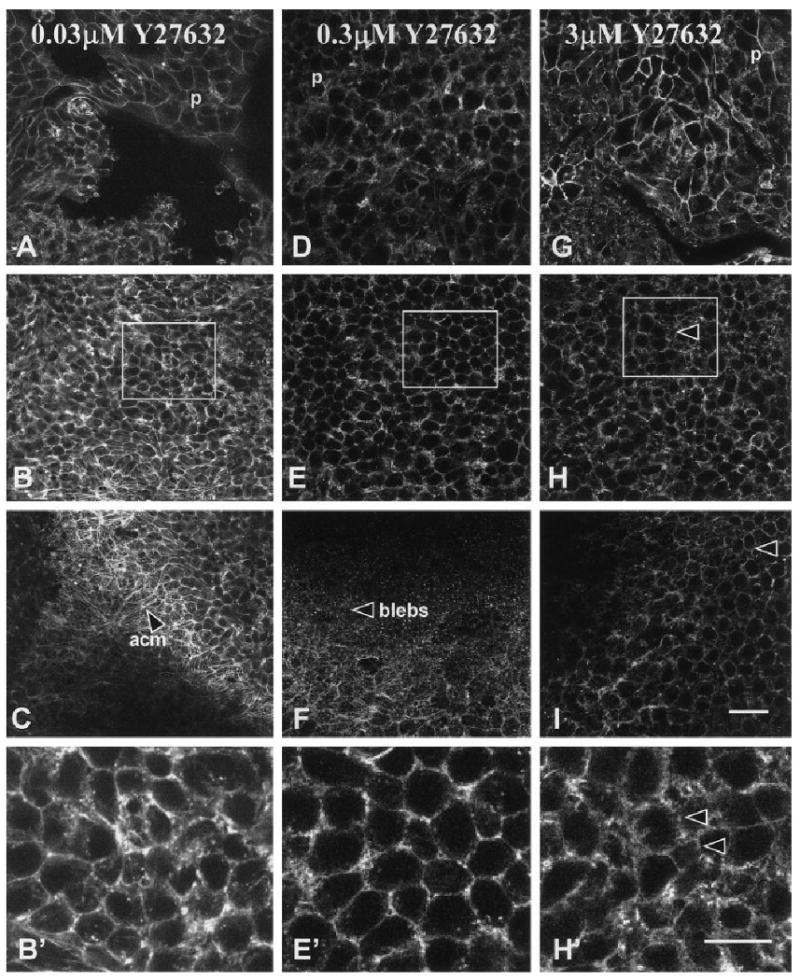
Single confocal optical sections through epithelia treated with Y27632 (0.03, 0.3, and 3 μM) stimulated with collagen and then stained with phalloidin. A–I: Three optical sections (Fig. 1A, dashed lines) were taken at the periderm–basal cell junction (A,D,G) central basal cells (B,E,H) and basal cell-filter interface (C,F,I). B′,E′,H′: Selected areas in B, E, and H (boxes) were enlarged. The actin had a normal distribution (A–C), and the cells reorganized the actin cortical mat (acm, C) in the presence of the lowest concentration of Y27632 (0.03 μM). Higher magnification of the actin distribution in the center of the basal cells showed that it was located along lateral cell membranes (B′). Although the actin distribution appeared normal (D, E, E′) in tissues treated with 0.3 μM Y27632, the actin cortical mat did not reorganize (F). Epithelium treated with 3 μM Y27632 had less polymerized actin in the cortical membrane areas (H, H′) and no actin cortical mat (I). In addition, the basal cell lateral membranes appeared wider (arrowheads, H, H′). p, periderm. Scale bars = 20 μm in I (applies to A–I), 10 μM in H′ (applies to B′,E′,H′).
Fig. 3.
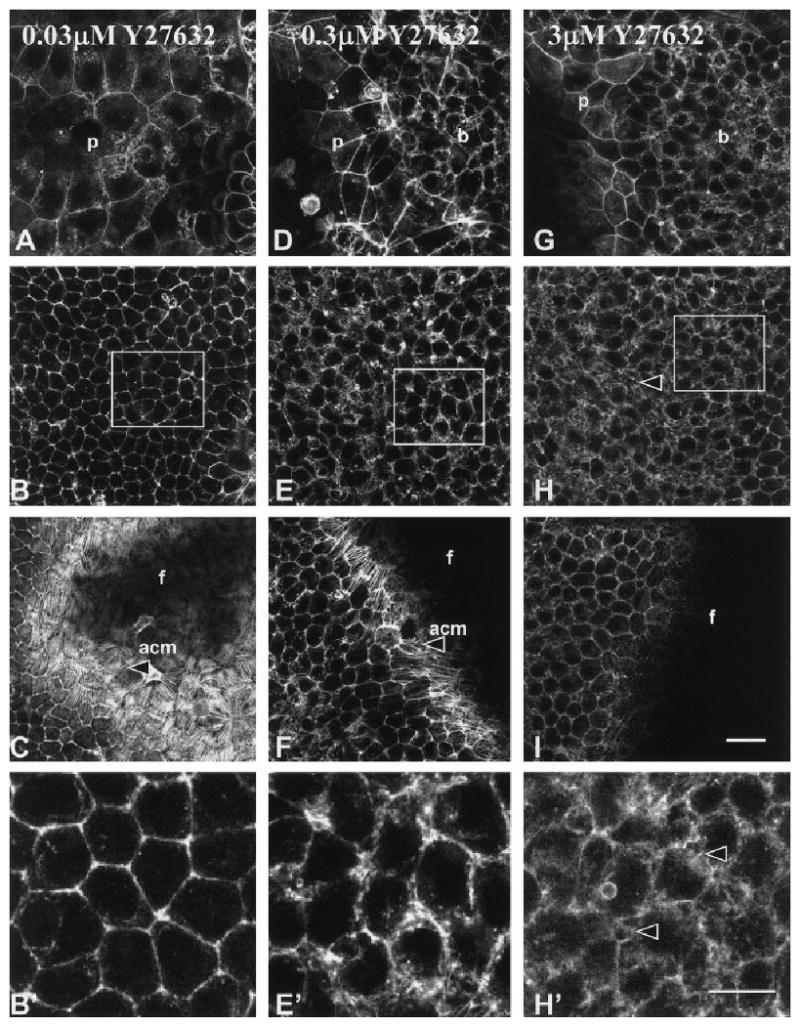
Single confocal optical sections through epithelia treated with Y27632 (0.03, 0.3, and 3 μM) stimulated with LPA and then stained with phalloidin. Three single optical sections (Fig. 1A, dashed lines) are shown from complete z-series for each treatment. A–I: The sections at the periderm-basal cell junction (A,D,G), central basal cells (B,E,H), and basal cell–filter interface (C,F,I) are shown to demonstrate the actin distribution throughout a representative epithelium. B′,E′,H′: Selected areas in B, E, and H (boxes) were enlarged. The actin in the periderm and cortical areas of basal cells had a normal distribution (A–C) and the cells reorganized an extensive actin cortical mat (acm, arrowhead) in the presence of the lowest concentration of Y27632 (0.03 μM). The periderm cell shape (D, p) and lateral cell actin distribution (E, E′) appeared altered in tissues treated with 0.3 μM Y27632, and the actin cortical mat were sparsely organized (F, arrowhead). Epithelia treated with 3 μM Y27632 had less polymerized actin in the cortical membrane areas (H, H′) and no actin cortical mat (I). In addition, the basal cell lateral membranes appeared to have bubbles or blebs (arrowheads, H and H′). f, filter; p, periderm; b, basal cells. Scale bar = 20 μm in I (applies to A–I), 10 μm in H′ (applies to B′,E′,H′).
Fig. 5.
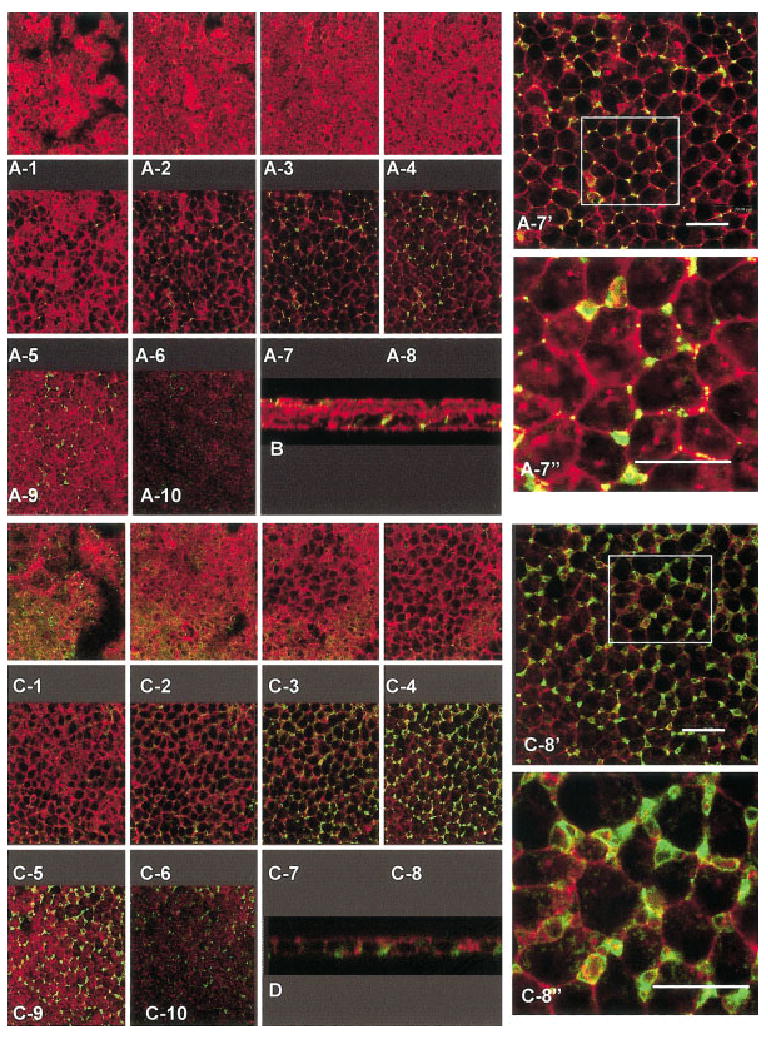
A–D: The complete z series through epithelium treated with 3 μM Y27632 + collagen (COL, A,B) or 10 μM Y27632 + COL (C,D) double labeled with annexin V (green) and phalloidin (red) demonstrates the total distribution of the exposed phosphatidylserine. The apical optical sections of the tissue treated with 3 μM Y27632 (A1–5) have little annexin V staining; however, the basal cells (A6–10) were positive for annexin V binding. Image A-7 was enlarged (A-7′) to demonstrate that the overall architecture of the tissue and distribution of annexin V. An additional area (box in A-7′) was enlarged again (A-7″) to view the distribution of actin and annexin V staining. The basal blebs below the plane of the basal cells (A-10, B) also contained annexin V staining. An xz image (cross-sectional view) also demonstrated that the distribution of annexin V staining (B) was in the basal lateral membranes. The actin cortical mat did not form in this epithelium (A-10). A complete z-series through an epithelium from the apical to the basal surface treated with 10 μM Y27632 had some annexin V staining in the apical regions of the tissue (C1–3), but the staining was more prominent in the basal lateral regions of the basal cells (C5–10). The region approximately 5 microns from the basal surface (C 8) appeared to contain the highest amount of annexin V binding and were shown at two higher magnifications (C 8′ and 8″). Cross-sectional analysis of different epithelia with the same exposure to Y27632 also demonstrated that the annexin V staining was more extensive in basal cells of epithelium exposed to 10 μM Y27632 (D). Scale bars = 20 μm.
One characteristic of apoptosis is that the plasma membrane bubbles or blebs. As our tissues treated with Y27632 appeared to have wider lateral membranes in the basal cells (Figs. 2, 3E′,H′), we examined an apoptosis marker, annexin V. Annexin V binds to phosphatidylserine that is “flipped” to the outer membrane leaflet when cells are undergoing apoptosis. We found that epithelia isolated without the basal lamina had some annexin V binding (Fig. 4A), predominately in the basal cell membranes (Fig. 4A6–9,8′,8″) and in the blebs that extended into the filter (Fig. 4A9–10). The complete z series from an epithelium that was scanned from the apical to basal surface demonstrated that the periderm layer (Fig. 4A1–3) did not bind annexin V. The area with the highest annexin V binding (Fig. 4A8) was enlarged (Fig. 4A8′) and then a selected area (square) was enlarged again (Fig. 4A8″) to demonstrate the spatial localization of the annexin V binding. The xz optical section of the same epithelium (Fig. 4B) also demonstrated that the distribution of annexin V was primarily in the basal cell lateral membranes. In contrast, a complete z series of an epithelium treated with COL and scanned from the apical to the basal surface (Fig. 4C,D) had nearly no annexin V binding in either the periderm (Fig. 4C1–2) or basal cells (Fig. 4C3–7). The ACM (Fig. 4C5–7) had reorganized, producing a flat basal surface (Fig. 4D). A single optical section of the basal cells (Fig. 4C5) was enlarged (Fig. 4C5′) and a selected area (square) was enlarged again (Fig. 4C5″) to demonstrate that annexin V did not bind to the basal lateral membranes in this specimen.
Fig. 4.
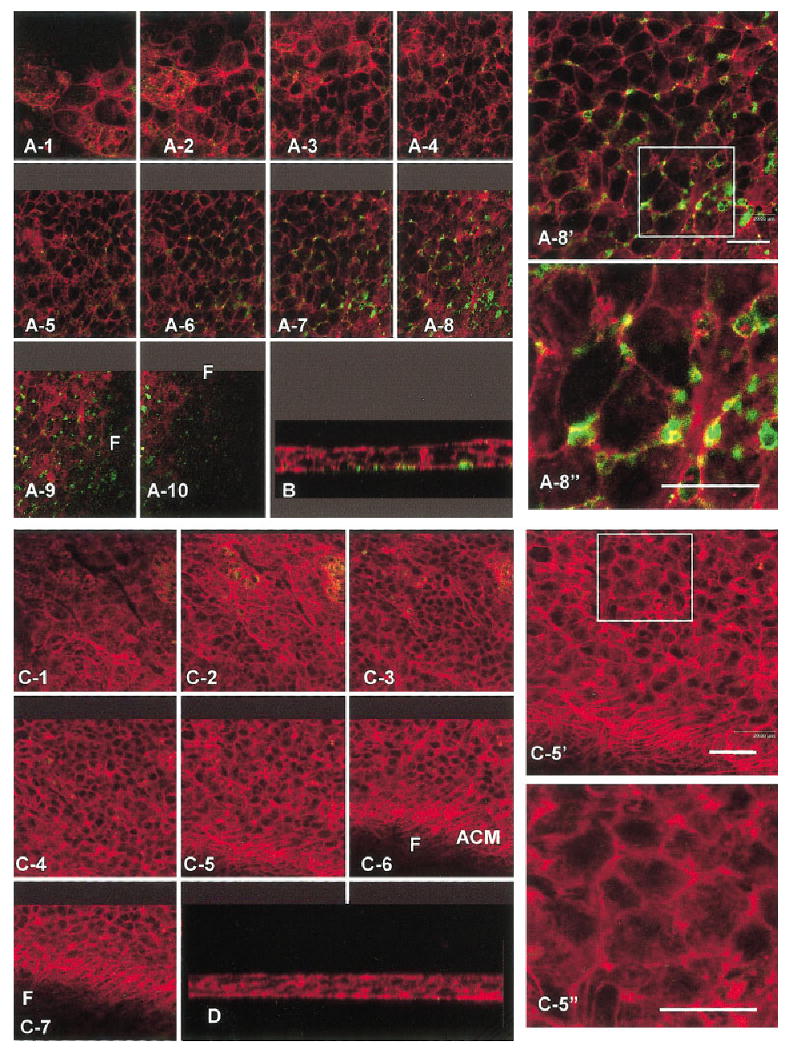
A–D: The complete z series of corneal epithelia cultured in medium with no treatment (NT, A,B) or collagen (COL, C,D) for 2 hr, were incubated with annexin V (green), and then fixed and stained with phalloidin (red). The epithelium that was isolated without the basal lamina and not treated with extracellular matrix or inhibitors was scanned from the apical (A-1) to the basal surface (A-10). This tissue bound some annexin V in the basal cell membrane areas (A6–9, B) and in the cytoplasmic blebs (A9–10, B). Optical section 8 was enlarged (A-8′), and a section (square) was enlarged again (A-8″). There was some overlap in the annexin V-positive areas and actin (yellow) visible in the enlarged area (A-8″). A cross-sectional optical section of the same tissue (B) also demonstrated that the annexin V binding was in the basal areas and cytoplasmic blebs along the basal surface of the epithelium. The complete z series through an epithelium treated with COL (C1–7) had very little annexin V binding and the actin cortical mat reorganized (C5–7). One basal optical section was enlarged (C-5′) and a region (square) was enlarged again (C-5″) to demonstrate that this tissue did not have detectable levels of annexin V binding. A cross-section of this tissue demonstrates that the basal surface was flat and annexin V did not bind to the basal cell membranes (D). Scale bars = 20 μm.
Epithelia treated with 3 or 10 μM Y27632 and type I collagen were annexin V-positive in the basal cell lateral membranes (Fig. 5A7–10,7′7″; C6–10,8′,8″). Complete z-series taken from the apical to the basal surface (Fig. 5A1–10 and C 1–10) through the epithelia demonstrated that basal cells had annexin V binding in the lateral cell membrane areas and in the blebbing cytoplasmic basal surface after exposure to either 3 (Fig. 5A7–10,B) or 10 μM Y27632 (Fig. 5C6–10,D). The annexin V-positive areas had very little overlap (yellow areas in Fig. 5) with F-actin in either single xz optical sections (Fig. 5B,D) or xy optical sections (Fig. 5A7′,7″ and C 8′,8″). In the enlarged images from selected areas (squares in Fig. 5A7′ and C8′), the annexin V staining appeared to be associated with lateral cell membrane blebs or bubbles (Fig. 5A7″ and C8″).
The morphologic experiments were confirmed with a quantitative binding assay for activated caspase-3. Epithelia isolated without the basal lamina and not treated (NT) with either ECM or Y27632 had moderate levels of caspase-3 activity (Fig, 6 [NT]). ECM- and LPA-treated epithelia (Fig. 6, fibronectin [FN], COL, LPA) had lower levels of active caspase-3 than untreated epithelia (NT). In contrast, epithelia exposed to 10 μM Y27632 had significantly higher amounts of activated caspase-3 (Fig. 6). Treating epithelia with both COL and Y27632 did decrease the level of caspase-3 compared with epithelia treated with Y27632 alone, but the caspase-3 levels were higher than COL-treated or NT epithelia (Fig. 6). Pretreating the epithelia with the caspase-3 inhibitor, V-ZAD-FMK (ZVAD), decreased caspase activity in all treatment groups (Fig. 6, ZVAD-NT, ZVAD-COL, ZVAD+C+Y, ZVAD+Y27632) compared with the treatment (NT, COL, COL+Y27632, Y27632), indicating that the assay was specific for caspase activity.
Fig. 6.
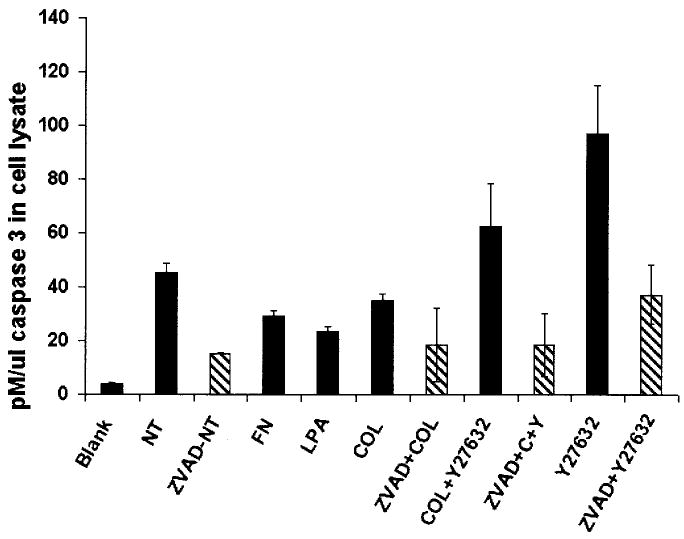
CaspACE assay results: extracellular matrix proteins and lysophosphatidic acid (LPA) decreased caspase-3 activity, and Y27632 increased caspase-3 activity. Cell lysates from corneal epithelia (n = 30/treatment group) isolated without the basal lamina and maintained in culture without treatment (NT), or stimulated with fibronectin (FN), LPA, and collagen (COL, with or without 10 μM Y27632) or 10 μM Y27632 only were compared with epithelia that were pretreated for 16 hr with the caspase-3 inhibitor ZVAD. Tissues treated with FN, COL, or LPA had lower pM/μL caspase-3 compared with the untreated epithelia (NT), demonstrating that the ECM and LPA could decrease apoptosis activity in the sheets of epithelia. The tissues treated with Y27632 had significantly higher caspase-3 levels and cotreating the tissues with COL decreased the caspase-3, but not to either untreated epithelia levels or COL alone. A blank and tissues pretreated with the caspase-3 inhibitor ZVAD were used as controls. All experimental groups represent the results of two experiments with duplicate samples (n = 4). Error bars are standard deviations of the mean.
Discussion
In the adult, corneal epithelial stem cells are located in the limbus. Epithelial cells that will differentiate migrate from the limbus to the central cornea (Collinson et al., 2002). The limbal stem cell population is established between 8 and 10 postnatal weeks in the mouse (Collinson et al., 2002). Before the establishment of the limbal stem cells, dividing cells are found throughout the corneal epithelium (Collinson et al., 2002).
In addition to normal centripetal migration of epithelial cells to replace the sloughing cells, corneal epithelial cells respond to wounding by migrating over the wound bed primarily by sliding of the whole epithelium (Zhao et al., 2003). Many factors contribute to the normal centripetal migration (Zieske, 1994) and successful closure of corneal wounds, including extracellular matrix components, growth factors and cytokines (Gipson and Inatomi, 1995; Nishida and Tanaka, 1996), and intracellular signal molecules that mediate the extracellular signals. In addition, the actin cytoskeleton and associated cell–cell adhesion proteins have been shown to be involved in corneal wound closure (Gipson and Inatomi, 1995; Danjo and Gipson, 1998). Recently, several laboratories have identified Rho and its associated downstream kinases (Rho-associated kinase, ROCK-1, ROCK-2; SundarRaj et al., 1998) as being necessary for centripetal corneal epithelial migration associated with cell cycle progression (Anderson and SundarRaj, 2001) and migration during corneal wound healing (Nakamura et al., 2001).
In contrast to these migration studies, the current investigation focuses on the embryonic corneal epithelial actin reorganization in freshly isolated whole epithelial sheets in response to specific extracellular stimuli. ECM stimulated changes in actin cytoskeleton organization have been well documented in the corneal epithelial organ culture system (Sugrue and Hay, 1981, 1986; Svoboda and Hay, 1987; Svoboda et al., 1999; Chu et al., 2000) and in cells grown in traditional tissue culture (Horwitz et al., 1986; Burridge et al., 1988, 1990; Bockholt and Burridge, 1995).
The small GTPase Rho is implicated in cytoskeletal rearrangements, including stress fiber and focal adhesion formation. One mechanism for the actin rearrangement is that the downstream kinase activated by RhoGTP, ROCK phosphorylates both myosin light chain and myosin phosphatase. This double action activates myosin ATPase and inactivates myosin phosphatase, respectively, resulting in actin–myosin contraction and the formation of stress fibers and focal adhesions in fibroblasts (Narumiya et al., 1997; Watanabe et al., 1999). In this study, we show that ROCK is important for actin bundle formation in the embryonic corneal epithelium as inhibiting this kinase blocked ECM and LPA stimulated actin cortical mat reformation. These conclusions were based on using a highly specific pharmacologic agent, Y27632, which specifically inhibits the ROCK family of kinases in the specific dose ranges used in these experiments (Narumiya, 1999; Davies et al., 2000).
It has been established that vascular endothelial and other epithelial cells undergo apoptosis if the cells are cultured in suspension and integrin-mediated matrix contacts are lost, in a process termed “anoikis” (Meredith et al., 1993; Frisch and Francis, 1994; Meredith and Schwartz, 1997; Frisch and Screaton, 2001). In this study, we found that embryonic corneal epithelial cells maintained in sheets were also dependent upon ECM for survival. We also found that blocking ECM stimulated actin reorganization with the ROCK inhibitor (Y27632) increased the apoptosis markers, caspase 3, and annexin V binding in the corneal epithelial sheets. Interestingly, we found that very low concentrations of Y27632 disrupted the actin cytoskeleton (0.3 μM); however, the increase in annexin V binding was observed at a 10-fold higher dose (3 μM) and caspase 3 activation was recorded at 10 μM. By comparison, most in vitro experiments use 10 μM Y27632 or higher doses (Rao et al., 2001).
Matrix-dependent cell survival has been studied extensively in Madin-Darby canine kidney (MDCK) cells in either two-dimensional tissue culture or filter-suspended systems. The caspase cascade was initiated after cell–matrix detachment in MDCK cells by both release of cytochrome c from mitochondria (Rytomaa et al., 2000) and increased activity of caspase 8, which physically associates with death receptors. Matrix detachment also induces Bax translocation to mitochondria in a caspase-dependent manner. Silencer of death domains (SODD) and dominant-negative FAS-associated death domain protein (FADD) efficiently inhibited cell–matrix detachment mediated anoikis in MDCK cells (Frisch, 1999). Expression of activated Ras or PKB/Akt blocks all the detectable events in the detachment-induced apoptosis signalling pathway, suggesting that PKB/Akt acts at an early point in the pathway, providing the signal normally generated by matrix attachment. Strong activation of Raf can also protect MDCK cells from detachment-induced apoptosis, but this occurs at a point downstream of cytochrome c release from mitochondria and is distinct from the PKB/Akt (Rytomaa et al., 2000).
In another cell type, mammary epithelial cells, basement membrane ECM, but not fibronectin or collagen was shown to suppress apoptosis. In these cells, antibodies to β1 integrins or overexpression of stromelysin 1, a metalloproteinase that degrades ECM, induced apoptosis (Boudreau et al., 1995). However, in the systems described (vascular endothelial cells, MDCK, and mammary epithelia), the cells were disrupted from their normal sheet or trabecular architecture.
In the current study, epithelial sheets were maintained as organ cultures with the apical–basal polarity and cell–cell junctions intact at the air–medium interface. The epithelial sheets were separated from the stroma and basal lamina then deprived of ECM stimulation (NT groups). Annexin V bound to the untreated tissues and activated caspase-3 was detected in an in vitro assay. Treating these blebbing epithelia with substrates that stimulated integrins (FN, COL) decreased the caspase-3 levels but did not reduce it to below the caspase-3 level observed in epithelia treated with the caspase-3 inhibitor, ZVAD. Therefore, the sheets of corneal epithelia were also matrix-dependent for survival, similar to vascular endothelia or other epithelial cells. Furthermore, we observed that caspase-3 levels were significantly higher in epithelia exposed to the ROCK inhibitor, Y27632. The increase in cell death was observed both by visualizing an apoptosis marker (annexin V) and a quantitative assay that measured caspase-3 activity. Interestingly, the annexin V-positive areas were the basal cell lateral membranes that also appeared to be altered in F-actin distribution.
Experimental Procedures
Tissue Isolation
Fertile White Leghorn chicken eggs (SPAFAS, Inc., Norwich, CT) were incubated for 8 days at 39°C. The embryos (Hamburger-Hamilton stages 27–34) were removed from the eggs and rinsed in Hanks' balanced saline solution (HBSS; GIBCO Laboratories, Grand Island Biological Co., Grand Island, NY).
Whole corneas, with some surrounding sclera, were removed from the embryos with fine forceps. Corneal epithelia isolated without the basal lamina, were treated for 2–4 min at 37°C in 0.8 mg/ml trypsin and 0.8 mg/ml collagenase (Sigma, St. Louis, MO) in calcium-magnesium–free HBSS. After the enzyme treatment, the corneas were rinsed in Ham's F12 medium (GIBCO Laboratories, Grand Island Biological Co.) and then HBSS to stop the action of the enzyme. A continuous sheet of corneal epithelial cells was dissected from the underlying stroma with fine tweezers and placed basal-side down on a black polycarbonate filter (3-mm diameter, 0.4-μm pore size; Poretics, Livermore, CA; Svoboda and Hay, 1987; Svoboda, 1992).
Organ Culture
Epithelial sheets (n = 30/treatment group) were suspended at the air–media interface by a triangular-shaped wire grid in an organ culture dish (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ; Svoboda et al., 1999; Chu et al., 2000). The medium height barely covered the apical surface of the tissue. Cultures were incubated at 37°C in a humidified gas mixture (5% CO2 and 95% air). The control culture medium was composed of Hank's F12 medium (GIBCO), antibiotic/antimycotic (GIBCO), and 50 μM ascorbic acid (Sigma). Epithelia fed only this medium were called no treatment controls (NT). The stimulatory molecules used were 10 μg/ml LPA (Sigma), 50 μg/ml human plasma FN (BD Biosciences, San Diego, CA), and 100 μg/ml type I collagen (COL, BD Biosciences). These treated cultures were referred to as FN, COL, or LPA treated and were controls for ECM/LPA+Y27632-treated epithelia. After the stimulatory incubation (2 hr), the epithelia were either fixed for immunohistochemistry or stained with phalloidin. In experiments using the ROCK inhibitor, Y27632, some corneal epithelial groups were cultured in medium with Y27632 (0, 0.03, 0.3, 3, or 10 μM; Yoshitomi Pharmaceutical Industries, Ltd.) 2 hr before stimulation with LPA or ECM. To visualize apoptosis, epithelia were incubated in the presence of annexin V for 15 min at 37°C, then rinsed and viewed on the confocal microscope immediately or fixed and stained with phalloidin.
Organ culture experiments were repeated at least four times, 20–30 epithelia were pooled in each treatment group. The epithelia were fixed and stained immediately for morphology or placed in extraction buffer for biochemical analysis.
Actin Labeling
The F-actin in the cells was labeled with fluorescently tagged phalloidin (Molecular Probes, Inc., Eugene, OR; Svoboda, 1992). The epithelia were incubated with FITC-phalloidin (1:20 dilution of phalloidin stock solution in 3.7% formaldehyde, 0.01% lysopalmitate [Sigma]) for 30 min at room temperature, followed by three 15-min rinses in phosphate buffered saline (PBS). A subset of each experiment was examined with confocal microscopy to determine whether the actin cortical mat reorganized. These experiments were repeated at lease four times, all images are representative samples from experiments. The fluorescent phallotoxins were kept as stock solutions containing 300 units/3 ml methanol (approximately 3.3 μM, Molecular Probes). A unit is defined, as the amount of material needed to stain one microscope slide of fixed cells (Molecular Probes).
Apoptosis Assays
Annexin V labeling
To visualize apoptosis, cells were incubated in Alexa fluor 488-labeled annexin V, as it binds to phosphatidylserine that normally resides on the cytoplasmic surface of the plasma membrane. A characteristic of apoptosis is that this lipid gets “flipped” to the outer membrane leaflet. In the last 15 min of incubation, labeled annexin V was added to the culture medium.
After incubation with Alexa fluor-labeled annexin V, some of the tissues were examined immediately by using the confocal microscope. Additional tissues were rinsed, fixed, and stained the Texas Red phalloidin as described above. Whole tissues were morphologically analyzed by using a Leica TSP SP2 microscope for actin and annexin V. All experiments were repeated at least four times with similar results.
CaspACE assay
Organ cultured corneal epithelia (n = ∼30 epithelia/treatment group) were treated with FN, LPA, COL, with or without Y27632 as described in the “organ culture” section. For these experiments, additional epithelial groups were incubated in the caspase-3 inhibitor, 50 μM Z-VAD-FMK for 16 hr before the addition of Y27532 (2 hr) and/or ECM (2 hr). The tissues were rinsed in cold PBS and then harvested on ice in 25 μl of lysis buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1% Triton X-100, protease cocktail inhibitor (Sigma) and phosphatase cocktail inhibitor I (Sigma)). Lowry assays were used to determine total protein. Equal amounts of protein were incubated with the colorimetric substrate (CaspACE Assay, Promega) overnight at 24°C, and read in a 96-well spectrophotometer at 405 nm. The results are compared with pNA calibration curve. All experimental groups were assayed as duplicate samples, and the experiments were repeated at least three times with similar results.
Confocal Laser Scanning Microscopy
The filters were placed basal side down in antifade mounting medium (Molecular Probes) on slides and viewed on a Leica TCS SP2 confocal microscope equipped with one argon and two helium neon lasers (458, 488, 514, 533, 643 excitation wavelengths). The continuously variable detection pinhole was set at the minimum size for optimal signal (Pawley, 1990; Wilson, 1990). The typical z-series was composed of optical sections in the xy optical plane. These images were en face optical sections through the vertical axis of the tissue. Specimens were also scanned in the xz plane, allowing the tissues to be visualized in cross-section. The point-spread function for the confocal microscope is greater in the xz than the xy focal plane (Pawley, 1990), causing the images to be more distorted.
Images were analyzed, enhanced, and stored. Black and white and color photographs were computer generated with minimal computer enhancement. These images were printed directly from Photoshop or PageMaker (Adobe) documents. All controls were collected, enhanced, and photographed with the same conditions as the positive tissue.
Acknowledgments
We thank Tracie Robinson and Robert Spears for their assistance with the caspACE assay. The Baylor Oral Health Foundation and NIH provided the funds to purchase the Baylor College of Dentistry Leica TCS SP2 microscope.
Grant sponsor: NIH NEI; Grant number: EY08886.
References
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Anderson SC, SundarRaj N. Regulation of a Rho-associated kinase expression during the corneal epithelial cell cycle. Invest Ophthalmol Vis Sci. 2001;42:933–940. [PubMed] [Google Scholar]
- Blankenberg FG, Katsikis PD, Tait JF, Davis RE, Naumovski L, Ohtsuki K, Kopiwoda S, Abrams MJ, Strauss HW. Imaging of apoptosis (programmed cell death) with 99mTc annexin V. J Nucl Med. 1999;40:184–191. [PubMed] [Google Scholar]
- Blankenberg FG, Tait JF, Strauss HW. Apoptotic cell death: its implications for imaging in the next millennium. Eur J Nucl Med. 2000;27:359–367. doi: 10.1007/s002590050046. [DOI] [PubMed] [Google Scholar]
- Bockholt SM, Burridge K. An examination of focal adhesion formation and tyrosine phosphorylation in fibroblasts isolated from src-, fyn-, and yes-mice. Cell Adhes Commun. 1995;3:91–100. doi: 10.3109/15419069509081279. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Sympson C, Werb Z, Bissell M. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Fath K. Focal contacts: transmembrane links between the extracellular matrix and the cytoskeleton. Bioessays. 1989;10:104–108. doi: 10.1002/bies.950100403. [DOI] [PubMed] [Google Scholar]
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K, Nuckolls G, Otey C, Pavalko G, Simon K, Turner C. Actin-membrane interaction in focal adhesions. Cell Differ Dev. 1990;32:337–342. doi: 10.1016/0922-3371(90)90048-2. [DOI] [PubMed] [Google Scholar]
- Chihara K, Amano M, Nakamura N, Yano T, Shibata M, Tokui T, Ichikawa H, Ikebe R, Ikebe M, Kaibuchi K. Cytoskeletal rearrangements and transcriptional activation of c-fos serum response element by Rho-kinase. J Biol Chem. 1997;272:25121–25127. doi: 10.1074/jbc.272.40.25121. [DOI] [PubMed] [Google Scholar]
- Chu CL, Reenstra RW, Orlow DL, Svoboda KKH. Erk and PI3 kinase are necessary for collagen binding and actin reorganization in avian corneal epithelia. Invest Ophthalmol Vis Sci. 2000;41:3374–3382. [PMC free article] [PubMed] [Google Scholar]
- Collinson JM, Morris L, Reid AI, Ramaesh T, Keighren MA, Flockhart JH, Hill RE, Tan SS, Ramaesh K, Dhillon B, West JD. Clonal analysis of patterns of growth, stem cell activity, and cell movement during the development and maintenance of the murine corneal epithelium. Dev Dyn. 2002;224:432–440. doi: 10.1002/dvdy.10124. [DOI] [PubMed] [Google Scholar]
- Danjo Y, Gipson IK. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci. 1998;111:3323–3332. doi: 10.1242/jcs.111.22.3323. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laurenzi V, Melino G. Apoptosis. The little devil of death. Nature. 2000;406:135–136. doi: 10.1038/35018190. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Olivo C, Venturino M, Dolce L, Silengo L, Tarone G. Actin cytoskeleton organization in response to integrin-mediated adhesion. Microsc Res Tech. 1999;47:67–78. doi: 10.1002/(SICI)1097-0029(19991001)47:1<67::AID-JEMT7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Frisch SM. Evidence for a function of death-receptor-related, death-domain-containing proteins in anoikis. Curr Biol. 1999;9:1047–1049. doi: 10.1016/s0960-9822(99)80455-2. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- Gipson I, Inatomi F. Extracellular matrix and growth factors in corneal wound healing. Curr Opin Ophthalmol. 1995;6:3–10. doi: 10.1097/00055735-199508000-00002. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;297:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hay ED, Revel JP. Fine structure of the developing avian cornea. Basel, Switzerland: S. Karger A.G; 1969. [PubMed] [Google Scholar]
- Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K. Interaction of plasma membrane fibronectin receptor with talin-a transmembrane linkage. Nature. 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Khoory W, Wu E, Svoboda KKH. Intracellular relationship between actin and alpha-actinin in a whole corneal epithelial tissue. J Cell Sci. 1993;106:703–717. doi: 10.1242/jcs.106.3.703. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- Meredith JE, Schwartz MA. Integrins, adhesion and apoptosis. Trends Cell Biol. 1997;7:146–150. doi: 10.1016/S0962-8924(97)01002-7. [DOI] [PubMed] [Google Scholar]
- Meredith JE, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nagano T, Chikama T, Nishida T. Role of the small GTP-binding protein Rho in epithelial cell migration in the rabbit cornea. Invest Ophthalmol Vis Sci. 2001;42:941–947. [PubMed] [Google Scholar]
- Narumiya S. Cellular functions & pharmacological manipulations of the small GTPase Rho & Rho effectors. Nippon Yakurigaku Zasshi. 1999;114(Suppl 1):1P–5P. doi: 10.1254/fpj.114.supplement_1. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Ishizaki T, Watanabe N. Rho effectors and reorganization of actin cytoskeleton. FEBS Lett. 1997;410:68–72. doi: 10.1016/s0014-5793(97)00317-7. [DOI] [PubMed] [Google Scholar]
- Nicholson DW. Apoptosis. Baiting death inhibitors. Nature. 2001;410:33–34. doi: 10.1038/35065201. [DOI] [PubMed] [Google Scholar]
- Nishida T, Tanaka T. Extracellular matrix and growth factors in corneal wounding. Curr Opin Ophthalmol. 1996;7:2–11. doi: 10.1097/00055735-199608000-00002. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Integrin-mediated signalling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr Opin Cell Biol. 1996;8:146–152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- Pawley JB, editor. Fundamental limits in confocal microscopy. New York: Plenum; 1990. pp. 19–37. [Google Scholar]
- Petch LA, Bockholt SM, Bouton A, Parsons JT, Burridge K. Adhesion-induced tyrosine phosphorylation of the p130 src substrate. J Cell Sci. 1995;108:1371–1379. doi: 10.1242/jcs.108.4.1371. [DOI] [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–1037. [PubMed] [Google Scholar]
- Re F, Zanetti A, Sironi M, Polentarutti N, Lanfrancone L, Dejana E, Colotta F. Inhibition of anchorage-dependant cell spreading triggers apoptosis in cultured human endothelial cells. J Cell Biol. 1994;127:537–545. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenstra WR, Orlow DL, Svoboda KKH. ECM stimulated signaling and actin reorganization in embryonic corneal epithelia are Rho dependent. Invest Ophthalmol Vis Sci. 2002;43:3181–3189. [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471–477. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J. 1994;13:2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytomaa M, Lehmann K, Downward J. Matrix detachment induces caspase-dependent cytochrome c release from mitochondria: inhibition by PKB/Akt but not Raf signalling. Oncogene. 2000;19:4461–4468. doi: 10.1038/sj.onc.1203805. [DOI] [PubMed] [Google Scholar]
- Sugrue SP, Hay ED. Response of basal epithelial cell surface and cytoskeleton to solubilized extracellular matrix molecules. J Cell Biol. 1981;91:45–54. doi: 10.1083/jcb.91.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue SP, Hay ED. The identification of extracellular matrix (ECM) binding sites on the basal surface of embryonic corneal epithelium and the effect of ECM binding on epithelial collagen production. J Cell Biol. 1986;102:1907–1916. doi: 10.1083/jcb.102.5.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SundarRaj N, Kinchington PR, Wessel H, Goldblatt B, Hassell J, Vergnes JP, Anderson S. A Rho-associated protein kinase: differentially distributed in limbal and corneal epithelia. Invest Ophthalmol Vis Sci. 1998;39:1266–1272. [PubMed] [Google Scholar]
- Svoboda KKH. Embryonic corneal epithelial actin alters distribution in response to laminin. Invest Ophthalmol Vis Sci. 1992;33:324–333. [PMC free article] [PubMed] [Google Scholar]
- Svoboda KKH, Hay ED. Embryonic corneal epithelial interaction with exogenous laminin and basal lamina is F-actin dependent. Dev Biol. 1987;123:455–469. doi: 10.1016/0012-1606(87)90403-9. [DOI] [PubMed] [Google Scholar]
- Svoboda KKH, Reenstra W. Approaches to studying cellular signaling: a primer for morphologists. Anat Rec (New Anat) 2002;269:123–139. doi: 10.1002/ar.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KKH, Orlow DL, Chu CL, Reenstra WR. ECM stimulated actin bundle formation in embryonic corneal epithelia is tyrosine phosphorylation dependent. Anat Rec. 1999;254:348–359. doi: 10.1002/(sici)1097-0185(19990301)254:3<348::aid-ar5>3.0.co;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- Whyte M. ICE/CED-3 proteases in apoptosis. Trends Cell Biol. 1996;6:245–248. doi: 10.1016/0962-8924(96)20025-x. [DOI] [PubMed] [Google Scholar]
- Wilson T. The role of the pinhole in confocal imaging systems. New York: Plenum; 1990. pp. 113–126. [Google Scholar]
- Wu XY, Svoboda KKH, Trinkaus-Randall V. Intracellular distribution of cytoskeletal and adhesion proteins in response to corneal substrata. Exp Eye Res. 1995;60:445–458. doi: 10.1016/s0014-4835(05)80101-0. [DOI] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Forrester JV, McCaig CD. Direct visualization of a stratified epithelium reveals that wounds heal by unified sliding of cell sheets. FASEB J. 2003;17:397–406. doi: 10.1096/fj.02-0610com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD. Perpetuation of stem cells in the eye. Eye. 1994;8:163–169. doi: 10.1038/eye.1994.41. [DOI] [PubMed] [Google Scholar]


