Abstract
OBJECTIVE
Hormonal contraceptives may adversely affect bone mineral density . However, racial differences and the reversibility of these changes are poorly understood. This study measured bone mineral density changes during hormonal contraceptive use and after discontinuation in a triethnic population.
METHODS
Bone mineral density was measured every 6 months for up to 3 years in 703 white, black, and Hispanic women using oral contraceptives (OCPs), depot medroxyprogesterone acetate (DMPA), or nonhormonal contraception, and in 68 DMPA discontinuers for up to 2 additional years. Mixed-model regression analyses were used to estimate the percentage change in bone mineral density for each contraceptive method.
RESULTS
Over 3 years, DMPA and OCP users lost more bone mineral density than nonhormonal contraception users (−3.7% and −0.5% vs. +1.9% at lumbar spine, and −5.2% and −1.3% vs. +0.6% at femoral neck, respectively). No differences were observed by race in bone mineral density changes that resulted from DMPA or OCP use. However, DMPA users aged 16–24 years lost more bone mineral density at the spine (4.2% vs. 3.2%, P=.006) and femoral neck (6.0% vs. 4.2%, P=.001) than those aged 25–33 years. After DMPA discontinuation, women who selected nonhormonal contraception gained bone mineral density (+4.9% at spine; +3.2% at femoral neck) while those who selected OCP recovered spinal (+2.3%), but not femoral neck bone mineral density (−0.7%).
CONCLUSIONS
Use of very low-dose OCP may result in a small amount of bone loss. DMPA use results in greater bone loss, but this is largely reversible at the spine. Use of very low-dose OCPs after DMPA discontinuation may slow bone recovery.
INTRODUCTION
Recent longitudinal studies indicate that a woman’s contraceptive choice may affect her bone mineral density (BMD). For example, research demonstrated that use of depot medroxyprogesterone acetate (DMPA) for 2 years results in a 5.7–6.8% loss of BMD at the spine and 3.6–5.8% at the hip.(1–4) As a result, the Food and Drug Administration issued a warning in 2004 advising women to limit its use to ≤2 years. However, recent prospective studies have demonstrated that once DMPA is discontinued, BMD increases more among prior DMPA users than nonusers, suggesting that DMPA-related bone loss is reversible.(3,5,6) Although encouraging, more information is needed to better assess this recovery of BMD. In addition, information is lacking on minority women as most subjects included in prior studies were white, and the effect of age on BMD recovery has not been determined. Finally, studies have not assessed the effect of hormonal contraceptive use after DMPA discontinuation on reversibility.
Oral contraception (OC) containing only 20 μg ethinyl estradiol (EE) may also adversely affect bone health, especially if used during adolescence. This may occur if young OC users experience less increase in BMD than normally occurs in the absence of hormonal contraceptive use.(7–9) For example, Polatti observed a 7.8% increase over 5 years among 19–22-year-olds not using OC, compared with no change in BMD among those who used OC containing 20 μg EE.(9) In comparison, Cromer noted a significant difference in BMD between adolescents using OC with 20 μg EE and those not using hormonal contraception after 12 months of use, but not after 2 years.(10) This study, however, was limited to girls ≤18 years of age who were still forming bone, and thus may not apply to adults. As no studies on OC with 20 μg EE have simultaneously included women ≤18 and >18 years of age, it is difficult to assess the effects of age on this relationship. Furthermore, the effect of race on this relationship is not known as studies on OC containing 20 μg EE have been limited to primarily white populations or did not include data on race or ethnicity.(8,9,11,12)
The purpose of this study was to estimate the influence of age, race, and other factors on the relationship between BMD or bone mineral apparent density (BMAD) and use of DMPA or OC containing 20 μg EE in a triethnic sample of women 16–33 years old. Furthermore, we examined whether DMPA-related bone loss is reversible and if the amount of bone re-accumulated is influenced by the contraceptive used after DMPA discontinuation.
METHODS
Non-Hispanic white, non-Hispanic black, and Hispanic women 16–33 years old were recruited between October 9, 2001, and September 14, 2004. A recruitment grid was designed to guide our efforts toward achieving a sample that was balanced across race/ethnicity (white, black, Hispanic), age group (16–24 years and 25–33 years), and contraceptive method (OC, DMPA, nonhormonal). This 3 × 2 × 3 grid depicted target sample sizes within each of 18 “cells” that were based on the overall sample size dictated by a power analysis. Once the targeted number was achieved in a given cell, the cell was essentially closed to recruitment. Criteria for exclusion were: pregnant or breastfeeding; pregnancy planned within 3 years; DMPA injection in last 6 months or OC use in past 3 months; current use of intrauterine device; medical contraindication to OC; oligomenorrhea; bilateral oopherectomy; phytoestrogen supplement use; dietary isoflavone intake >84 mg/day; illness or medication known to affect BMD; or strict vegetarian diet.
Of 2,999 women who responded to advertisements and mailed announcements, 1,404 met general inclusion criteria and matched an “open” recruitment cell. Of these, 805 women provided written informed consent (or assent and parental permission if <18 years of age) to undergo further screening, including a blood draw and bone scan. Fasting blood samples were collected between 0700 and 1000 h. Of the 805 women, 5 withdrew prior to completing the first visit, 92 had abnormal laboratory results, and 5 had a t-score of -2.5 or less on their bone scan (Figure 1). Thus, baseline data were analyzed for 703 women. Those excluded (n=102) did not differ from women included in the analyses (n=703) on age, marital status, parity, or education (all P >.05).
Figure 1.
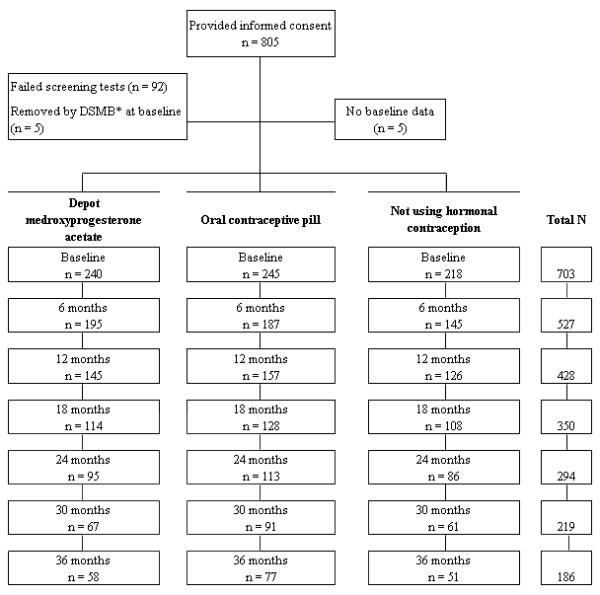
Flow of recruitment and retention across the study. *Removed by Data Safety Monitoring Board (DSMB) due to a t-score of -2.5 or less (t-score is measured as the standard deviation between patient and average peak young adult bone mass).
At the first visit, standardized counseling was provided regarding the risks and benefits of contraception, including its potential effects on bone. Following this discussion, women chose one of the following methods for contraceptive use during the next 36 months: OC (0.15 mg desogestrel + 20 μg EE for 21 days, followed by 2 days of placebo and 5 days of 10 μg EE), DMPA, or nonhormonal contraception (NH). A total of 245 women selected OC, 240 chose DMPA, and 218 NH.
Contraception was dispensed every 3 months. Anthropometry, phlebotomy, urine pregnancy testing, written questionnaires, calcium checklist,(13) and bone densitometry were completed every 6 months. A registered dietitian conducted a 24-hour recall of dietary intake annually. All participants received free well-woman care and contraception during the study as well as monetary compensation. Those who did not return for scheduled visits were reminded by phone and certified letters. All procedures were approved by the Institutional Review Board of the University of Texas Medical Branch.
Weight was measured with women wearing light indoor clothing using a digital scale accurate to the nearest 0.1 kg. Height was measured using a wall-mounted stadiometer (Heightronic, Snoqualmie, WA) accurate to the nearest 0.001 m.
Demographic information obtained included age, race/ethnicity, marital status, education, income, national origin, gravidity, and parity. Behavioral measures included prior breastfeeding and hormonal contraceptive use, smoking, alcohol use, and physical activity. Tobacco use was measured with questions from the MONICA Smoking Assessment.(14) For analytic purposes, current smokers were those who reported regular or occasional smoking while nonsmokers were those who currently did not smoke. Alcohol use was calculated from questions on the Diet History Questionnaire regarding how often subjects drank alcohol (either beer, wine or wine coolers, or liquor or mixed drinks) during the past 12 months and the amount usually consumed when drinking.(15)
Weight-bearing physical activity was taken from a measure that included a list of 56 common activities, and questions on the frequency and duration of up to two physical activities performed during the past month. Kolle and colleagues have reported that the total number of minutes per week devoted to weight-bearing exercise should be ≥121 minutes to positively impact BMD levels in reproductive-aged women.(16) Therefore, we categorized weight-bearing exercise into two groups: ≤120 minutes/week and ≥121 minutes/week.
Of the 240 initial DMPA users, 182 discontinued this method, 68 of whom remained in the study for up to 2 additional years. There were no differences in baseline characteristics between DMPA discontinuers who remained in the study (n=68) and those who did not (n=114) with regard to age, race/ethnicity, height, weight, lean mass, age at menarche, lifestyle variables, calcium intake, pregnancy/breast feeding, previous exposure to OC or DMPA, and bone density measurements. However, the former were more likely to have a higher BMI, exercise more, and have higher baseline fat mass and percent body fat. Of the 68 women who were followed after DMPA discontinuation, 44 began OC and were given the same formulation used in the study, while the remaining 24 chose NH. DMPA discontinuers also underwent bone scanning at the time of discontinuation.
Bone densitometry was conducted using dual-energy X-ray absorptiometry (DXA; Hologic QDR 4500W Elite fan-beam densitometer). Long-term accuracy of the instrument was assessed through the use of a phantom spine calibrated daily prior to the scanning of participants. The coefficient of variation (CV) of this machine was 0.27 g/cm2±0.003. All scanning and analyses were conducted by certified radiologic technologists using a standardized protocol recommended by the International Society for Clinical Densitometry. The same technologist scanned 78% of the subjects; two additional technologists scanned the remaining 19% and 3% of the subjects, respectively. The reliability of the primary and secondary technologists was evaluated by scanning 28 women twice in the same day by the same technologist, with <1 hour between scans and complete repositioning as has been reported in the literature.(17,18) The site-specific percent coefficient of variation for the reliability sample was 0.78% for the hip, 1.95% for the femoral neck, and 0.55% for the lumbar spine.
Densitometry measurements included bone mineral density (BMD) (g/cm2) measured at the lumbar spine (L1—L4) and total left hip (Ward’s triangle, greater trochanter, intertrochanter, and femoral neck). Hip data are presented separately for the femoral neck, as this particular site is highly predictive of hip fracture.(19) Calculations for BMD (BMC[g]/projected area of the bone [cm2]) have been shown to be influenced by bone size as they are based on two of three dimensions of bones (length and width without depth). To address this issue, we also calculated spine BMAD (BMC/A3/2)(20) and femoral neck BMAD (BMC/A2),(21) which are approximations of the volumetric density of bone estimated from the BMC and the projected area of the bone (A). Estimates of total fat mass (g), percent fat mass, and lean mass (g) were generated from DXA scans of the whole body.
Over the 36-month study period, 257 women were lost to follow-up, 137 women stated they desired a different contraceptive method, and 123 did not complete the study due to other reasons. There were differences with regard to reasons for discontinuation among the three contraceptive groups; NH users were more likely to be lost to follow-up (NH 44%, DMPA 35%, OC users 32%, P<.018) while DMPA users were more likely to seek a different contraceptive method (DMPA 36%, OC 15%, NH 6%, P<.001) than their counterparts. Furthermore, the frequency of discontinuation due to pregnancy or a desire to become pregnant was higher among OC users than DMPA users (7% vs. 2%, P<.006), but was similar to NH users (7% vs. 5%, P=.281).
We used one-way analysis of variance with Bonferroni corrections for continuous variables and chi-square tests for categorical variables to compare the three contraceptive groups at baseline. We also used longitudinal analysis to estimate the BMD and BMAD changes by different contraceptive methods along with their correlates over the period of the study. To accommodate the repeated measurements, the data were modeled using Stata’s mixed effects regression procedure (xtmixed module, STATA 9, Stata Corporation, College Station, TX), which allowed us to obtain regression coefficients for various predictors while adjusting for the estimated errors for the repeated measurements. This class of model also allows inclusion of time-dependent covariates and accommodates subjects with incomplete data due to variation in number and spacing in observations over the period of follow-up, which frequently occurs in longitudinal studies.
The primary outcome was percentage change of BMD or BMAD at spine, femoral neck, or hip measured every 6 months over 36 months from the baseline values. We estimated percentage changes of BMD and BMAD at each 6-month increment across 36 months by contraceptive method, after adjusting for baseline status of BMD or BMAD, race/ethnicity, age, age at menarche, parity, previous use of pills and DMPA, and lifestyle variables (smoking, alcohol use, weight-bearing exercise). Whole body lean mass and calcium intake were included as time-dependent variables in the models. The interaction term between time and contraceptive method was included to estimate the BMD or BMAD change over time in different contraceptive users. Time as a quadratic term was included in all models to examine the linearity of BMD/BMAD changes over time. Other two-way interactions were then examined and retained when P≤.05. Similar models were also constructed to estimate the BMD and BMAD recovery after discontinuation of DMPA. A prestudy power analysis was performed to achieve a power of 83–97% with an alpha of 0.05. This analysis revealed that a minimum of 45 women per group would be required after 36 months of follow-up (229 per group at baseline) to detect a 4–6% difference in BMD (compared to baseline values) between DMPA and NH users over a range of reasonable estimates of standard deviations.
RESULTS
At baseline, the total sample had a mean age of 24.3 years. NH users were more likely to have higher parity, OC users were less likely to have used DMPA previously, and DMPA users were more likely to report current smoking (Table 1).
Table 1.
Sample Characteristics According to Contraceptive Selected at Baseline
| Characteristic | OC (n = 245) |
DMPA (n = 240) |
NH (n = 218) |
|---|---|---|---|
| Age, % | |||
| 16–24 y | 54.3 | 56.7 | 45.9 |
| 25–33 y | 45.7 | 43.3 | 54.1 |
| Race % | |||
| Black | 29.8 | 30.0 | 25.2 |
| White | 33.5 | 34.2 | 38.1 |
| Hispanic | 36.7 | 35.8 | 36.7 |
| Height, cm, mean (SE) | 162.0 (0.4) | 162.2 (0.4) | 160.8 (0.5) |
| Weight, kg, mean (SE) | 73.3 (1.1) | 71.8 (1.2) | 73.2 (1.3) |
| BMI, kg/m2, mean, (SE) | 27.9 (0.4) | 27.2 (0.4) | 28.3 (0.5) |
| Lean mass, kg, mean (SE) | 44.5 (0.5) | 44.4 (0.5) | 44.1 (0.5) |
| Fat mass, kg, mean (SE) | 27.0 (0.7) | 25.6 (0.8) | 27.2 (0.8) |
| Fat mass, % of total, mean (SE) | 36.4 (0.5) | 34.8 (0.5) | 36.7 (0.5) |
| Age at menarche, y, mean (SE) | 12.2 (0.1) | 12.5 (0.1) | 12.2 (0.1) |
| Parity, mean (SE) | 0.86 (0.07)a | 1.13 (0.08)b | 1.56 (0.10) |
| Months of lactation, mean (SE) § | 2.22 (0.31) | 3.02 (0.44) | 2.39 (0.30) |
| Past use of pill (months), mean (SE) | 21.9 (2.04) | 16.86 (1.91) | 17.70 (1.94) |
| Past use of DMPA injection (no), mean (SE) | 1.40 (0.22)c | 3.47 (0.42)d | 2.61 (0.35) |
| High school graduate, % | 78.8 | 71.7 | 79.8 |
| Relative with shortened height, %¶ | 31.4 | 33.3 | 34.9 |
| Relative with fracture history, %† | 11.8 | 12.5 | 17.4 |
| Current smoker, % | 23.3e | 36.3f | 22.0 |
| Alcohol use, gm/day, mean (SE) | 1.6 (0.6) | 1.2 (0.4) | 2.3 (1.0) |
| Calcium intake, mg/day, mean (SE) | 601.8 (21.0) | 627.4 (24.7) | 653.9 (24.1) |
| Weight-bearing exercise >120 min/wk,% | 43.2 | 36.3 | 37.5 |
| Spine BMD, g/cm2, mean (SE) | 1.065 (0.007) | 1.055 (0.007) | 1.051 (0.007) |
| Spine BMAD, g/cm3, mean (SE) | 0.144 (0.001) | 0.141 (0.001) | 0.142 (0.001) |
| Femoral neck BMD, g/cm2, mean (SE) | 0.921 (0.008) | 0.912 (.009) | 0.900 (.008) |
| Femoral neck BMAD, g/cm3, mean (SE) | 0.204 (0.002) | 0.200 (0.002) | 0.203 (0.003) |
| Hip BMD, g/cm2, mean (SE) | 1.007 (0.008) | 0.997 (.008) | 0.987 (0.008) |
OC = oral contraceptive; DMPA = depot medroxyprogesterone acetate; NH = nonhormonal contraception; BMD = bone mineral density; BMI = body mass index; SE = standard error; BMAD = bone mineral apparent density.
P<.001 for OC vs. NH
P<.001 for DMPA vs. NH
P<.034 for OC vs. NH.
P<.001 for DMPA vs. OC
P<.01 for OC vs. DMPA
P<.01 for DMPA vs. NH.
One-way analysis of variance with Bonferroni correction was used for continuous variables and chi-square tests were used for categorical variables.
Only those who were ever pregnant were included as denominator.
One or more close female relative (mother, sister, grandmother, or aunt) lost height (gotten shorter) as they grew older.
One or more close female relative (mother, sister, grandmother, or aunt) suffered a broken hip, wrist, spine, or shoulder after the age of 45.
Estimates based on mixed-model regression showed that the mean percentage change of BMD was significantly different at the spine among DMPA and OC users at all follow-up visits compared to that of NH users (Table 2 and Figure 2). Over 36 months, the BMD of DMPA and OC users consistently declined from baseline values (except for the first 18 months at the spine among OC users). The difference between DMPA and NH users, as well as the difference between OC and NH users, increased over the 3-year interval. A similar scenario was observed for femoral neck BMD among DMPA users from 12 months onward and among OC users from 18 months onward. A significant difference was also found between DMPA and OC users at each follow-up visit for spine BMD and after 6 months for femoral neck BMD (P<.05).
Table 2.
Estimated Mean Percentage Change in Bone Mineral Density from Baseline across 36 Months by Contraceptive Methoda,b
| OC | DMPA | NH | |
|---|---|---|---|
| Spine BMD | |||
| 6 mo | 0.18* | −1.02*** | 0.51 |
| 12 mo | 0.20** | −1.95*** | 0.91 |
| 18 mo | 0.08*** | −2.75*** | 1.33 |
| 24 mo | −0.01*** | −3.22*** | 1.66 |
| 30 mo | −0.19*** | −3.48*** | 1.93 |
| 36 mo | −0.54*** | −3.73*** | 1.94 |
| Femoral Neck BMD | |||
| 6 mo | −0.22 | −0.65 | 0.05 |
| 12 mo | −0.30 | −1.30* | 0.15 |
| 18 mo | −0.54* | −2.21*** | 0.29 |
| 24 mo | −0.76* | −3.10*** | 0.54 |
| 30 mo | −1.00* | −4.15*** | 0.66 |
| 36 mo | −1.29** | −5.16*** | 0.61 |
BMD = bone mineral density; OC = oral contraceptive; DMPA = depot medroxyprogesterone acetate; NH = nonhormonal contraception.
P<.05
P<.01
P<.001 when compared to nonhormonal group.
Adjusted by baseline BMD (gm/cm2), age (16–24 years vs 25–33 years), age at menarche (years in continuous scale), race/ethnicity (black, white, and Hispanic), parity, total body lean mass (kg), daily calcium intake (mg/day), weight-bearing exercise (≤120 min/wk vs >120 min/wk), alcohol use (gm/day), smoking status (current smoker vs not), months of pill use, and previous use of DMPA.
Mixed-model regression analyses were used for the adjustment, and p values are based on the difference in the coefficients at the particular follow-up visit generated from the model.
Figure 2.
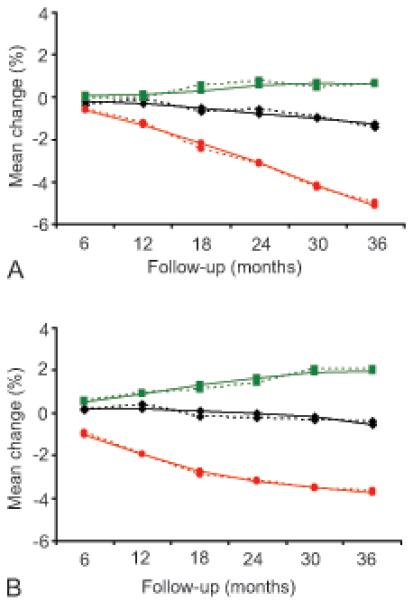
Mean percentage bone mineral density change across 36 months of follow-up by contraceptive method for (A) femoral neck bone mineral density and (B) spine bone mineral density. Solid lines represent the estimated mean percentage (modeled) changes and dotted lines represent the unadjusted values. Green squares: nonhormonal contraception; black diamonds: oral contraceptives; red circles: depot medroxyprogesterone acetate.
DMPA users had the highest BMD loss at the spine during the first year, after which the loss slowed during the second and third years. At the femoral neck, BMD loss was slower during the first year, and increased during the second and third years. OC users had a slight BMD increase at the spine during the first 12 months, followed by a slow and gradual decrease in the second and third years, and had a slow and consistent decrease over time at the femoral neck. For total hip BMD, the changes were −3.5%, −0.3%, and +1.6% among DMPA, OC, and NH users, respectively, over the 3 years (data not shown).
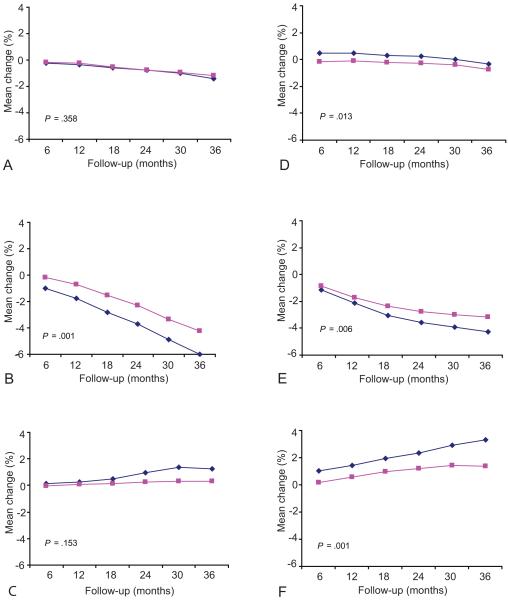
Age was found to be an important determinant of BMD change by contraceptive method. Over 36 months DMPA users 16–24 years old lost significantly more bone at the spine (4.2% vs. 3.2%, P=.006) and femoral neck (6.0% vs. 4.2%, P=.001) than those 25–33 years old (Figure 3); however, OC users 16–24 years old lost significantly less bone density at the spine (0.4% vs. 0.8%, P=.013) than women 25–33 years of age. In contrast, NH users 16–24 years old gained significantly more bone at the spine (3.3% vs. 1.3%, P=.001) than those 25–33 years of age.
Figure 3.
Modeled mean percentage change from baseline across 36 months by age group in femoral neck bone mineral density for (A) oral contraception, (B) depot medroxyprogesterone acetate, and (C) nonhormonal contraception; and spine bone mineral density for (D) oral contraception, (E) depot medroxyprogesterone acetate, and (F) nonhormonal contraception. Blue diamonds = 16–24 year olds; pink squares = 25–33 year olds. P values indicate the difference in coefficient between the two age groups derived from the method-specific mixed-model regression analyses.
We also estimated bone changes by contraceptive method based on BMAD to adjust for volumetric changes in bone. The mean percentage change in BMAD at the lumbar spine among DMPA users was −1.1%, −2.1%, and −3.0% by the end of the first, second, and third year, respectively, when compared with baseline values. The respective changes for OC and NH users were: +0.2%, +0.2%, and +0.3%, and +0.6%, +1.2%, and +1.7%. The percentage change for femoral neck BMAD for DMPA, OC, and NH users was −1.0%, −0.5%, and +0.5% in year 1, −3.0%, −1.7%, and +0.3% in year 2, and −5.9%, −3.4%, and −1.4% in year 3.
In addition to contraceptive method, several other predictors were found to be significantly associated with BMD changes (Table 3). BMD loss at the spine among DMPA users was not linear as the quadratic term (time variable) was significant. NH users had a significant increase in BMD at both sites over the duration of the study, while the reverse was true for DMPA or OC users. Also, DMPA users who were current smokers were more likely to lose BMD at the spine compared to NH users. Irrespective of contraceptive method, previous pregnancy had a positive effect on bone at the spine and femoral neck and lean mass had a positive effect on bone mass at the femoral neck. Moreover, NH users who had a history of DMPA use were more likely to gain BMD than those who had never used DMPA.
Table 3.
Predictors of Percentage Change Bone Mineral Density in Spine (L1—L4) and Femoral Neck during 36 Months of Contraceptive Usea,b,c
| Variable | Coefficient | 95% Confidence Intervals | P value | |
|---|---|---|---|---|
| Spine | ||||
| Baseline BMD (g/cm2) | −4.2516 | −5.7895 | −2.7137 | <.001 |
| DMPA§ | 0.0722 | −0.5311 | 0.6756 | .814 |
| OC§ | 0.3450 | −0.2622 | 0.9521 | .265 |
| White† | −0.1990 | −0.5864 | 0.1883 | .314 |
| Hispanic† | −0.1635 | −0.5497 | 0.2228 | .407 |
| Age (1=16–24 y; 2=25–33 y)* | −0.7316 | −1.2731 | −0.1901 | .008 |
| DMPA x Age§ | 0.7765 | 0.0588 | 1.4942 | .034 |
| OC x Age§ | 0.1294 | −0.5692 | 0.8280 | .717 |
| Time in study2 | −0.0009 | −0.0020 | 0.0002 | .112 |
| DMPA x Time in study2§ | 0.0031 | 0.0016 | 0.0046 | <.001 |
| OC x Time in study2§ | −0.0001 | −0.0016 | 0.0013 | .859 |
| Time in study (mo) | 0.0820 | 0.0451 | 0.1189 | <.001 |
| DMPA x time in study§ | −0.2695 | −0.3203 | −0.2188 | <.001 |
| OC x time study§ | −0.0571 | −0.1068 | −0.0075 | .024 |
| Parity | 0.2080 | 0.0759 | 0.3402 | .002 |
| Current smoker | 0.4533 | −0.1685 | 1.0751 | .153 |
| DMPA x current smoker§ | −1.0568 | −1.8514 | −0.2621 | .009 |
| OC x current smoker§ | −0.3971 | −1.2326 | 0.4384 | .352 |
| Past use of DMPA (#injection) | 0.0634 | 0.0132 | 0.1135 | .013 |
| DMPA x previous use of DMPA | −0.0397 | −0.1019 | 0.0224 | .210 |
| OC x previous use of DMPA | −0.0591 | −0.1449 | 0.0266 | .177 |
| Whole body lean mass (kg) | 0.0093 | −0.0117 | 0.0304 | .384 |
| Constant | 4.1715 | 2.0740 | 6.2690 | <.001 |
| Femoral Neck | ||||
| Baseline femoral neck BMD (g/cm2) | −4.6684 | −6.3527 | −2.9841 | <.001 |
| DMPA§ | −0.0699 | −0.7229 | 0.5832 | .834 |
| OC§ | 0.1313 | −0.5268 | 0.7894 | .696 |
| White† | 0.2172 | −0.2595 | 0.6939 | .372 |
| Hispanic† | 0.2774 | −0.1907 | 0.7455 | .245 |
| Age (1=16–24 y; 2=25–33 y)* | −0.6120 | −1.2737 | 0.0498 | .070 |
| DMPA x Age§ | 0.9446 | 0.0746 | 1.8147 | .033 |
| OC x Age§ | 0.3517 | −0.5046 | 1.2080 | .421 |
| Time in study (mo) | 0.0184 | 0.0023 | 0.0346 | .025 |
| DMPA x time in study§ | −0.1640 | −0.1863 | −0.1418 | <.001 |
| OC x time study§ | −0.0533 | −0.0746 | −0.0320 | <.001 |
| Parity | 0.2500 | 0.0884 | 0.4116 | .002 |
| Current smoker | 0.2266 | −0.1722 | 0.6254 | .265 |
| Past use of DMPA (# injection) | −0.0252 | −0.0597 | 0.0093 | .153 |
| Whole body lean mass (kg) | 0.0755 | 0.0481 | 0.1028 | <.001 |
| Constant | 0.9573 | −1.2488 | 3.1633 | .395 |
BMD = bone mineral density; DMPA = depot medroxyprogesterone acetate; NH = nonhormonal contraception.
Separate mixed models were used for spine and femoral neck bone mineral density.
Dependent variable: Percentage change BMD from baseline values.
The following variables were also included in each model, but were not significant in any model: weight bearing exercise (>120 min/wk vs ≤120 min/wk), alcohol use (gm/day), calcium intake (mg/d), age at menarche (y), and past use of pill (months).
Reference category: black.
Reference group: nonhormonal method users.
Reference group: 16–24-year-olds.
Separate mixed models for hip BMD, spine BMAD, and femoral neck BMAD revealed other notable findings: 16–24-year-olds were more likely to lose BMD at the hip compared to 25–33-year-olds; NH users had a significant increase in BMD and BMAD at the hip and spine over 3 years; DMPA and pill users lost BMD and BMAD over time at all sites; calcium intake was positively associated with hip BMD and spine BMAD; current smoking had synergistic effects on spine BMAD loss among DMPA users; and whole body lean mass had a positive effect for both spine and femoral neck BMAD (data not shown). No notable racial differences were observed in BMD or BMAD changes that resulted from DMPA or OC use.
Those who chose NH contraception after discontinuing DMPA experienced faster recovery at both the spine and femoral neck than those who chose OC (Figure 4). Over 2 years, the NH group had an estimated 4.9% increase (raw value 3.7%) in mean spine BMD and a 3.2% increase (raw value 2.6%) in mean femoral neck BMD, while OC users experienced a 2.3% (raw value 2.2%) increase in spine BMD and a 0.7% (raw value 0.5%) decrease in femoral neck. The BMD increase at the spine and femoral neck for NH users over time was not linear as it reached a plateau at 18 months after DMPA discontinuation. OC users experienced a slower recovery, which was linear at the lumbar spine, but did not regain BMD at the femoral neck. The corresponding changes for NH and OC users for total hip BMD, spine BMAD, and femoral neck BMAD were +2.6%, +5.7%, and +2.6% (NH), and +0.3%, +1.3%, and −0.1% (OC), respectively (data not shown).
Figure 4.
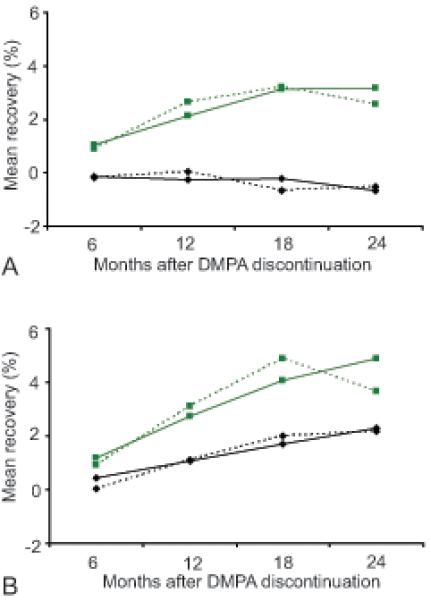
Mean percentage bone mineral density recovery after depot medroxyprogesterone acetate discontinuation across 24 months for (A) femoral neck bone mineral density and (B) spine bone mineral density. Solid lines represent the estimated (modeled) mean percentage changes and dotted lines represent the unadjusted values. Green squares: depot medroxyprogesterone acetate to nonhormonal contraception; black diamonds: depot medroxyprogesterone acetate to oral contraceptives.
Predictors of BMD recovery based on mixed-model regression analyses are shown in Table 4. Those who chose NH after DMPA discontinuation had a significantly greater rate of recovery of their BMD at both sites than those who changed to OC during the first 2 years after DMPA discontinuation. In addition, for NH users, longer duration of DMPA use before discontinuation resulted in faster recovery at the spine. This was also true at the femoral neck irrespective of the method adopted after DMPA discontinuation. With regard to racial differences, BMD recovery at the femoral neck for whites and Hispanics was significantly lower than for blacks. NH users also recovered total hip BMD and spine BMAD more quickly than DMPA discontinuers who became OC users (data not shown).
Table 4.
Predictors of Bone Mineral Density Recovery at Spine (L1—L4) and Femoral Neck during 24 Months after Depot Medroxyprogesterone Acetate Discontinuationa,b,
| Variable | Coefficient | 95% Confidence intervals |
P value | |
|---|---|---|---|---|
| Spine | ||||
| Spine BMD at DMPA discontinuation (g/cm2) | −2.8269 | −5.5956 | −0.0583 | .045 |
| Method after DMPA discontinuation (DMPA to NH)§ | −0.7528 | −2.1024 | 0.5967 | .274 |
| Age (1=16–24 y; 2=25–33 y)* | −0.3716 | −1.0719 | 0.3288 | .298 |
| White† | −0.2281 | −1.0404 | 0.5842 | .582 |
| Hispanic† | 0.3575 | −0.4390 | 1.1540 | .379 |
| Time (after DMPA discontinuation), mo | 0.1049 | 0.0717 | 0.1381 | <.001 |
| Time x NH user§ | 0.1033 | 0.0444 | 0.1622 | .001 |
| Duration of DMPA use (prior to discontinuation), mo | −0.0093 | −0.0439 | 0.0252 | .596 |
| Duration of DMPA use x NH§ | 0.0562 | 0.0047 | 0.1076 | .032 |
| Constant | 3.3628 | 0.1411 | 6.5845 | .041 |
| Femoral Neck | ||||
| Femoral neck BMD at DMPA discontinuation (g/cm2) | 0.6395 | −4.1112 | 5.3902 | .792 |
| Method after DMPA discontinuation (DMPA to NH)§ | −0.3177 | −1.6936 | 1.0582 | .651 |
| Age (1=16–24 y; 2=25–33 y)* | 1.0138 | −0.1670 | 2.1947 | .092 |
| White† | −1.8553 | −3.2458 | −0.4648 | .009 |
| Hispanic† | −1.4115 | −2.7464 | −0.0767 | .038 |
| Time (after DMPA discontinuation), mo | −0.0302 | −0.0830 | 0.0227 | .263 |
| Time x NH user§ | 0.1985 | 0.1047 | 0.2923 | <.001 |
| Duration of DMPA use (prior to discontinuation), mo | 0.0468 | 0.0007 | 0.0929 | .047 |
| Constant | −1.8226 | −7.0242 | 3.3790 | .492 |
BMD = bone mineral density; DMPA = depot medroxyprogesterone acetate; NH = nonhormonal contraception.
Separate mixed models were used for spine and femoral neck bone mineral density.
Dependent variable: Percentage change bone mineral density at different follow-up visits from the values at the time of depot medroxyprogesterone acetate discontinuation.
Reference group: black.
Reference group: depot medroxyprogesterone acetate to oral contraceptive.
Reference group: 16–24-year-olds.
DISCUSSION
This study evaluated the independent effects of two different types of hormonal contraception on BMD over a 3-year interval. Use of OC containing 20 μg EE resulted in BMD loss at the spine during the third year of contraceptive use and at the femoral neck all 3 years. The loss we observed at the spine (0.54% over 36 months) was consistent with that observed by Endrikat in a prior study of 20–35-year-olds using OC containing 20 μg EE (0.4% over 36 months).(11) While these two studies suggest that use of a very low dose birth control pill may decrease BMD, it should be noted that the amount of bone loss we observed at the spine is very small. A slightly greater loss occurred at the femoral neck. However, bone loss at this site (0.3% in 12 months and 1.3% in 36 months) is still less than that which occurs during early menopause. In comparison, Guthrie et al observed that women lost 1.7% of their femoral neck BMD during the first year of menopause, which is almost six times more than we observed after one year of DMPA use.(22) Thus, it is unlikely that the loss associated with OC use significantly increases a woman’s risk of fracture. Furthermore, the reversibility of this loss and its long-term effects has not been determined.
Women who chose DMPA for contraception experienced greater bone density loss (3.7% at the spine and 5.2% at the femoral neck) than OC users. Furthermore, DMPA users 16–24 years of age had greater bone loss than those aged 25–33 years. This supports the findings of Scholes et al who observed that spinal BMD loss resulting from DMPA use was greater in 18–21 year old women than in those 22–39 years old.(23) The reason for greater bone loss among younger DMPA users is not known, but may be due to age-related differences in estrogen levels. Levels of estradiol have been observed to be lower in DMPA users 18–25 than those 35–45 years of age.(24) Since a decline in estradiol causes high bone resorption and lower bone formation, this may explain the differences we observed. In addition, younger women experienced a greater relative difference when compared to those using NH contraception, as the latter group was actively forming bone. When considered together, these observations suggest that DMPA may prevent women who use this method at a young age from reaching their peak bone density.
However, data from women who discontinued DMPA suggest that these adverse effects on bone are temporary, at least with regard to the spine. In fact, similar to Clark et al, (5) we observed that women who used DMPA longer experienced greater gains in spinal BMD after discontinuation than those who used it for less time. The gain in spinal BMD we observed among discontinuers who subsequently used NH was actually higher (2.4% per year) than that reported by Clark et al (1.0–1.9% per year), although those who used OC after discontinuation had a comparable recovery (1.1% per year on average in our study). These two studies provide reassurance that DMPA use does not cause long-term adverse effects at the spine, even when used for an extended period.
Findings at the femoral neck were more concerning. First, DMPA users lost more bone at the femoral neck than at the spine over the 3-year observation period (5.2% vs. 3.7%). While most spinal bone loss occurred during the first year of use, BMD loss at the femoral neck was greater during the second and third years of use. This is of concern as women may use DMPA for multiple years. Similar to prior investigators, (5,6) we also observed that after DMPA was discontinued, BMD recovery was slower at the femoral neck than at the spine. Most concerning was our observation that among women who used OC after DMPA discontinuation, BMD did not recover at the femoral neck during the 2 years of follow up, suggesting that OC use immediately after DMPA discontinuation may interfere with bone recovery at this site. These findings are limited, however, by the small number of OC users we followed after DMPA discontinuation and the fact that only one OC formulation was studied. It is also possible that bone recovery occurred once these women discontinued OC. Future studies should address whether OCs containing higher amounts of estrogen similarly interfere with BMD recovery and whether this relationship is affected by age, amount of time on DMPA, and amount of time that OC is used.
Another important finding was that tobacco use resulted in greater bone loss among DMPA users at the spine. This relationship between current smoking and BMD loss could have implications for many women, as those who smoke are more likely to select DMPA for contraception than methods containing estrogen. In fact, 36% of women in our study who chose DMPA as their contraceptive method reported current smoking. Too few women were in the reversibility arm to stratify by smoking status, so it is unclear if tobacco use interferes with bone recovery. It is reassuring, however, that the relationship between tobacco use and bone loss was observed only at the spine, where recovery is likely. This topic merits further study so that health care providers can adequately counsel their patients regarding the risk of smoking while using DMPA.
No differences were observed by race/ethnicity in BMD changes that occurred as a result of DMPA or OC use. With regard to reversibility, blacks recovered bone better than whites or Hispanics at the femoral neck regardless of the method selected after discontinuation. Thus, it appears that black women who elect to begin OC after DMPA discontinuation may be able to regain their BMD at the femoral neck. However, this conclusion is based on 11 black women who used OC after DMPA discontinuation and thus must be interpreted with caution.
This study has several limitations. First, we did not randomize women to one of the three contraceptive groups as these methods have different efficacies and randomization could have led to unintended pregnancies. Second, discontinuation rates for all contraceptive methods were high. However, this is common in contraceptive studies as there are many reasons women may choose to change or discontinue their method.(25–27) Third, too few women were followed after DMPA discontinuation to stratify our analysis by race/ethnicity, smoking status, and other important variables. Finally, we studied only one formulation of OC so our findings cannot be generalized to other types of birth control pills with different amounts of estrogen or other progestins.
In conclusion, we observed that both DMPA and OC containing 20 μg EE caused BMD loss. We also found that DMPA-related changes were largely reversible at the spine regardless of the contraceptive method used after DMPA discontinuation and at the femoral neck if NH contraception was used. However, use of OC immediately after DMPA discontinuation may impede the ability to regain bone at the femoral neck, at least temporarily. This finding appears to have greatest relevance for white women who are at high risk of hip fracture after menopause. Further studies are needed to confirm these findings and to determine if they are robust across women of different races and ages. Furthermore, different formulations of OC should be studied as they may have different effects on BMD. Most importantly, it will be critical to determine if observed contraceptive-related changes in BMD affect the risk of fracture during use or in later years.
It is important to remember that the reason women use DMPA and OC is to avoid pregnancy. The typical failure rate of DMPA and OC after 12 months of use is 7% and 9%, respectively, as compared with failure rates of up to 17% for condom users.(28) As clinicians, it is important to weigh the theoretical risk of fracture from decreased BMD in DMPA and OC users against the very real risk of pregnancy in women if their contraceptive choices are limited.
Acknowledgments
Supported by the National Institute of Child Health & Human Development grants R01HD39883 and K24HD043659 and General Clinical Research Centers (GCRC) program, National Center for Research Resources, NIH, M01RR000073.
Footnotes
Financial Disclosure: The authors have no potential conflicts of interest to disclose.
Précis Spinal bone loss resulting from depot medroxyprogesterone acetate use is largely reversible; bone recovery is slower if a 20 μg oral contraceptive is used after depot medroxyprogesterone acetate discontinuation.
References
- 1.Clark MK, Sowers M, Nichols S, Levy B. Bone mineral density changes over two years in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2004;82:1580–6. doi: 10.1016/j.fertnstert.2004.04.064. [DOI] [PubMed] [Google Scholar]
- 2.Berenson AB, Radecki Breitkopf C, Grady JJ, Rickert VI, Thomas A. Effects of hormonal contraception on bone mineral density after 24 months of use. Obstet Gynecol. 2004;103:899–906. doi: 10.1097/01.AOG.0000117082.49490.d5. [DOI] [PubMed] [Google Scholar]
- 3.Scholes D, Lacroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139–44. doi: 10.1001/archpedi.159.2.139. [DOI] [PubMed] [Google Scholar]
- 4.Lara-Torre E, Edwards CP, Perlman S, Hertweck SP. Bone mineral density in adolescent females using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol. 2004;17:17–21. doi: 10.1016/j.jpag.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Clark MK, Sowers M, Levy B, Nichols S. Bone mineral density loss and recovery during 48 months in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2006;86:1466–74. doi: 10.1016/j.fertnstert.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Scholes D, Lacroix AZ, Ichikawa LE, Barlow WE, Ott SM. Injectable hormone contraception and bone density: results from a prospective study. Epidemiology. 2002;13:581–7. doi: 10.1097/00001648-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Cromer BA, Stager M, Bonny A, et al. Depot medroxyprogesterone acetate, oral contraceptives and bone mineral density in a cohort of adolescent girls. J Adolesc Health. 2004;35:434–41. doi: 10.1016/j.jadohealth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Mais V, Fruzzetti F, Ajossa S, Paoletti AM, Guerriero S, Melis GB. Bone metabolism in young women taking a monophasic pill containing 20 mcg ethinylestradiol: a prospective study. Contraception. 1993;48:445–52. doi: 10.1016/0010-7824(93)90134-s. [DOI] [PubMed] [Google Scholar]
- 9.Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221–4. doi: 10.1016/0010-7824(95)00036-a. [DOI] [PubMed] [Google Scholar]
- 10.Cromer BA, Bonny AE, Stager M, et al. Bone mineral density in adolescent females using injectable or oral contraceptives: a 24-month prospective study. Fertil Steril. 2008;90:2060–7. doi: 10.1016/j.fertnstert.2007.10.070. [PubMed:18222431] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endrikat J, Mih E, Düsterberg B, et al. A 3-year double-blind, randomized, controlled study on the influence of two oral contraceptives containing either 20 μg or 30 μg ethinylestradiol in combination with levonorgestrel on bone mineral density. Contraception. 2004;69:179–87. doi: 10.1016/j.contraception.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Nappi C, Di Spiezio Sardo A, Acunzo G, et al. Effects of a low-dose and ultra-low-dose combined contraceptive use on bone turnover and bone mineral density in young fertile women: a prospective controlled randomized study. Contraception. 2003;67:355–9. doi: 10.1016/s0010-7824(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 13.Hertzler AA, Frary RB. A dietary calcium rapid assessment method (RAM) Top Clin Nutr. 1994;76:85. [Google Scholar]
- 14.World Health Organization Smoking Questionnaire. MONICA Manual (1998–1999), Part III, Section 1. [Accessed July 11, 2008];The WHO MONICA (Multinational Monitoring Trends in Cardiovascular Disease) Project. http://www.ktl.fi/publications/monica/manual/part3/smquest3.htm
- 15.National Cancer Institute . Diet History Questionnaire (DHQ) National Institutes of Health; [Accessed July 10, 2008]. http://riskfactor.cancer.gov/DHQ/ [Google Scholar]
- 16.Kolle E, Torstveit MK, Sundgot-Borgen J. Bone mineral density in Norwegian premenopausal women. Osteoporos Int. 2005;16:914–20. doi: 10.1007/s00198-004-1783-2. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TV, Sambrook PN, Eisman JA. Sources of variability in bone mineral density measurements: implications for study design and analysis of bone loss. J Bone Miner Res. 1997;12:124–35. doi: 10.1359/jbmr.1997.12.1.124. [DOI] [PubMed] [Google Scholar]
- 18.Ravaud P, Reny JL, Giraudeau B, Porcher R, Dougados M, Roux C. Individual smallest detectable difference in bone mineral density measurements. J Bone Miner Res. 1999;14:1449–56. doi: 10.1359/jbmr.1999.14.8.1449. [DOI] [PubMed] [Google Scholar]
- 19.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–9. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–45. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 21.Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73:1332–9. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]
- 22.Guthrie JR, Ebeling PR, Hopper JL, Barrett-Connor E, Dennerstein L, Dudley EC, Burger HG, Wark JD. A prospective study of bone loss in menopausal Australian-born women. Osteoporos Int. 1998;8:282–90. doi: 10.1007/s001980050066. [DOI] [PubMed] [Google Scholar]
- 23.Scholes D, Lacroix AZ, Ott SM, Ichikawa LE, Barlow WE. Bone mineral density in women using depot medroxyprogesterone acetate for contraception. Obstet Gynecol. 1999;93:233–8. doi: 10.1016/s0029-7844(98)00447-5. [DOI] [PubMed] [Google Scholar]
- 24.Walsh JS, Eastell R, Peel NFC. Effects of depot medroxyprogesterone acetate on bone density and bone metabolism before and after peak bone mass: A case-control study. J Clin Endocrin Metab. 2008;93:1317–23. doi: 10.1210/jc.2007-2201. [DOI] [PubMed] [Google Scholar]
- 25.Berenson AB, Odom SD, Radecki Breitkopf C, Rahman M. Physiologic and psychologic symptoms associated with use of injectable contraception and 20 microgram oral contraceptive pills. Am J Obstet Gynecol. 2008 Oct;199:351.e1–12. doi: 10.1016/j.ajog.2008.04.048. Epub 2008 Jul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy PA, Brixner D. Hormonal contraceptive discontinuation patterns according to formulation: investigation of associations in an administrative claims database. Contraception. 2008;77:257–63. doi: 10.1016/j.contraception.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: a prospective evaluation of frequency and reasons. Am J Obstet Gynecol. 1998;179:577–582. doi: 10.1016/s0002-9378(98)70047-x. [DOI] [PubMed] [Google Scholar]
- 28.Kost K, Singh S, Vaughan B, Trussell J, Bankole A. Estimates of contraceptive failure from the 2002 National Survey of Family Growth. Contraception. 2008;77:10–21. doi: 10.1016/j.contraception.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]