Abstract
Uveitis is an inflammatory ocular disease characterized by the infiltration of T lymphocytes and other leukocytes into the eye. The recruitment of these inflammatory cells from systemic vasculature to ocular tissue is a well-coordinated multistep process including rolling, firm adhesion and transmigration. CXCL12 (SDF-1α) is an endothelial cell-derived cytokine interacting with CXCR4 and CXCR7, two chemokine receptors mainly expressed in T cells, neutrophils and monocytes. Recent studies have shown that CXCR4, CXCR7 and their ligand, CXCL12, are important for the regulation of leukocyte mobilization and trafficking. However, it is unclear whether these two chemokine receptors are implicated in the pathogenesis of uveitis. In this study, we used DO11.10 mice, whose CD4+ T cells are genetically engineered to react with ovalbumin (OVA), to investigate the role of CXCR4 and CXCR7 in an animal model of uveitis. Intravital microscopy revealed that intravitreal OVA challenge of DO11.10 mice caused the infiltration of both T cells and neutrophils. The invasion of these inflammatory cells coincided with the detection of transcriptional upregulation of CXCR4 and CXCR7 in the eye. In addition, both real time-PCR and immunohistochemistry revealed an enhanced expression of endothelial CXCL12. Furthermore, intraperitoneal injection of AMD3100 (a specific CXCR4 antagonist) significantly attenuated OVA-induced uveitis and CXCL12-mediated transwell migration. In contrast, intraperitoneal administration of CXCR7 neutralizing antibody did not significantly alter ocular infiltration of inflammatory cells caused by OVA challenge. Our data suggest that CXCR4 but not CXCR7 plays a critical role in antigen-induced ocular inflammation by facilitating leukocyte infiltration. This study not only enhances our knowledge of the immunopathological mechanism of uveitis but also provides a novel rationale to target CXCR4 as an anti-inflammatory strategy to treat uveitis.
Keywords: CXCL12, CXCR4, CXCR7, neutrophils, monocytes, ocular inflammation, T cells, uveitis
1. Introduction
Noninfectious uveitis is characterized by intra-ocular inflammation. It is often associated with systemic autoimmune diseases such as Behcet's disease, sarcoidosis, spondyloarthropathy or inflammatory bowel disease (Becker, 2000; Martin et al., 2002). According to recent studies, the prevalence of uveitis is approximately 115.3 patients per 100 000 in the general population. The annual incidence averages 52.4 per 100 000 (Gritz and Wong, 2004). The often chronic or recurrent nature of noninfectious uveitis predisposes the patients to a high risk of permanent visual impairment, causing a significant health-related economic impact.
The histological hallmark of uveitis is sequential and well-orchestrated infiltration by different leukocyte populations into the eye, leading to ocular tissue inflammation and damage (Becker et al., 2001; Ke et al., 2007; Xu et al., 2003; Xu et al., 2004). The recruitment of inflammatory cells from the systemic vasculature to local tissue consists of a chain of well-coordinated events including tethering, rolling, activation, firm adhesion, and transmigration (Ley et al., 2007). This complicated process is tightly and temporally controlled by interaction between various adhesive molecules of activated leukocytes and their counterparts expressed on the surface of endothelial cells (Steeber and Tedder, 2000). Among them, CXCR4 and its ligand, CXCL12 (also called SDF (stromal derived factor)-1 α) have received recent attention for their crucial role in leukocyte trafficking (Chan et al., 2003; Ding et al., 2006; Fernandis et al., 2003; Link, 2005; Ottoson et al., 2001; Thelen and Thelen, 2008).
CXCR4 is a 7 transmembrane domain receptor coupled G protein. This chemokine receptor is mainly expressed by T lymphocytes, neutrophils and monocytes (Feng et al., 1996; Loetscher et al., 1994; Patrussi and Baldari, 2008). CXCL12 is a 68 amino acid CXC chemokine which specifically interacts with CXCR4 (Oberlin et al., 1996; Bleul et al., 1996). Bone marrow stromal elements and endothelial cells are the major source of CXCL12 expression (Möhle and Moore, 1998; Möhle and Rafii, 1999). It has been shown that mobilization of leukocytes from the bone marrow is largely influenced by interference with the engagement of CXCR4 with CXCL12 (so-called CXCR4/CXCL12 axis) (Juarez et al., 2004; Lataillade et al., 2004). Furthermore, recent studies have demonstrated that this CXCR4/CXCL12 axis is also implicated in transendothelial migration of inflammatory cells (Möhle and Moore, 1998; Möhle and Rafii, 1999). In addition, up-regulation of CXCR4 is reported in many inflammatory diseases such as rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease (Bajetto et al., 2001; Haringman et al., 2006; Mikami et al., 2008).
Recently, CXCR7 was found to be a second receptor binding to CXCL12. CXCR7 is a G protein-coupled orphan receptor. It is highly conserved in mammals, and expressed by a broad spectrum of tissues including T lymphocytes and monocytic cells (Burns et al., 2006; Law and Rosenzweig, 1994). CXCR7 monoclonal antibody has been shown to inhibit CXCL12-induced T cell chemotaxis (Balabanian et al., 2005). Furthermore, a recent study demonstrated the involvement of both CXCR4 and CXCR7 in the transendothelial migration of renal progenitor cells, and CXCR7 is particularly important for the cell adhesion to the endothelium (Mazzinghi et al., 2008).
In this study, we aimed to define the role of both CXCR4 and CXCR7 in the pathogenesis of uveitis. Utilizing an antigen-specific uveitis model that has been established in our laboratory recently (Zhang et al., 2009), we demonstrate that the expression of transcripts for CXCR4, CXCR7 and their ligand, CXCL12, is up-regulated in the eyes after antigen challenge. Although blocking CXCR4 or CXCR7 attenuated CXCL12-induced transmigration in vitro, only CXCR4 inhibitor AMD3100 but not CXCR7 antibody significantly ameliorates ocular inflammation in vivo. Collectively, these results indicate that CXCR4-mediated leukocyte infiltration is an important step in the development of this model of uveitis.
2. Materials and Methods
2.1. Mice and Induction of Uveitis
Mice whose myelomonocytic cells were labeled with enhanced green fluorescent protein (EGFP) by inserting EGFP gene into the murine lysozyme M (lys) locus were provided by Dr. Thomas Graf, and T-Red transgenic mice carrying dsRedII fluorescent protein gene driven by CD4 promoter were a generous gift of Dr. Ulrich von Andrian's laboratory. The mice expressing both red fluorescent T lymphocytes and EGFP-labeled monocytic cells were generated by crossing these 2 strains to DO11.10 mice on a BALB/c background. The phenotype of dual fluorescence and ovalbumin (OVA) specific TCR recognized by anti-mouse DO11.10 TCR antibody (Invitrogen, Carlsbad, CA) in the new strain was confirmed by flow cytometry analysis. In addition to the transgenic mice described above, six- to 8-week-old BALB/c mice and DO11.10 mice on a BALB/c background (Jackson Laboratory, Bar Harbor, Maine) were used for the experiments. The animal experimental protocols are approved by our institutional animal care and use committee.
DO11.10 mice were injected with OVA or control solutions into the vitreous chamber of each eye, respectively. Some mice also received anti-CXCR7 antibody along with the antigen intravitreally. In order to adequately deliver OVA and testing antibody together, we prepared the injection solution of OVA or OVA with anti-CXCR7 antibody in total 4 μl phosphate-buffered saline (PBS). The injections were performed with ultra-thin, pulled borosilicate glass needles (outer diameter about 50 μm) and Hamilton syringes under direct visualization through a surgical microscope. To avoid any potential eye damage that could be due to the increase of intraocular pressure caused by the relatively large injection volume, we first inserted the needle intravitreally and allowed some vitreous fluid to be released. We then replaced it with the 4 μl injection solution. These mice received 100 μg of OVA (Sigma, St. Louis, MO) and 1.5 μg of rabbit polyclonal anti-CXCR7 GPCR RDC1 antibody (Catalog number: ab72100) (Abcam, Cambridge, MA).. Uveitis was evaluated by intravital microscopy at various time points after the OVA challenge. As shown in Figure 1, the injection of 4 μl control solution did not cause ocular inflammation. Furthermore, we did not observe abnormal histological change of ocular structure in the eyes that were administrated 4 μl PBS in our previous study (Zhang et al. 2009).
Figure 1.
Intravitreal injection of OVA elicits uveitis in DO11.10 mice. OVA, PBS or BSA were administered intravitreally to DO11.10 mice (n = 3). In addition, BALB/c mice received the same amount of OVA intravitreally. Twenty four hours later, circulating leukocytes were labeled with rhodamine by intraperitoneal injection, and ocular inflammation was assessed by intravital microscopy. Representative image of a single frame from intravital microscopy videos taken of the iris at 24 hours during the course of inflammation induced by OVA. Note: Influx and adhesion of rhodamine-labeled leukocytes only in OVA-challenged eyes.
2.2. Intravital Microscopy
One hundred fifty μl of rhodamine (0.2% in PBS) was administered intraperitoneally (i.p.) into the mice to label intravascular leukocytes right before intravital microscopy as we have previously described (Becker, 2000; Dullforce et al., 2006; Lim et al., 2006; Zhang et al., 2009). Labeled inflammatory cells in the iris and ciliary/limbal region were observed by intravital epifluorescence videomicroscopy of anesthetized animals with a modified DM-LFS microscope (Leica) and either a CF 84/NIR black and white camera from Kappa, Gleichen, Germany, or a color Optronics DEI-750CE camera (Optronics International, Chelmsford, MA). Real-time videos were recorded in NTSC format for 10 seconds each. Both rolling and adherent leukocytes in the iris vessels were identified as a marker for anterior chamber uveitis. These cells were further quantified to assess the severity of the ocular inflammation. Briefly, the intravital microscopy movie files were loaded to ImageJ software. Each 20X magnified view of intravital microscopy represents approximately 18,000 μm2 of filmed iris, and was projected to a computer monitor. All vessels in an area between 3,000 and 4,000 μm2 were evaluated for all leukocyte traffic that were in, entered, or exited the outlined are during the 10 second video. All rollers and stickers were counted. Rollers were the cells that appeared to be crawling along the side of the outlined vessels. These cells were distinct from the regular circulating cells due to their slow flow speed. Stickers were defined as the cells that adhered to the vessels during the course of the entire movie period. The average numbers of stickers and rollers were counted and divided by the average □m2 area of the vessels. This value was then multiplied by 1 000 to convert to the concentration in mm2.
2.3. RT-PCR of Murine CXCR4 and CXCL12
Total RNA from eye homogenates was isolated with RNAeasy Mini kit (Qiagen, Valencia, CA). First-strand cDNA synthesis was accomplished with oligo (dT)-primed Omniscript reverse transcriptase kit (Qiagen, Valencia, CA). Gene-specific cDNA was amplified by a hot-start touchdown PCR procedure, with Platinum Taq DNA Polymerase kit (Invitrogen) and mouse specific primer pairs (CXCR4 sense, 5′- TGG TCA TAT GGA GGG TAT GT -3′, and CXCR4 antisense, 5′- CCT GTA ATG GTC AAG GTT GT -3′, 168 bp amplicon; CXCR7 sense, 5′- TCT GGT TGC TTG AGT GGT -3′, and CXCR7 antisense, 5′- CTG GTG CTG GCT TTG ATA -3′, 212 bp amplicon; CXCL12 sense, 5′- ATC ACA GAC GGC CCT GGT -3′, and CXCL12 antisense, 5′-CTG GCA TTA CTA TGG CTC CAC-3′, 155 bp amplicon). PCR thermal cycler conditions were as follows: 1 × 15 min 95°C, 35 cycles denaturation (45 sec, 94°C), annealing (45 sec, 61°C), and extension (45 sec, 72°C). A primer pair for a constitutively expressed gene, β-actin (sense, 5′- ATG CCA ACA CAG TGC TGT CT -3′, and antisense, 5′- AAG CAC TTG CGG TGC ACG AT -3′), was included in each assay as an internal control. PCR products were separated by electrophoresis in a 2% agarose gel and analyzed by densitometry.
2.4. Ex vivo whole-mount preparation and imaging
Iris whole-mounts were prepared 24 hours after intravitreal challenge with specific antigen (OVA) or control in DO11.10 mice, as described previously (Rosenbaum et al, 2007). Briefly, eyes were enucleated, dissected to remove the posterior of the eye and lens, and fixed in 4% paraformaldehyde at 4°C overnight. Following fixation, the remaining anterior segments were washed in tris-buffered saline (TBS) and incubated for 60 minutes in 2% rabbit serum. Then, anterior segments were incubated with 10 μg/ml rat anti-mouse CXCL12 antibody (R&D Systems) overnight at 4°C. After washing in TBS 5 times, the excised eye segments were incubated with HRP-conjugated goat anti-rat IgG (1:500) at 4°C overnight, followed by extensive washing in TBS. Then, the CXCL12 staining was developed with the substrate DAB (50 ml/ml) and DAB enhancer, respectively, for 3-5 minutes using Liquid DAB-Plus Substrate Kit according to the manufacturer's instruction (Invitrogen, Carlsbad, CA). After stopping the reaction with the rinse of distilled water, the anterior segments of the eyes were cut into the shape of a clover leaf. Then, it was mounted flatly on SuperFrost® Plus coated glass microscope slides (Fisher Scientific, Pittsburgh, PA). After applying a coverslip with mounting media, samples were imaged using a Leica DM5000B epifluorescent microscope and photographed with a Leica DC500 digital camera (Leica).
2.5. Flow Cytometry
Splenocytes from DO11.10 mice were obtained by crushing the spleen through a 70 μm pore size strainer. Freshly isolated splenocytes, peripheral leukocytes and bone marrow cells were incubated with Red Blood Cell Lysing Buffer (Sigma-Aldrich, St. Louis, MO) for 5 min. These cells were suspended in PBS containing 1% FBS, 0.5 mM EDTA and 0.1% sodium azide. Anti-CD4, anti-Ly6G and anti-Ly6G/C antibodies conjugated with different fluorescent colors were used to label these cell surface markers. Data acquisition was performed on a FACSCalibur flow cytometer, and data were analyzed using CellQuest software.
2.6. Isolation of Neutrophil and Monocyte-Predominant Leukocytes
DO11.10 mice were intraperitoneally injected with 500 μL of a 6% solution of oyster glycogen type II (Sigma-Aldrich) prepared in sterile PBS. After 3 hours of glycogen solution stimulation, total 1 ml PBS was used to lavage the peritoneal cavity and collect exudate cells. Neutrophils and monocytes in the cell exudates were identified by flow cytometry analysis of Ly6G and Ly6G/C expression for further experiment.
2.7. Transwell Migration Assay
Transwell migration assays were performed using 8 μm pore size transwell inserts (Corning Inc., Corning, NY) suspended by the outer rim within individual wells of 24-well ultralow plates (Costar). The bottom wells were filled with 0.7 ml of RPMI containing 10% FBS in the presence and absence of 30 ng/ml CXCL12 (Peprotech, Rocky Hill, NJ). Two hundred fifty thousand cells (per each well) were incubated with various concentrations of AMD3100 (Sigma-Aldrich), 25 μg/ml anti-mouse CXCR7 antibody (R and D Systems, San Diego, CA) or control vehicle for 30 min, and added to the transwell, which was placed in contact with the medium in the bottom well. The migration assay plates were placed in a humidified incubator (37°C, 5% CO2) for up to 3 hours. At different time points, medium and cells from the lower wells were collected. Harvested cells were stained with trypan blue to assess cell viability, and the number of viable cells was counted by a hemacytometer.
2.8. Statistics
Data are expressed as the average ± SEM, and a representative experiment is shown for each figure. Statistical probabilities were evaluated by Student's t-test, with a value of p < 0.05 considered significant.
3. Results
3.1. Ocular Infiltration of Leukocytes in OVA-induced Uveitis
We intravitreally administrated OVA into the eyes of in DO11.10 mice on BALB/c background. Ocular inflammation was assessed at 24 hours after the antigen challenge. Compared to the controls that received PBS, OVA injection evoked marked leukocyte influx and adhesion in the iris vascular beds (Figure 1). Transgenic T cells of DO11.10 mice are well known for their response to OVA through specific TCR recognition. However, in order to confirm that this anterior uveitis is antigen specific, we also treated DO11.10 mice intravitreally with equal amount of bovine serum albumin (BSA) as a control with irrelevant antigen challenge. As illustrated in Figure 1, BSA did not cause ocular leukocyte infiltration in the DO11.10 mice. Moreover, we challenged regular BALB/c mice intravitreally with OVA, and these mice did not exhibit ocular inflammation as assessed by intravital microscopy (Figure 1). These results further support the fact that OVA-induced uveitis in DO11.10 mice is mediated by specific TCR recognition.
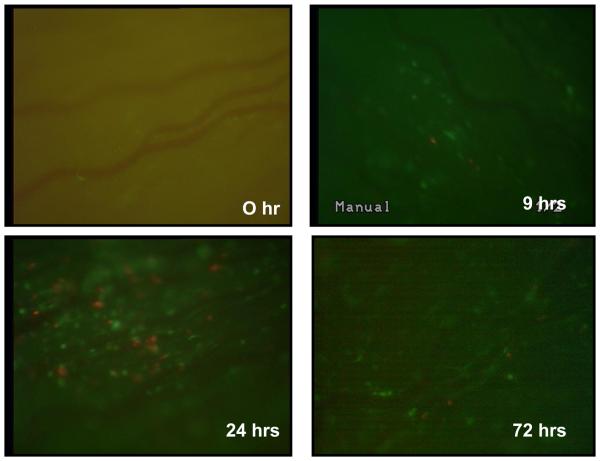
In addition, we previously showed that this antigen-specific uveitis is mediated by CD4+ T lymphocytes as evidenced by the fact that systemic depletion of CD4+ cells by GK1.5 antibody blocks the ocular inflammation (Zhang et al, 2009). In addition, the uveitis is signified by marked rolling and adhesion of neutrophil-predominant leukocytes in the vasculature of the eyes. In order to further characterize the kinetics of infiltration of different leukocyte subsets in the iris of OVA-induced uveitis, we cross-bred DO11.10 mice that have transgenic dsRedII and EGFP under the control of CD4 promoter and lysozyme promoter, respectively (Mempel et al, 2006; Faust et al., 2000). Therefore utilizing intravital microscopy, this model allowed us to specifically study the time course of T cell, neutrophil, and monocyte trafficking in the eye after antigen challenge. After intravitreal injection of OVA to the eyes of DO11.10 mice, influx of fluorescent leukocytes in the iris was monitored by intravital microscopy. As shown in Figure 2, the infiltration of red T cells and green neutrophils was observed along ocular vasculature as early as 9 hours after OVA stimulation. The inflammatory cell migration reached the peak at 24 hours and persisted beyond 72 hours (Figure 2). No rolling or adherent T cells or neutrophils were observed in the eyes at 0 hour before OVA injection or in the control animals that received sham injection of PBS at these time points (data not shown).
Figure 2.
Leukocyte infiltration occurs in the eye of DO11.10 mice over time after OVA challenge. OVA was administered intravitreally into the DO11.10 mice which have transgenic dsRed and green fluorescent proteins under the control of a CD4 promoter and lysozyme promoter, respectively. Ocular infiltrating cells were assessed by intravital microscopy at 0, 9, 24, and 72 hours. Representative image of a single frame from intravital microscopy videos taken of the iris from 2 independent experiments during the course of inflammation induced by OVA. Note: T cells displayed red fluorescence, whereas neutrophils and monocytes were green.
3.2. Up-regulation of CXCR4, CXCR7 and CXCL12 in OVA-induced Uveitis
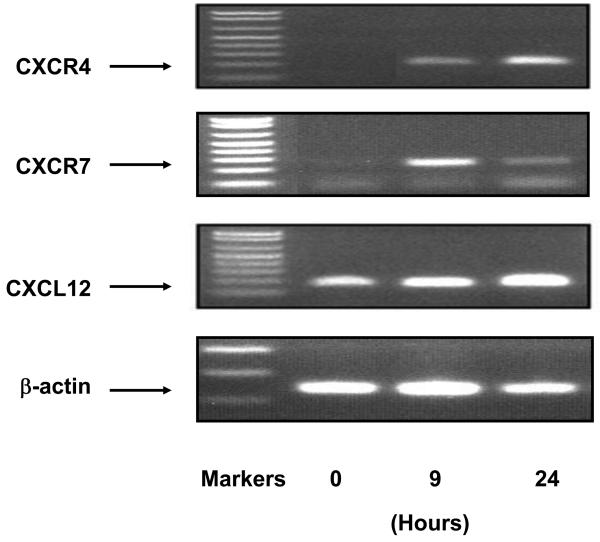
It is well documented that CXCR4 and CXCR7 are mainly expressed by T cells, neutrophils and monocytes. Since we showed that T lymphocytes and myeloid cells are implicated in OVA-induced uveitis, it was feasible to speculate that CXCR4, CXCR7 and their ligand, CXCL12, played a role in the ocular infiltration of these cells. To test this assertion, we examined the transcription of these 3 genes in the eyes at different time points after OVA challenge. RT-PCR did not detect CXCR4 signal at 0 hour point, indicating no endogenous expression of CXCR4 in naïve eyes (Figure 3). Nevertheless, transcription of CXCR4 in the eye was observed at 9 hours, and became more predominant at 24 hours after OVA administration (Figure 2). In addition, a significant induction of CXCR7 occurred at 9 hours after OVA challenge. The ocular expression of mRNA for CXCR7 attenuated somewhat at 24 hours (Figure 3). The induction of CXCR4 and CXCR7 transcripts coincides with the local infiltration of leukocytes as we observed previously (Figure 2).
Fig. 3.
Transcription of CXCR4, CXCR7 and CXCL12 is up-regulated in the eye challenged with OVA. OVA was administered intravitreally. The eyes were harvested at different time points, and total RNA was collected. RT-PCR analysis was performed to examine the expression of CXCR4, CXCR7 and its specific ligand CXCL12 (n = 3 per group). Note: Induction of CXCR4, CXCR7 and CXCL12 in OVA-induced uveitis.
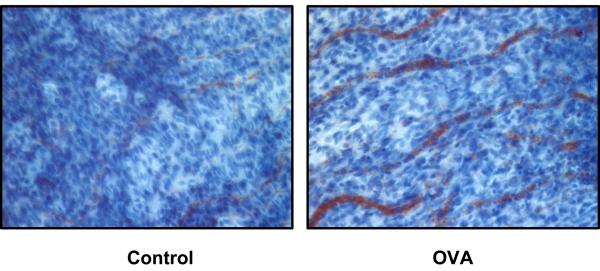
In light of the induction of CXCR4 and CXCR7 in OVA-induced uveitis, we then examined the expression of CXCL12, a specific ligand for these 2 CXC receptors. As shown in Figure 3, normal eye displayed a baseline CXCL12 mRNA expression, and OVA challenge enhanced the level of CXCL12 transcripts at 24 hours. Moreover, to confirm CXCL12 expression at a protein level, we used ex vivo staining of CXCL12 in excised iris tissues. Immunohistochemistry with CXCL12 antibody showed specific faint CXCL12 staining in the control group, whereas the CXCL12 production was markedly augmented in the eyes at 24 hours after OVA challenge (Figure 4). The CXCL12 signal was localized along the ocular vessels where endothelial cells are situated.
Figure 4.
Enhancement of CXCL12 expression occurs in the eye of DO11.10 mice at 24 hours after OVA challenge. OVA or PBS as a control vehicle was administered intravitreally (n=2). Twenty-four hours later, the iris was dissected and fixed in paraformaldehyde. Immunohistochemical analysis was performed to detect CXCL12 protein expression in the eye. Note: Augmented CXCL12 staining detected within ocular vessels of OVA-treated eye.
3.3. In Vitro Suppression of CXCL12-induced Leukocyte Trafficking by Blocking CXCR4 and CXCR7
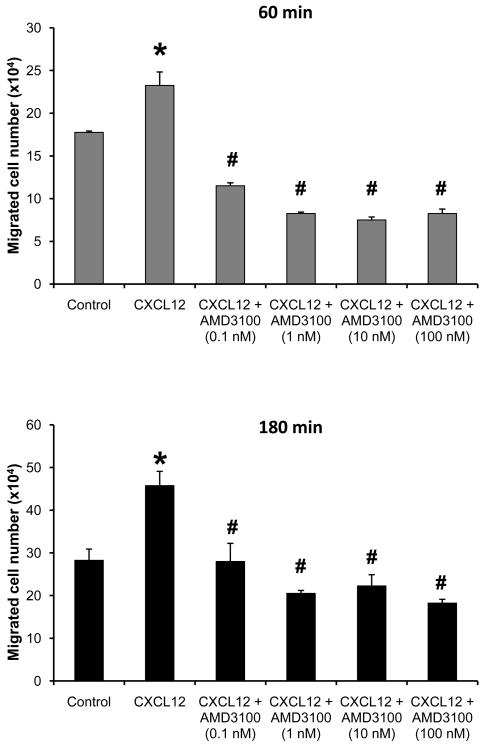
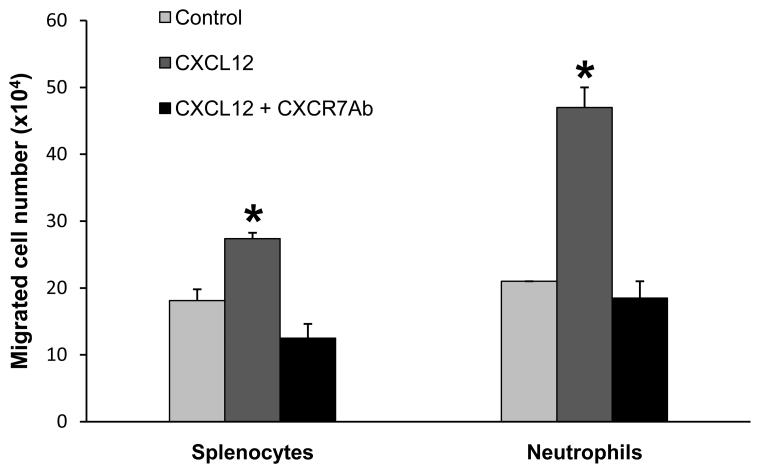
To further define the role and mechanism of CXCR4 and CXCR7 in the uveitis, we first examined whether blocking CXCR4 or CXCR7 inhibited inflammatory cell chemotaxis and migration in vitro. We utilized a transwell migration assay to simulate the in vivo situation of ocular transmigration. Since we previously demonstrated the influx of both T cells and myeloid leukocytes in OVA-induced uveitis, we intended to study if a specific CXCR4 inhibitor, AMD3100, affected the trafficking of these two cell populations. In this study, we found that more than 85% of splenocytes of DO11.10 mice were CD4+. Thus, the splenocytes were used to represent peripheral T cells. Oyster glycogen has been widely used to induce neutrophils and monocytes from mouse peritoneal cavity. However, the cellular composition of oyster glycogen-induced peritoneal exudates changes over time, and the peak influx of neutrophils and monocytes usually occurs between 2 and 4 hours after the irritant inoculation (Mulligan et al., 1998). Therefore, we harvested neutrophil and monocyte-predominant peritoneal leukocytes at 3 hours after oyster glycogen challenge, and these cell populations were further confirmed by flow cytometry for the expression of Ly6G (a marker of neutrophils) and Ly6G/C (a marker of both granulocytes and monocytes). Then, we tested CXCL12-induced migration of the splenocytes and myeloid predominant leukocytes separately. Compared to the control group, addition of CXCL12 induced a noticeable transwell migration of the neutrophil/monocyte-predominant cells and splenocytes beginning at 60 and 120 min, respectively, after the chemotactic stimulation (Figure 5). This suggests that the kinetics of CXCL12-elicited cellular response is different among lymphocytes, neutrophils and monocytes. However as shown in Figure 5, the transwell migration of these leukocytes was significantly inhibited by pretreatment with AMD3100 in a dose dependent manner. Furthermore, to rule out the possibility that potential cytotoxicity of AMD3100 could result in decreased cell migration, trypan blue assay did not reveal an increase of cell death in all AMD3100-treated groups. Likewise, DO11.10 splenocytes were pretreated with anti-mouse CXCR7 antibody (R and D Systems). The CXCR7 antibody (10 μg/ml) suppressed CXCL12-induced cell transwell migration at 2 hours for both neutrophils and splenocytes (Figure 6) but not at 3 hours (data not shown), revealing that the differential involvement of CXCR4 and CXCR7 in the timing of CXCRL12-mediated cell trafficking.
Figure 5.
AMD3100 suppresses CXCL12-induced transwell migration of leukocytes. DO11.10 splenocytes and neutrophil-predominant leukocytes were pre-incubated with AMD3100 for 30 min at 37°C. Then, these cells were placed in the top of the transwell, which was in contact with the medium containing CXCL12 in the bottom chamber. The top and bottom wells were separated by a filter membrane. At different time points, migrated cells in the bottom well were collected and counted (n = 3 per group). Note: CXCL12 significantly augmented transwell migration of T cell-predominant splenocytes (A) and neutrophil and monocyte-predominant leukocytes (B) (*p < 0.05). Furthermore, AMD3100 inhibited CXCL12-induced cell migration in a dose dependent manner (# p < 0.05).
Figure 6.
Anti-CXCR7 antibody attenuated CXCL12-induced transwell migration of leukocytes. T cell-predominant DO11.10 splenocytes and neutrophil-predominant leukocytes were pre-incubated with CXCR7 antibody (25 μg/ml) for 30 min at 37°C. Then, these cells were placed in the top of the transwell, which was in contact with the medium containing CXCL12 in the bottom chamber. The top and bottom wells were separated by a filter membrane. At 2 hour time point, migrated cells in the bottom well were collected and counted (n = 2 per group). Note: Anti-CXCR7 antibody attenuated CXCL12-induced chemotaxis of leukocytes. CXCL12 induced significant cell migration (*p < 0.05) and antibody treated cells migrated significantly less than those without antibody (*p < 0.05).
3.4. Attenuation of OVA-induced Uveitis by Blocking CXCR4 but Not CXCR7
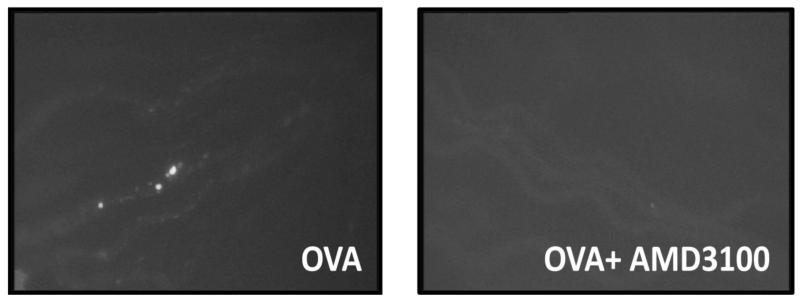
Next, we went further to confirm the role of CXCR4 and CXCR7 in OVA-induced uveitis, by comparing the severity of OVA-induced uveitis in mice treated with and without the CXCR4 inhibitor or CXCR7 antibody. Daily 100 μg injection of AMD per mouse was administered intraperitoneally for 2 consecutive days prior to the induction of uveitis. Twenty-four hours after intraveal challenge of OVA, circulating leukocytes were labeled with rhodamine, and ocular inflammation was assessed by intravital microscopy. Compared to OVA stimulation alone, AMD3100 significantly reduced rolling and adherent leukocytes in the vasculature of the eyes (Figures 7A and 7B). Interestingly, blocking CXCR4 appeared to suppress more adherent cells than rollers. This result further validates the role of CXCR4 in inflammatory cell trafficking during uveitis.
Figure 7.
Blocking CXCR4 by AMD3100 attenuates OVA-induced uveitis in DO11.10 mice. DO11.10 mice received 100 μg of AMD3100 intraperitoneally for consecutive 2 days (n=6 per group). Then, OVA was delivered intravitreally to induce uveitis. Twenty four hours later, circulating leukocytes were labeled with rhodamine, and ocular inflammatory cells were assessed by intravital microscopy. (A) Representative image of a single frame from intravital microscopy videos taken of the iris at 24 hours during the course of inflammation induced by OVA. Note: AMD3100 prevented OVA-induced inflammatory cell infiltration in the eye. (B) Quantification of rolling and adherent cells in the vasculature of the iris treated with and without AMD3100 antibody (*p < 0.05).
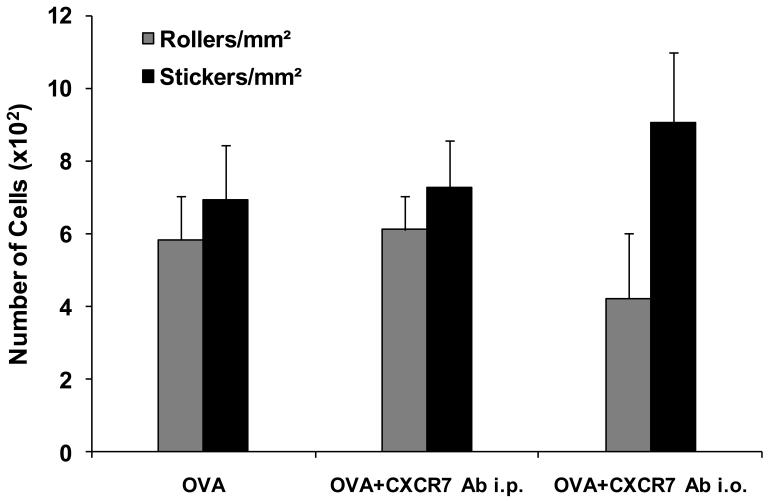
We also tested the effect of anti-CXCR7 antibody on OVA-induced uveitis. Recently, Mazzinghi et al. reported that treating mice with a single dose of 10 μg polyclonal anti-CXCR7 antibody (Abcam) inhibits transferred renal progenitor cell migration in vivo (Mazzinghi et al., 2008). In our experiments, DO11.10 mice received intraperitoneal injection of 15 μg the neutralizing polyclonal CXCR7 antibody once a day for 2 total days before and during intravitreal OVA challenge. To ensure the adequate amount of the antibody was delivered to local tissue, an additional group of DO11.10 mice received direct intravitreal injection of the anti-CXCR7 antibody (1.5 μg). The ocular inflammation was assessed 24 hours later. Although the CXCR7 antibody had attenuated CXCL12-induced cell migration in vitro, both systemic and local administrations of this neutralizing antibody did not significantly alter the severity of uveitis in vivo at 24 hours after OVA stimulation (Figure 8). This suggests that CXCR4 but not CXCR7 is primarily implicated in the development of uveitis in this model.
Figure 8.
Anti-CXCR7 antibody did not mitigate OVA-induced uveitis in DO11.10 mice. DO11.10 mice received a total of 30 μg of polyclonal CXCR7 antibody intraperitoneally over two days (n = 5) or 1.5 μg of CXCR7 antibody intravitreally (n = 3). Twenty four hours after intravitreal OVA challenge, circulating leukocytes were labeled with rhodamine, and ocular inflammatory cells were assessed by intravital microscopy. (A) Representative images of a single frame from intravital microscopy videos taken of the iris at 24 hours during the course of inflammation induced by OVA. Note: CXCR7 antibody did not significantly alter OVA-induced inflammatory cell infiltration in the eye. (B) Quantification of rolling and adherent cells in the vasculature of the iris treated with and without CXCR7 antibody.
3.5. No Change of Systemic Leukocyte Level by AMD3100
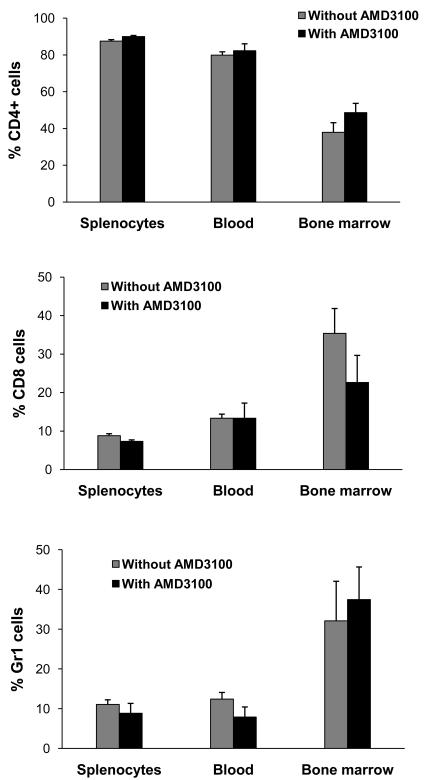
CXCR4/CXCL12 axis plays an important role in the mobilization of systemic leukocytes expressing CXCR4. In addition, interference of CXCR4-mediated G-protein signaling might affect cell proliferation and survival. Thus, AMD3100 could diminish the supply of inflammatory cells by reducing the number of circulating leukocytes. Therefore, we examined if blocking CXCR4 by AMD3100 decreased the proportion of systemically circulating lymphocytes or neutrophils. Peripheral leukocytes, splenocytes and bone marrow cells were isolated from the mice treated with or without AMD3100 as described above. These cells were further stained for CD4, CD8 and Ly6G, respectively. Flow-cytometry revealed no difference in the number of systemic T cells and neutrophils between control and AMD3100-treated animals (Figure 9). This indicates that the reduction of ocular inflammation by AMD3100 is not due to its effect on the production or systemic redistribution of leukocytes.
Figure 9.
AMD3100 does not affect the numbers of systemic leukocytes. DO11.10 mice were treated with or without AMD3100 for 2 days (n = 4 per group). Then, peripheral leukocytes, splenocytes and bone marrow cells were harvested and stained for CD4, CD8 and Ly6G, respectively. Note: AMD did not the change total percentage of systemic lymphocytes and neutrophils.
4. Discussion
In this study, we have shown that CXCR4 and CXCR7 are up-regulated in a unique antigen-specific uveitis model. Moreover, CXCR4 plays an important role in leukocyte trafficking during the ocular inflammation. Currently, there are several antigen specific models of uveitis. In addition to conventional experimental autoimmune uveitis, Gery and his colleagues developed transgenic mice expressing hen egg lysozyme (HEL) under the control of the alphaA-crystallin promoter. Adoptive transfer of these mice with the lymphocytes from syngeneic wild-type donors sensitized against HEL can induce subacute and chronic intraocular inflammation. The uveitis is mainly mediated by a Th1 response and exhibits neutrophil and monocyte-predominant infiltration (Lai et al., 1999; Kim et al., 2002). In addition, Gregerson et al. established transgenic mice expressing beta-galactosidase (β-gal) in the photoreceptor cells of the retina. They found that the transgenic mice with high level retinal β-gal expression developed autoimmune uveoretinitis after receiving T cells from normal mice immunized with β-gal (Gregerson et al., 1999). Compared to these models, direct injection of OVA into the eyes of DO11.10 mice causes a rapid onset of anterior uveitis and it does not require adoptive cell transfer. Most importantly, the anterior uveitis allows us to perform intravital microscopy, a powerful means to assess ocular leukocyte trafficking for the purpose of this study.
In various inflammatory responses, local invasion of leukocyte is a critical convergent step leading to consequent tissue damage, and the inflammatory cascade is determined by sequential influx of the leukocytes with different functions. For instance, in an adaptive immune response, antigen presenting cells recruit and interact with T helper cells first. The activated T cells then elicit additional components of the inflammatory response including attracting neutrophils and monocytes. Both human and animal studies have shown that activation of uveitogenic T lymphocytes and their effector cells plays a pivotal role in the pathogenesis of uveitis (Zhang et al, 2009). In this study, we found a rapid invasion of T cells in the eyes of DO11.10 mice as early as 9 hours after OVA challenge. This T cell response is essential for the ocular infiltration of neutrophils and monocytes (Zhang et al, 2009). Theoretically, the majority of CD4+ T cells in DO11.10 mice carry a transgenic T cell receptor that specifically reacts with OVA. Thus, it is unclear whether the kinetics of T cell response in this uveitis model is comparable to that in non-transgenic mice that have far fewer total antigen specific lymphocytes. Nevertheless, DO11.10 mice have been commonly used to study many inflammatory diseases. McGargill et al. generated double transgenic mice that express OVA peptide under a control of human keratin 14 promoter as well as a TCR specific for this antigen. These animals spontaneously developed severe skin lesion and lethal inflammation (McGargill et al., 2002). In an asthmatic model, Wilder and her colleagues reported that compared to OVA-primed BALB/c mice, DO11.10 mice do not develop eosinophilia or OVA specific IgE in response to OVA aerosol exposure (Wilder et al., 2001). In fact, these DO11.10 mice display an early T cell activation and neutrophil infiltration in the lung (Wilder et al., 2001). This study appears to be consistent with our observation of neutrophil and monocyte-dominated response in the uveitis at 24 hours after OVA challenge.
The local invasion of inflammatory cells is a complicated process temporally and tightly orchestrated by many immune mediators and cellular molecules. First, patrolling leukocytes are signaled and attracted to the target tissue. To set a stage for the leukocyte extravasation, expression of selectin causes cell rolling (or slowdown) along the vascular surface (Ley et al., 2007). Then, chemokines, integrins and other adhesive molecules all participate in the facilitation of cell activation, firm adhesion and transmigration (Ley et al, 2007). This multistep cascade is indispensable for the exit of the inflammatory cells from the systemic circulation to the tissue. Recently, cumulative evidence suggests that CXCR4, CXCR7 and their specific ligand, CXCL12, play an important role in the cell trafficking (Jourdan et al., 2000; Broxmeyer et al., 2007; Ding et al., 2003).
In this study, we demonstrated the increase of CXCR4 and CXCR7 transcripts in OVA-induced uveitis. Nevertheless, the expression kinetics of these 2 chemokine receptors is different. As shown by RT-PCR, the peak of CXCR7 transcription appears to occur earlier and diminish at 24 hours when CXCR4 reaches its maximal expression. This observation is consistent with a recent report (Wang et al., 2008). In that study, Wang et al. found that overexpression of CXCR4 reduces CXCR7 level. However, a reciprocal regulation of CXCR4 by CXCR7 was not observed as alteration of CXCR7 did not affect CXCR4 expression (Wang et al., 2008). These data indicate that CXCR4 influences CXCR7 production and possibly its function.
Several studies showed that CXCR7 is involved in cell trafficking and migration (Burns et al., 2006; Law and Rosenzweig, 1994; Mazzinghi et al., 2008; Thelen and Thelen, 2008; Wang et al., 2008). In this study, anti-CXCR7 antibody reduced CXCL12-induced transwell migration, suggesting that the antibody is functional in terms of its activity of blocking the receptor. However, systemic and intraocular administrations of anti-CXCR7 antibody at an optimal dose did not significantly mitigate intraocular leukocyte infiltration. In addition, Hartmann et al. reported that CXCR7 is dispensable for CXCL12-triggered signaling in human T lymphocytes. Nevertheless, CXCR7 blockers attenuate CXCR4-mediated integrin activation (Hartmann et al., 2008), suggesting that CXCR7 plays an adjunctive role in cell migration. These findings indicate that CXCR7 plays a disposable role in the early inflammatory process during OVA-induced uveitis. The discrepancy between our observation and other reports could be due to the differential involvement of CXCR7 in different tissues and disease settings.
CXCR4 is an inducible chemokine receptor expressed by lymphocytes, neutrophils and monocytes. However, naïve T cells express CXCR4 before they are differentiated to different T helper lineage (Bromley et al., 2008). In this study, we found no baseline CXCR4 mRNA expression in normal eyes. The timing of appearance of CXCR4 signal is correlated nicely with that of leukocyte invasion in OVA-induced uveitis, and CXCR4 transcription is further enhanced at 24 hours when marked cell infiltration was observed. The temporal relationship between CXCR4 mRNA induction and leukocyte trafficking suggests a role of CXCR4 in cell migration. Recent studies demonstrate that CXCR4 is essential for cell homing (Juarez et al, 2004; Lataillade et al., 2004; Möhle and Moore, 1998; Möhle and Rafii, 1999).
CXCL12 is a CXCR4 specific ligand, which is constitutively expressed in the bone marrow and by the endothelial cells. Here, we showed that the normal eye produces baseline CXCL12, and the antigen stimulation enhanced the CXCL12 expression. This suggests that normal eye and the endothelial cells of ocular vasculature express CXCL12 in anticipation of the arrival of activated CXCR4+ cells. Moreover, the increase of CXCL12 transcripts during ocular inflammation further facilitates leukocyte trafficking/migration. The interaction between CXCR4 and CXCL12 plays an important role in cell migration and other biological functions (Epstein, 2004; Juarez et al., 2004; Lataillade et al., 2004; Möhle and Moore, 1998; Möhle and Rafii, 1999). Thus it is referred as “CXCR4/CXCL12 axis”.
The production and release by the bone marrow as well as the extent of transendothelial migration determine the number of leukocytes accumulating in inflamed tissue. Numerous studies have demonstrated that CXCR4/CXCL12 axis is essential for the regulation of bone marrow mobilization of stem cells and neutrophils (Christopher and Link, 2007; Aiuti et al., 1999). However, our study showed that blocking CXCR4 within 24 hours did not change the leukocyte composition of peripheral blood, and inhibition of transmigration contributes to the reduction of OVA-induced uveitis by the CXCR4 inhibitor.
AMD3100 is a bicyclam compound. It has been shown to be a specific CXCR4 antagonist by interacting with aspartic acid residues at position 171, 182, 193, and 262 of the chemokine receptor (DeClercq, 2003). Thus, AMD3100 prevents the docking of CXCR4 with CXCL12, thereby inhibiting CXCR4-mediated signaling and function (DeClercq, 2003). AMD3100 has been successfully tested in treating HIV, which utilizes CXCR4 as one of its co-receptors for entering host cells (DeClercq, 2003). Furthermore, AMD3100 is effective for suppressing inflammation in both experimental arthritis and asthma (DeClercq, 2003; Epstein, 2004). Here, we report that blocking CXCR4 by AMD3100 also mitigated OVA-induced uveitis. Using intravital microscopy, we were able to assess the effect of AMD3100 on the ocular trafficking of leukocytes. Although it attenuated both rolling and adherent cells, AMD3100 appears to have a more profound suppression on adherent cells. This implies that CXCR4 is involved in both rolling and adhesion processes. However, adhesion requires more involvement of CXCR4. CXCR4 is a G protein-coupled receptor. G protein-mediated signaling is also intertwined with apoptosis pathways. CXCL12 is shown to promote cell proliferation through CXCR4 (Hartmann et al., 2005; Vlahakis et al., 2002). However, we did not observe significant cell death or cytotoxicity even after the cells were exposed to 100 nM AMD3100. Our result suggests that suppression of leukocyte transmigration is related to direct blockage of the engagement between CXCR4 and its ligand.
Taken together, we previously established an antigen-induced uveitis model (Zhang et al., 2009). Using this model, we have demonstrated an up-regulation of CXCR4 and CXCR7 in the eye. Echoing the complexity of leukocyte migration, our study reveals novel functional discrepancies in immune uveitis resulting from in vivo disruption of signaling through CXCR 4 versus CXCR7
CXCR4 plays a major role in infiltration of different leukocyte subsets during early ocular inflammation. Blocking CXCR4 by AMD3100 suppresses transmigration of lymphocyte, neutrophils and monocytes, leading to attenuation of uveitis. This study not only enhances our knowledge of the immunopathological mechanism of uveitis but also tests the feasibility of targeting CXCR4 as a novel therapeutic strategy for the control of ocular inflammation.
Acknowledgments
This study was supported by National Institutes of Health Grants EY016788 (ZZ), EY013093 (JTR), and EY006484 (JTR). JTR is a Scholar supported by Research to Prevent Blindness. Funds from the Stan and Madelle Rosenfeld Family Trust also contributed to this work.
References
- Aiuti A, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999;29(6):1823–31. doi: 10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bajetto A, et al. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22(3):147–84. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- Balabanian K, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760–6. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- Becker MD. The role of T cells in autoimmune uveitis. Ocul Immunol Inflamm. 2000;8(2):93–100. [PubMed] [Google Scholar]
- Becker MD, et al. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci.Oct. 2001;42(11):2563–6. [PubMed] [Google Scholar]
- Becker MD, et al. Intraocular in vivo imaging of activated T-lymphocytes expressing green-fluorescent protein after stimulation with endotoxin. Graefes Arch Clin & Exp Ophthalmol. 2001;239(8):609–12. doi: 10.1007/s004170100320. [DOI] [PubMed] [Google Scholar]
- Bleul CC, et al. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184(3):1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9(9):970–80. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, et al. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. Ann N Y Acad Sci. 2007;1106:1–19. doi: 10.1196/annals.1392.013. [DOI] [PubMed] [Google Scholar]
- Burns JM, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, et al. Expression of chemokine receptors, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology. 2003;110(2):421–6. doi: 10.1016/S0161-6420(02)01737-2. [DOI] [PubMed] [Google Scholar]
- Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14(1):3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2(7):581–7. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- Ding Z, et al. TK. L-selectin stimulation enhances functional expression of surface CXCR4 in lymphocytes: implications for cellular activation during adhesion and migration. Blood. 2003;101(11):4245–52. doi: 10.1182/blood-2002-06-1782. [DOI] [PubMed] [Google Scholar]
- Dullforce PA, et al. Antigen-specific accumulation of naive, memory and effector CD4 T cells during anterior uveitis monitored by intravital microscopy. Cellular Immunology. 2006;239(1):49–60. doi: 10.1016/j.cellimm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Epstein RJ. The CXCL12-CXCR4 chemotactic pathway as a target of adjuvant breast cancer therapies. Nat Rev Cancer. 2004;4(11):901–9. doi: 10.1038/nrc1473. [DOI] [PubMed] [Google Scholar]
- Faust N, et al. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96(2):719–26. [PubMed] [Google Scholar]
- Feng Y, et al. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272(5263):872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Fernandis AZ, Cherla RP, Ganju RK. Differential regulation of CXCR4-mediated T-cell chemotaxis and mitogen-activated protein kinase activation by the membrane tyrosine phosphatase, CD45. J Biol Chem. 2003;278(11):9536–43. doi: 10.1074/jbc.M211803200. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, et al. Retinal expression of a neo-self antigen, beta-galactosidase, is not tolerogenic and creates a target for autoimmune uveoretinitis. J Immunol. 1999;163(2):1073–80. [PubMed] [Google Scholar]
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500. doi: 10.1016/j.ophtha.2003.06.014. discussion 500. [DOI] [PubMed] [Google Scholar]
- Haringman JJ, et al. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Ann Rheum Dis. 2006;65(3):294–300. doi: 10.1136/ard.2005.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann TN, et al. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24(27):4462–71. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- Hartmann TN, et al. A crosstalk between intracellular CXCR7 and CXCR4 involved in rapid CXCL12-triggered integrin activation but not in chemokine-triggered motility of human T lymphocytes and CD34+ cells. J Leukoc Biol. 2008;84(4):1130–40. doi: 10.1189/jlb.0208088. [DOI] [PubMed] [Google Scholar]
- Jourdan P, et al. Cytokines and cell surface molecules independently induce CXCR4 expression on CD4+ CCR7+ human memory T cells. J Immunol. 2000;165(2):716–24. doi: 10.4049/jimmunol.165.2.716. [DOI] [PubMed] [Google Scholar]
- Juarez J, Bendall L, Bradstock K. Chemokines and their receptors as therapeutic targets: the role of the SDF-1/CXCR4 axis. Curr Pharm Des. 2004;10(11):1245–59. doi: 10.2174/1381612043452640. [DOI] [PubMed] [Google Scholar]
- Ke Y, et al. Suppression of established experimental autoimmune uveitis by anti-LFA-1alpha Ab. Invest Ophthalmol Vis Sci. 2007;48(6):2667–75. doi: 10.1167/iovs.06-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, et al. Induction of ocular inflammation by T-helper lymphocytes type 2. Invest Ophthalmol Vis Sci. 2002;43(3):758–65. [PubMed] [Google Scholar]
- Lai JC, et al. Uveitis induced by lymphocytes sensitized against a transgenically expressed lens protein. Invest Ophthalmol Vis Sci. 1999;40(11):2735–9. [PubMed] [Google Scholar]
- Law NM, Rosenzweig SA. Characterization of the G-protein linked orphan receptor GPRN1/RDC1. Biochem Biophys Res Commun. 1994;201(1):458–65. doi: 10.1006/bbrc.1994.1723. [DOI] [PubMed] [Google Scholar]
- Lataillade JJ, Domenech J, Le Bousse-Kerdilès MC. Stromal cell-derived factor-1 (SDF-1)\CXCR4 couple plays multiple roles on haematopoietic progenitors at the border between the old cytokine and new chemokine worlds: survival, cell cycling and trafficking. Eur Cytokine Netw. 2004;15(3):177–88. [PubMed] [Google Scholar]
- Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Lim LL, et al. Intravital microscopy of leukocyte-endothelial dynamics using the Heidelberg confocal laser microscope in scleritis and allergic conjunctivitis. Molecular Vision. 2006;12:1302–5. [PubMed] [Google Scholar]
- Link DC. Neutrophil homeostasis: a new role for stromal cell-derived factor-1. Immunol Res. 2005;32(13):169–78. doi: 10.1385/IR:32:1-3:169. [DOI] [PubMed] [Google Scholar]
- Loetscher M, et al. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269(1):232–7. [PubMed] [Google Scholar]
- Martin TM, Smith JR, Rosenbaum JT. Anterior uveitis: current concepts of pathogenesis and interactions with the spondyloarthropathies. Curr Opin Rheumatol. 2002;14(4):337–341. doi: 10.1097/00002281-200207000-00001. [DOI] [PubMed] [Google Scholar]
- Mazzinghi B, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205(2):479–90. doi: 10.1084/jem.20071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGargill MA, et al. A spontaneous CD8 T cell-dependent autoimmune disease to an antigen expressed under the human keratin 14 promoter. J Immunol. 2002;169(4):2141–7. doi: 10.4049/jimmunol.169.4.2141. [DOI] [PubMed] [Google Scholar]
- Mempel TR, et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25(1):129–41. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Möhle R, Rafii S, Moore MA. The role of endothelium in the regulation of hematopoietic stem cell migration. Stem Cells. 1998;16(Suppl 1):159–65. doi: 10.1002/stem.5530160819. [DOI] [PubMed] [Google Scholar]
- Möhle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. Regulation of transendothelial migration of hematopoietic progenitor cells. Ann N Y Acad Sci. 1999 Apr 30;872:176–85. doi: 10.1111/j.1749-6632.1999.tb08463.x. discussion 185-6. [DOI] [PubMed] [Google Scholar]
- Mikami S, et al. Blockade of CXCL12/CXCR4 axis ameliorates murine experimental colitis. J Pharmacol Exp Ther. 2008 Nov;327(2):383–92. doi: 10.1124/jpet.108.141085. [DOI] [PubMed] [Google Scholar]
- Mulligan MS, et al. Cytokine and adhesion molecule requirements for neutrophil recruitment during glycogen-induced peritonitis. Inflamm Res. 1998;47(6):251–5. doi: 10.1007/s000110050326. [DOI] [PubMed] [Google Scholar]
- Oberlin E, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382(6594):833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- Ottoson NC, et al. Cutting edge: T cell migration regulated by CXCR4 chemokine receptor signaling to ZAP-70 tyrosine kinase. J Immunol. 2001;167(4):1857–61. doi: 10.4049/jimmunol.167.4.1857. [DOI] [PubMed] [Google Scholar]
- Patrussi L, Baldari CT. Intracellular mediators of CXCR4-dependent signaling in T cells. Immunol Lett. 2008;115(2):75–82. doi: 10.1016/j.imlet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JT, et al. T cell-antigen-presenting cell interactions visualized in vivo in a model of antigen-specific inflammation. Clin Immunol. 2007 doi: 10.1016/j.clim.2007.10.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeber DA, Tedder TF. Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol Res. 2000;22(23):299–317. doi: 10.1385/IR:22:2-3:299. [DOI] [PubMed] [Google Scholar]
- Thelen M, Thelen S. CXCR7, CXCR4 and CXCL12: an eccentric trio? J Neuroimmunol. 2008;198(12):9–13. doi: 10.1016/j.jneuroim.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Vlahakis SR, et al. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169(10):5546–54. doi: 10.4049/jimmunol.169.10.5546. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283(7):4283–94. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- Wilder JA, et al. Ovalbumin aerosols induce airway hyperreactivity in naïve DO11.10 T cell receptor transgenic mice without pulmonary eosinophilia or OVA-specific antibody. J Leukoc Biol. 2001;69(4):538–47. [PubMed] [Google Scholar]
- Xu H, et al. Leukocyte trafficking in experimental autoimmune uveitis: breakdown of blood-retinal barrier and upregulation of cellular adhesion molecules. Invest Ophthalmol Vis Sci. 2003;44(1):226–34. doi: 10.1167/iovs.01-1202. [DOI] [PubMed] [Google Scholar]
- Xu H, et al. Recruitment of IFN-gamma-producing (Th1-like) cells into the inflamed retina in vivo is preferentially regulated by P-selectin glycoprotein ligand 1:P/E-selectin interactions. J Immunol. 2004;172(5):3215–24. doi: 10.4049/jimmunol.172.5.3215. [DOI] [PubMed] [Google Scholar]
- Zhang Z, et al. Interleukin-17 Causes Neutrophil Mediated Inflammation in Ovalbumin-Induced Uveitis in DO11.10 Mice. Cytokine. 2009 doi: 10.1016/j.cyto.2008.12.019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]