Abstract
Coding of the complex tastes of ionic stimuli in humans was studied by combining taste confusion matrix (TCM) methodology and treatment with chlorhexidine gluconate. The TCM evaluates discrimination of multiple stimuli simultaneously. Chlorhexidine, a bis-biguanide antiseptic, reversibly inhibits salty taste and tastes of a subset of bitter stimuli, including quinine hydrochloride. Identifications of salty (NaCl, “salt”), bitter (quinine·HCl, “quinine”), sweet (sucrose, “sugar”), and sour (citric acid, “acid”) prototypes, alone and as components of binary mixtures, were measured under 4 conditions. One was a water-rinse control and the others had the salt and quinine tastes progressively reduced by treatment with 1 mM chlorhexidine, 3 mM chlorhexidine, and ultimately to zero by elimination of NaCl and quinine·HCl. Treatment with chlorhexidine perturbed identification of salt more than quinine; both were thereafter more often confused with “water” and unidentified when mixed with sucrose or citric acid. All pairwise discriminations that depended on the tastes of NaCl and quinine·HCl deteriorated, and although H2O was mistakenly identified as quinine after chlorhexidine, this may have been a decisional bias. Other confusions reflected “unprompted mixture analysis” and an obscuring of salt taste by a less-inhibited stronger quinine or sugar or acid tastes in mixtures. Partial inhibition of the tastes of NaCl and quinine·HCl by chlorhexidine was considered in the context of multiple receptors for the 2 compounds. Discrimination among prototypic stimuli with varying strengths was consistent with a gustatory system that evaluates a small number of independent tastes.
Keywords: binary mixtures, dynamic mixture analysis, mixture suppression, quinine inhibition, salt inhibition, taste confusion matrix
Introduction
Coding of ionic tastes in humans remains a puzzle that a taste inhibitor could help solve through documentation of perceptual consequences coinciding with loss of specific tastes (Gent et al. 1999, 2002). Behavioral studies of genetic “knockouts” missing T1R and T2R receptors have led to analogous progress for sweet and nonionic bitter tastes in animals (Chandrashekar et al. 2006; Frank et al. 2008). The diuretic amiloride, which blocks epithelial Na+ channels (ENaCs), has proven to be a powerful experimental tool (Brand et al. 1985; DeSimone and Ferrell 1985; Ninomiya and Funakoshi 1988; Hettinger and Frank 1990; Schiffman et al. 1990). Amiloride inhibition revealed 2 tastes of NaCl in rodents. Besides the inhibited Na+-specific taste, an amiloride resistant “bitter” taste difficult to distinguish from KCl, NH4Cl, or quinine·HCl is elicited by NaCl (Frank and Nowlis 1989; Hill et al. 1990; Frank 2008).
The salty prototype, NaCl, has a distinct taste to humans that is easily discriminated from the salty–bitter KCl and NH4Cl (McBurney and Shick 1971; Frank et al. 2001). Unfortunately, amiloride has little impact in humans (Ossebaard and Smith 1995, 1996). And although NaCl adaptation reduces all saltiness (Smith and McBurney 1969; Smith and van der Klaauw 1995), it has “side” tastes, a sweet taste at threshold (Bartoshuk et al. 1978) and a sour taste at moderately strong concentrations (Ossebaard and Smith 1995, 1996).
Chlorhexidine gluconate (CHX), a substantive (Briner et al. 1994), bitter, bis-biguanide cationic antiseptic (Gilbert and Moore 2005), reversibly blunts the human salty taste for hours at concentrations used to control periodontal disease (Helms et al. 1995; Frank et al. 2001). Like sweet stimuli following gymnemic acid (Gent et al. 1999), salty ionic stimuli lose their identities following CHX treatment (Gent et al. 2002). The persistent salty-taste hypogeusia matches the strongly positively charged CHX's persistent antimicrobial activity, which depends on tight binding to mucous membranes (Jones 1997). The slowly developing effect of CHX on cationic salty stimuli suggests its binding may reconfigure oral epithelial structure to block the transport required for salty-taste transduction (Breslin and Tharp 2001; Frank et al. 2001; Hettinger and Frank 2009). A corresponding slow “increase” in hamster chorda tympani baseline neural activity occurs during a 5-min 1 mM CHX rinse; however, magnitudes of neural responses to NaCl were unaffected (Hettinger et al. 2001).
CHX inhibition (Grover and Frank 2008), combined with taste confusion matrix (TCM) methodology (Hettinger et al. 1999) to measure identification of multiple taste stimuli simultaneously (Gent et al. 1999, 2002), may prove to be a powerful experimental strategy for efficient study of the multiple tastes of ionic stimuli in humans. The human gustatory system may have multiple NaCl-sensing receptors. CHX, applied locally, reduces NaCl intensity and alters its quality at oral regions innervated by cranial nerve (CN) VII more than at regions innervated by CN IX (Grover and Frank 2008). Analogous regional differences in the receptors involved in the detection of NaCl are well established in rodents (Formaker and Hill 1991; Kitada et al. 1998). Amiloride treatment inhibits a single class of CN VII, rodent afferent neuron. Salt responses of other CN VII and most CN IX afferents are unaffected (Ninomiya and Funakoshi 1988; Hettinger and Frank 1990; Formaker and Hill 1991; Kitada et al. 1998; Sollars and Hill 1998; Lundy and Contreras 1999).
Adaptation to bitter antimicrobial quinine hydrochloride, a compound reported to have local anesthetic and irritant properties (Bateman and Dyson 1986; AlKadi 2007), does not affect all bitter ligands (McBurney et al. 1972; Keast and Breslin 2002), perhaps because there are multiple human T2R receptors (Delwiche et al. 2001; Bufe et al. 2002; Kuhn et al. 2004; Hansen et al. 2006). Nonetheless, quinine adapts the bitter taste of CHX, and CHX treatment reduces quinine's taste while sparing bitter tastes of monovalent and divalent salts, as well as the distinct tastes of sweet or sour substances (Helms et al. 1995; Breslin and Tharp 2001; Frank et al. 2001). The strong effect of CHX on quinine taste may be a persistent sensory “adaptation” between the bitter amphiphilic CHX and other amphiphilic bitter ligands (Helms et al. 1995; Breslin and Tharp 2001; Frank et al. 2001). Persistent action and rapid self-adaptation make CHX's taste difficult to study. However, a 5-min treatment with 10 mM quinine·HCl followed by a 4-min water rinse reduced quinine's taste, but less than CHX treatment did, and left NaCl's taste unchanged (Breslin and Tharp 2001).
Quinine·HCl was tasteless at sites innervated by CN VII following CHX treatment but retained a bitter quality at CN IX sites (Grover and Frank 2008). Analogous regional differences in detecting bitter stimuli are well established in rodents (Frank 1991; Frank et al. 2004; Geran and Travers 2006; Travers and Geran 2009). However, sensitivity to particular bitter ligands is quite species dependent in mammals (Hettinger et al. 2007), reflecting great T2R evolutionary volatility (Shi and Zhang 2006). Bitter quinine·HCl, detected at 9 μM by humans (Schiffman et al. 1994), is detected at 300 μM by golden hamsters (Frank et al. 2004); hamsters also find CHX aversive at that level (Hettinger et al. 2001, 2007).
One aim of the present study was to see whether raising CHX concentration would increase inhibitory effects that would remain specific to NaCl and quinine·HCl. The selective inhibition of CHX would simplify the main aim: testing for the independence of human taste qualities. Interpretation requires consideration of known side tastes of NaCl and citric acid (McBurney and Shick 1971; McBurney et al. 1972; van der Klaauw and Smith 1995) and taste mixture suppression, which, in binary mixtures, especially weakens faint component tastes (Bartoshuk 1975; Frijters and Schifferstein 1994; Oram et al. 2001; Watson et al. 2001; Laing et al. 2002; Marshall et al. 2005; Frank 2008). Like humans, golden hamsters are more likely to detect both component tastes in binary mixtures if the tastes are equally intense (Nowlis and Frank 1981; Smith and Theodore 1984; Frank et al. 2003).
Treatment with 1.34 mM CHX reduces taste intensities of 100 mM NaCl and 0.1 mM quinine·HCl by 75% (Frank et al. 2001) and increases their misidentification, measured within the framework of a 10-stimulus TCM-experimental design. Under these conditions, there also is an increase in mislabeling H2O as the obtunded stimuli (Gent et al. 2002; Johar 2006). Two water-rinse controls were included in the present study. In the basic control, subjects were presented the 10 stimuli. In the “positive” control, NaCl and quinine·HCl were replaced by H2O to simulate a complete obliteration of NaCl and quinine·HCl tastes. This control determined the performance limit, given a complete and specific inhibition of NaCl and quinine tastes, and also tested whether subjects may try to use all 10 possible responses when confronted with multiple samples of the remaining 300 mM sucrose, 3 mM citric acid, and H2O.
Ten stimuli, including binary mixtures, each associated with a unique veridical response label, were presented 10 times, yielding a 10 × 10 TCM for each subject for the basic water control, 1 mM CHX treatment, 3 mM CHX treatment, and the positive water control, which progressively “dilute” the tastes of NaCl and quinine·HCl. Consistency of stimulus identification and pairwise stimulus discriminability, measured in bits of information (Attneave 1959), were computed from each TCM (Hettinger et al. 1999). In conjunction with patterns of errors, these information theoretic measures were used to determine how the gustatory system evaluates prototypic stimuli with strong or weak tastes, encountered alone or in binary mixtures. Unlike the visual system's evaluation of colors, each of which is mediated by outputs representing comparisons among the broad stimulus spectra of 3 cones, results were hypothesized to be consistent with the gustatory system's evaluation of a small number of tastes independently (Frank 2008).
Materials and methods
Subjects
Fourteen university students and research staff (10 women and 4 men, nonsmokers), aged 18–37 (mean ± standard deviation = 25.8 ± 5.5 years), were compensated for participation in this study, which was approved by Institutional Review Boards of Yale University and the University of Connecticut Health Center. One individual served as a dummy subject, blind to the test-stimulus arrangements, in order to train experimenters by familiarizing them with the experimental paradigm. Data are presented on the other 13 subjects. None of the subjects reported seasonal allergies or a history of taste or smell problems.
Stimulus sets and treatment rinses
The complete set of 10 test-stimulus compounds (Table 1), dissolved in deionized water, included 4 single components (component [label]): 300 mM sucrose [“sugar”], 3 mM citric acid [“acid”], 100 mM sodium chloride [“salt”], and 0.1 mM quinine hydrochloride [“quinine”], all possible binary mixtures of the single components (except sucrose plus citric acid) and deionized H2O [“water”]. Component concentrations were chosen to approximate the perceptual intensity of 100 mM NaCl (Frank et al. 2001), and mixture-component and single-component concentrations were equal. Average published component intensity ratings, all labeled “medium” on a 0–9 fixed interval scale, were 5.8 ± 0.4 for sucrose, 4.8 ± 0.1 for NaCl, 4.5 ± 0.5 for quinine·HCl, and 4.4 ± 0.2 for citric acid (Frank et al. 2001). To simulate total inhibition of NaCl and quinine·HCl, a “without NaCl or Quinine” (‘w/o-NQ’), 10-stimulus set was constructed in which all NaCl and quinine·HCl components were extracted. In this simulated positive control, both the sucrose + NaCl mix and sucrose + quinine·HCl mix, for example, were replaced by pure sucrose. Treatment rinses with 1 mM CHX and 3 mM CHX preceded stimulus testing with the complete set. Control rinses with deionized H2O preceded testing with either the complete (H2O(1)) or the w/o-NQ set (H2O(2)) (Table 1).
Table 1.
Rinses, test-stimulus sets, and labels
| Treatment rinse |
Control rinse |
Correct response | ||
| Chlorhexidine |
H2O(1) |
H2O(2)a |
||
| 1 mM | 3 mM | 0 mM | ||
| Complete test-stimulus set | w/o NaCl & quinine | Label | ||
| A. 100 mM NaCl (N) | H2O (W1) | Salt | ||
| 0.1 mM Quinine·HCl (Q) | H2O (W2) | Quinine | ||
| NaCl + quinine·HCl (NQ) | H2O (W3) | Salt + quinine | ||
| B. NaCl + sucrose (NS) | Sucrose (S1) | Salt + sugar | ||
| NaCl + citric acid (NH) | Citric acid (H1) | Salt + acid | ||
| Quinine·HCl + sucrose (QS) | Sucrose (S2) | Quinine + sugar | ||
| Quinine·HCl + citric acid (QH) | Citric acid (H2) | Quinine + acid | ||
| C. 300 mM sucrose (S) | Sucrose (S3) | Sugar | ||
| 3 mM citric acid (H) | Citric acid (H3) | Acid | ||
| H2O (W) | H2O (W4) | Water | ||
Letters in parentheses are abbreviations for stimuli used in text.
H2O(2) is a “positive control,” simulating total loss of NaCl and quinine·HCl tastes, containing 4 W (W1-W4), 3 S (S1-S3) and 3 H (H1-H3).
Psychophysical methods
Each subject participated in 4 sessions, presented in a counterbalanced order, to accommodate the 2 CHX treatment and the 2 H2O control rinse conditions. Sessions occurred on 4 different days at least 2 days apart to minimize possible persistence of CHX effects (Frank et al. 2001). Prior to each test session, subjects were trained to associate test stimuli with their labels with 2 replicates of the complete set of 10 stimuli. In the testing phase, each subject was presented with 10 replicates of the test stimuli (Table 1) without feedback (Hettinger et al. 1999). Subjects sampled 15 mL of stimuli for a few seconds using a “sip and spit” method. Stimuli, presented in random order within replicates, were paced at 1 per minute, with subjects washing their mouths several times with H2O following each stimulus.
Subjects were asked to identify stimuli from the list of 10 labels (“correct” responses) provided (Table 1). Before testing with replicate 1 and again before replicate 6, subjects rinsed (3 times, each for 1 min, 20-s pauses between rinses) with 15-mL aliquots of CHX or H2O. The rinses were repeated before replicate 6 to ensure effects were retained throughout the 1-h session (Gent et al. 2002). Following rinses, subjects washed their mouths thoroughly with H2O and, after a 5-min waiting period to allow any nonspecific discomfiture to abate (Frank et al. 2001), testing began. Subjects were entirely blind to the test-stimulus arrangements for each condition and were instructed that stimuli presented during training may or may not be presented in the testing session. The procedure resulted in a 10 stimulus by 10-response TCM for each subject for each condition, that is, 4 TCMs per subject.
Data analysis
Measures of “percent correct,” “information transmitted,” and “error response frequency” were calculated from the TCM. Average values are given as means ± standard errors (SEs) unless otherwise indicated.
Percent correct
Percent correct, the ratio of correct responses to total number of presentations × 100, measures the accuracy with which the subjects apply the labels correctly to the stimuli. Two-way repeated-measures analysis of variance (ANOVA), followed by planned comparisons, were used to evaluate the effect of CHX on correct identification. The 2 factors in the initial ANOVA were rinse (0 mM [H2O], 1, and 3 mM CHX) and stimulus (10 stimuli, the “complete” set listed in Table 1). Two additional 2-way ANOVAs separately evaluated the effect of CHX on identification of the 7 stimuli containing NaCl and/or quinine·HCl and the 3 stimuli containing neither NaCl nor quinine·HCl. A 1-way ANOVA evaluated ratios of correct identifications (%) following treatment with CHX to corresponding values following H2O multiplied by 100 to yield a percentage of control identification for the 3 subsets of stimuli listed in Table 1A–C.
Information theoretic measures: T10, T2, and T3
Information theory does not assume labels are used correctly; rather, it measures whether responses are used consistently (without overlap). From each 10 × 10 TCM, overall consistency of performance, T10, and “discriminations” of subsets of stimuli, T2 and T3, were derived (Gent et al. 1999, 2002; Hettinger et al. 1999). T10 is the average bits of information transmitted from the 10 test stimuli to the 10 responses (Attneave 1959). For equally likely stimulus alternatives and equally favored response alternatives, perfectly consistent identification would give a T10 of 3.32 bits (log2 10). By way of contrast, simulations of wholly random performance yield an average T10 of 0.70 bit (Hettinger et al. 1999). The measure T2 quantifies, in bits of information transmitted by any given pair of stimuli, pairwise stimulus discriminability and is derived from any 2 × 10 submatrix. For any 2 stimuli selected from the 10, nonoverlapping responses would give a T2 of 1.0 bit, and simulations of random performance give an average T2 of 0.40 bit (Hettinger et al. 1999). By analogy, the measure T3 quantifies “triad discriminability” derived from any 3 × 10 submatrix. For stimulus triads, perfect information transmission gives a T3 of 1.585 bits, and, based on 1000 trials, simulated random performance gives an average T3 of 0.53 ± 0.004 bit. Formulae for calculating transmitted information are in Hettinger et al. (1999).
A 1-way repeated-measures ANOVA, followed by planned comparisons, assessed the effect of CHX on overall response consistency, T10, with rinse (0, 1, and 3 mM CHX) the single factor. Values of T10 in the positive control, w/o-NQ condition were separately evaluated.
For analysis of T2, the 45 possible pairs of the 10 stimuli were divided into 2 groups: pairs that were predicted to become “indistinguishable” after CHX treatment and pairs that were predicted to remain “distinguishable” after CHX had removed the tastes of NaCl and quinine·HCl (Gent et al. 2002). T2 for each of 12 indistinguishable pairs was predicted to approach 0.40 bit, consistent with random performance. After CHX treatment, the subjects, for example, should have trouble identifying the NaCl + sucrose mixture as different from sucrose itself. In contrast, T2 for discrimination of each of the 33 distinguishable pairs should approach 1.0 bit under any of the conditions. After H2O or CHX rinse, for example, the subjects should not have trouble identifying the NaCl + citric acid mixture as different from sucrose.
CHX’ effect on values of T2 was examined for average indistinguishable and average distinguishable pairs in separate 1-way repeated-measures ANOVA with treatment rinse (0, 1, and 3 mM CHX) as the within-subjects factor. Measures of T2 were averaged for all indistinguishable and for all distinguishable pairs, for each rinse condition for each subject. Also, the effect of CHX on distinguishable pairs having low (<0.80) T2 control values was tested similarly with a 1-way repeated-measures ANOVA to test for possible post-CHX improvement. ANOVAs were followed by planned comparisons. t-Tests were used to evaluate changes in T3, post-CHX discriminability among sucrose, citric acid, and H2O.
Error response frequencies, consistencies in off-label responses
Patterns of incorrect responses were examined to address side tastes of prototypical stimuli, mixture interactions (with and without treatment by CHX), and effects of stimulus context (basic control vs. simulated positive control).
Differences in aggregate response frequencies were analyzed with a series of post hoc contrasts using χ2 tests. Overall, 66 2 × 2 tables were evaluated, yielding a corrected significant P = 0.05/66 (<0.0008). Using the Bonferroni method for correction assures that α will be no greater than 0.05.
Finally, data from the positive control were used to evaluate how subjects distributed responses to the H2O stimulus across the 10 stimulus labels when presented with excess water-like stimuli. Identification patterns for the H2O test stimulus in the 2 H2O rinse controls, H2O(2)–w/o-NQ and H2O(1)–basic, were compared. As shown in Table 1, H2O was presented 4 times per replicate and subjects chose correct labels for 3 distinct stimuli in the w/o-NQ positive control, whereas H2O was presented once per replicate and subjects chose correct labels for the complete set of 10 stimuli in the basic H2O control. In both cases, test stimuli followed control H2O rinses while the subjects had the entire list of 10 labels before them. This comparison addressed how the change in test-stimulus context, that is, eliminating NaCl and quinine·HCl, would affect responses to H2O stimuli. Subjects might try to use all 10 labels even when confronted with 3 stimuli. Aggregate response frequencies were evaluated with χ2 post hoc contrasts, to test for decreased use of water in favor of increased use of salt, quinine, and salt + quinine labels for the missing stimuli in the positive control.
Results
Confusion matrixes for the 2 experimental conditions and basic control are followed by comparisons of 1) identifications of the entire set of 10 test stimuli, 2) selected subsets of the stimuli across conditions, and 3) error analysis and the confusion matrix for the positive control.
The TCMs
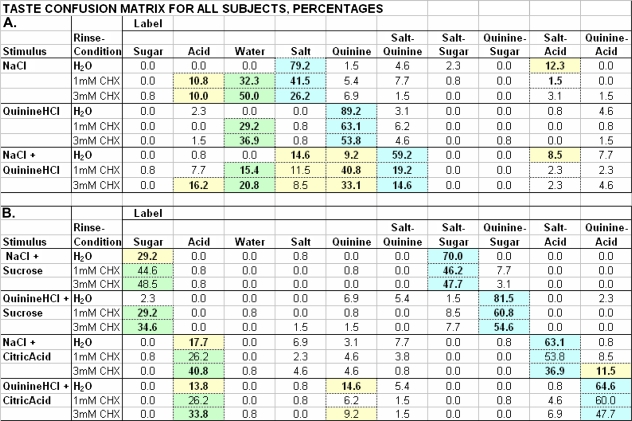
The aggregate TCMs for basic control and 2 CHX treatments, averaged across 13 subjects, are in Figures 1 and 2. Blue cells contain correct (on-label) responses, yellow cells consistent errors (off-label), and green cells predicted responses after CHX. The 2 Figures together encompass three 10 × 10 TCMs, entries representing average percentages of the 10 possible responses (columns) to the 10 stimuli (rows). Results for test stimuli containing “only NaCl and/or quinine·HCl” are in Figure 1A; results for test stimuli containing “binary mixtures of NaCl or quinine·HCl with sucrose or citric acid” are in Figure 1B; and results for “sucrose,” “citric acid,” and water are in Figure 2.
Figure 1.
H2O & CHX Rinse Taste Confusion Matrix: NaCl, Quinine·HCl and Mixes.
Values are aggregate (N = 13) response percentages for stimuli listed in Table 1 A and B, complete test-stimulus set, under rinse conditions of H2O, 1 and 3 mM chlorhexidine. Associated correct-label responses are on a blue background. Hypothesized label shifts with CHX rinses are on green. For H2O rinse, correct label and significant off-label values in yellow cells are in boldface type. For CHX rinse conditions, significant changes from label use in H2O rinse condition are in boldface type.
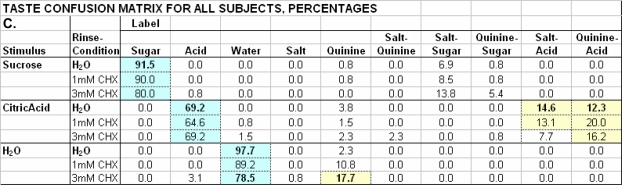
Figure 2.
H2O & CHX Rinse Taste Confusion Matrix: Sucrose, Citric Acid and H2O.
Values are aggregate (N = 13) response percentages for stimuli listed in Table 1C, complete test-stimulus set, under rinse conditions of H2O, 1 and 3 mM chlorhexidine. Associated correct-label responses are on a blue background. For H2O rinse, correct label and significant off-label values in yellow cells are in boldface type. For CHX rinse conditions, significant changes from H2O rinse label use are in boldface type.
The 10 test stimuli, all together
Chlorhexidine treatment impaired the accurate identification of the 10 stimuli, F(2,24) = 50.7, P < 0.0001. Average correct identification fell from 76 ± 11% with H2O rinse to 59 ± 13% after 1 mM CHX and 51 ± 10% after 3 mM CHX. Both concentrations of CHX reduced accurate identification, P < 0.0001, 3 mM more so than 1 mM, P < 0.001. Percent correct is calculated from the 10 blue cells in Figures 1 and 2 of the 100-cell TCM. All cells are used to calculate response consistency, T10, which quantifies distinct use of labels, namely, nonoverlapping labels for the 10 stimuli.
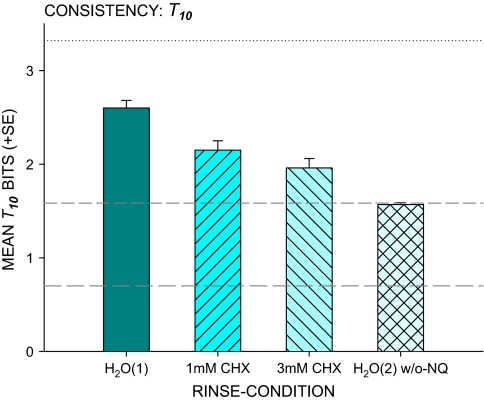
The average T10 of 2.6 bits of information for the basic control H2O rinse is equivalent to “perfect” transmission of 6 stimuli to 6 responses (Figure 3). This is well below the 3.3 bits available in 10 stimuli and the deficit likely stems from limitations imposed by the gustatory system—although more general cognitive constraints on information transmission cannot be excluded. CHX treatment reduced information transmission, F(2,24) = 43.21, P < 0.0001, to 2.2 bits at 1 mM and 2.0 bits at 3 mM, P < 0.007. Yet, the 2.0 bits after 3 mM CHX, equivalent to transmission of 4 stimuli to 4 responses, surpassed the 1.6 bits for 3 stimuli to 3 responses attained by the positive control (t = 4.20, P < 0.001). After 3 mM CHX, tastes of NaCl and/or quinine·HCl continued to provide information equivalent to recognizing 1 stimulus in addition to sucrose, citric acid, and H2O.
Figure 3.
Mean T10 (+SE), consistency of identification. Rinse conditions for trials with 10 distinct stimuli presented were the basic control H2O(1) rinse, 1 and 3 mM chlorhexidine treatment. Multiple replicates of sucrose, citric acid, and H2O were presented for the positive control H2O(2) w/o-NQ. Information in 10 distinguishable stimuli is 3.32 bits (upper dotted line); in 3 distinguishable stimuli, it is 1.6 bits (middle dashed line). Random performance averages 0.70 bit (lower dashed line). This figure appears in color in the online version of Chemical Senses.
The 10 stimuli, divided to test hypotheses
NaCl and/or quinine·HCl versus no NaCl or quinine·HCl—percent correct
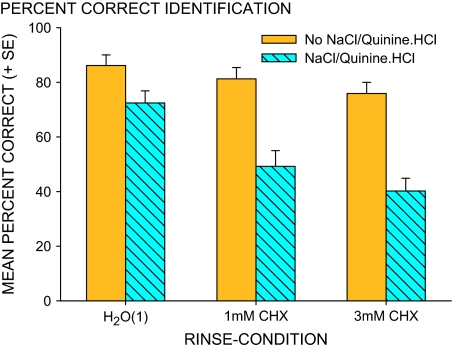
CHX treatment, more at 3 mM than 1 mM, reduced identification of stimuli containing “NaCl and/or quinine·HCl” (Figure 4). Average percent correct, 72% for stimuli that contained NaCl and/or quinine·HCl in basic controls, was reduced, F(2,24) = 48.4, P < 0.0001, falling by 23% after 1 mM CHX (P < 0.0001) and by 32% after 3 mM CHX (P < 0.00001). Identification of stimuli that contained “no NaCl or quinine·HCl,” 86% for controls, also fell, F(2,24) = 5.1, P < 0.02, by 10% after 3 mM CHX (P < 0.02). Identities of stimuli containing NaCl and quinine·HCl were much less distinct and, apparently, identities of stimuli not containing the 2 compounds were also less distinct but see “Analysis of errors,” below.
Figure 4.
Average percent correct identification (+SE) for NaCl–quinine and no NaCl–quinine stimuli. Bars, left to right, represent data for the basic control H2O(1) rinse and 1 and 3 mM CHX treatment. This figure appears in color in the online version of Chemical Senses.
NaCl and/or quinine·HCl only, NaCl or quinine·HCl mixed with sucrose or citric acid, no NaCl and quinine·HCl—percent correct ratios and triad discriminabilities
Although basic control identification of correct labels was much better for some stimuli than others, F(9,108) = 4.9, P = 0.00002 (Figures 1 and 2), the average 74% control identification of stimuli containing “only NaCl and quinine·HCl” was near the 70% control for “mixtures with sucrose and citric acid.” However, after CHX, percent control identification, averaged for 1 and 3 mM CHX, differed, F(2,24) = 20.4, P < 0.0001. “NaCl and quinine·HCl” fell to 47 ± 6% of control but “sucrose and citric acid mixtures” fell less, P < 0.002, to 71 ± 5% of control. This difference is further evaluated under Analysis of errors. For stimuli containing neither compound, percent control identification was 93 ± 5% after CHX, more than for stimuli containing NaCl or quinine·HCl, alone or in mixes with sucrose or citric acid (P < 0.0002 and <0.005).
To see how well stimuli containing neither NaCl nor quinine·HCl were discriminated from each other, triad discriminability, T3, for the sucrose, citric acid, and H2O rows in Figure 2 was calculated. Average discriminability of the 3 stimuli was a perfect 1.6 bits for the basic control and 1 mM CHX but dropped to 1.5 bits after 3 mM CHX treatment, t(12) = 3.01, P < 0.01. This small loss of transmitted information is further evaluated under Analysis of errors.
Indistinguishable or distinguishable after CHX treatment—Pairwise discriminability (T2) and multidimensional scaling (MDS)
Average discriminability of the 12 stimulus pairs predicted to be indistinguishable after CHX rinse dropped from 0.82 ± 0.02 bit on basic control trials toward the average 0.40-bit random transmission, that is, to 0.51 ± 0.04 bit for 1 mM CHX and 0.42 ± 0.05 bit for 3 mM CHX treatment, F(2,24) = 63.28, P < 0.0001. In contrast, discriminability of the 33 pairs predicted to be distinguishable after CHX rinse approached 1-bit perfect performance, irrespective of rinse treatment, averaging 0.93 ± 0.01 bit on basic control trials, 0.94 ± 0.02 bit after 1 mM CHX, and 0.93 ± 0.02 bit after 3 mM CHX treatment. T2 values for all pairwise discriminations are provided in Supplementary Online Material.
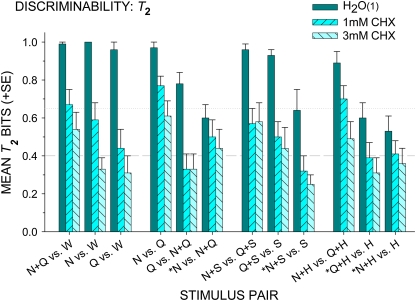
Figure 5 presents average T2 for each stimulus pair predicted to be indistinguishable after CHX. On average, discriminability fell after CHX, P < 0.0001, but remained higher for 1 mM CHX than for 3 mM CHX, P < 0.03. Four stimulus pairs predicted to be indistinguishable after CHX treatment were quite poorly discriminated on basic control rinse trials (T2 < 0.65 bit). These were 4 of the 6 pairwise comparisons of binary mixtures versus their components, discriminations considered under “Error Analysis.” Also, 4 distinguishable pairs (not plotted), each a citric acid–containing binary mixture versus NaCl and/or quinine·HCl, were “poorly” discriminated on basic control trials (T2 < 0.75) but “better” discriminated on CHX treatment trials, F(2,24) = 4.42, P = 0.02. Average T2 improved from controls of 0.68 ± 0.04 to 0.77 ± 0.03 bit after 1 mM CHX (P = 0.03) and to 0.75 ± 0.06 bit after 3 mM CHX (P = 0.03). Discrimination between citric acid and the post-CHX water-like taste of NaCl and quinine·HCl was easier than discriminations between citric acid and full-blown tastes of control NaCl and/or quinine·HCl.
Figure 5.
Mean T2 (+SE), bits of information transferred, for 12 stimulus pairs predicted to be indiscriminable after chlorhexidine (1 and 3 mM) treatment. Pairwise comparisons of NaCl (N) and/or quinine·HCl (Q) containing stimuli versus 1) H2O (W), 2) themselves, 3) sucrose (S), and 4) citric acid (H) are presented from left to right; and each ordered from high to low for basic control rinse [H2O(1)]. *Note low control values (<0.65 bit, dotted line) for comparisons between mixtures and their components. Average random performance is 0.40 bit, indicated by a dashed line.
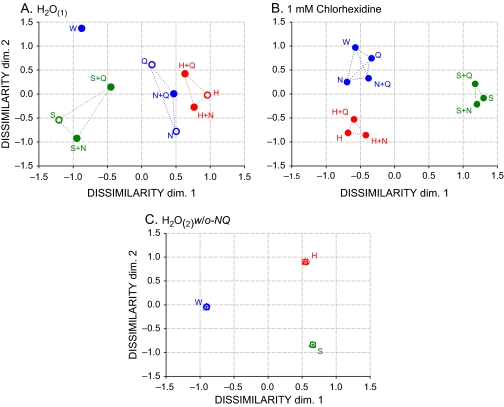
The 45 T2 values for each rinse condition were entered into Statistica 6© as dissimilarity measures (Youngentob et al. 2006) to reveal discriminations among the 10 stimuli with nonmetric MDS (Kruskal and Wish 1978). Key distances and stress values are given in Table 2. The 2D configurations shown in Figure 6 are for the (A) basic control H2O rinse, (B) 1 mM CHX treatment, and (C) the positive control H2O rinse. Table 2 also includes the unplotted MDS solution for 3 mM CHX treatment that fits degeneracy criteria of identical points for tightly clustered similar stimuli (Kruskal and Wish 1978).
Table 2.
Distances for 2D T2 nonmetric MDS configuration
| Row | 10 Stimuli | Treatment condition |
3 Stimuli | |||
| H2O | 1 mM CHX | 3 mM CHX | w/o-NQ | |||
| 1 | X–W | 1.91 | 0.57 | 0.00 | 0.01 | W–W |
| 2 | X–X | 0.97 | 0.45 | 0.00 | 0.01 | W–W |
| 3 | XS–XS | 0.95 | 0.30 | 0.00 | 0.01 | S–S |
| 4 | XH–XH | 0.53 | 0.31 | 0.00 | 0.01 | H–H |
| 5 | XS–W | 1.84 | 2.06 | 2.10 | 1.74 | S–W |
| 6 | XH–W | 2.13 | 1.70 | 1.70 | 1.74 | H–W |
| 7 | H–S | 2.22 | 2.11 | 2.10 | 1.74 | H–S |
| Stress | 2D | 0.14 | 0.07 | .00001a | .001 | |
| 3D | 0.03 | 0.02 | .002 | .005 | ||
Average MDS distances for 1) X versus W (n = 3), 2) X versus X (n = 3), 3) XS versus XS (n = 3), 4) XH versus XH (n = 3), 5) XS versus W (n = 3), 6) HX versus W (n = 3), and 7) H versus S (n = 1). X = N (NaCl), Q (quinine·HCl), NQ, or no N or Q; W (H2O); H (citric acid); and S (sucrose).
Low stress and 0 distances are signs of a degenerate MDS solution (Kruskal and Wish 1978).
Figure 6.
2D MDS stimulus configurations for (A). H2O(1), (B). 1 mM CHX, and (C). H2O(2) w/o-NQ rinse conditions. 1 mM CHX treatment drove stimuli containing only NaCl and quinine closer to water (blue) and stimuli containing sucrose (green) or acid (red) closer to each other. Dotted lines indicate distances included in Table 2 averages. W, H2O; N, NaCl; S, sucrose; H, citric acid; and Q, quinine·HCl. 45 T2 values were the dissimilarity measures for each solution.
In the basic H2O control shown in Figure 6A, tasteless H2O (W) is distant from the 4 prototypes, among which sucrose (S) is farther from the 3 electrolytes (quinine·HCl [Q], citric acid [H], and NaCl [N]) than they are from each other. The 5 binary mixtures fall between their 2 components, with individual distances consistent with selective “unprompted mixture analysis” and shared side tastes of prototypic stimuli as described under Analysis of errors below. Gustatory perceptual organization after 1 mM CHX treatment is quite different. Figure 6B depicts 3 stimulus clusters; stimuli containing only NaCl and quinine·HCl have joined H2O, and citric acid–containing and sucrose-containing stimuli have separately converged. Figure 6C for the positive control trials reveals multiple overlapping points representing the replicates of H2O, citric acid, and sucrose.
Analysis of errors, finding consistent confusions in controls and after CHX
Error analysis revealed that identification depended on dynamic perceptual boundaries between single stimuli and their mixtures in subjects trained to use customary labels for taste–stimulus prototypes.
NaCl and/or quinine·HCl only, side tastes, mixture analysis, and mixture suppression
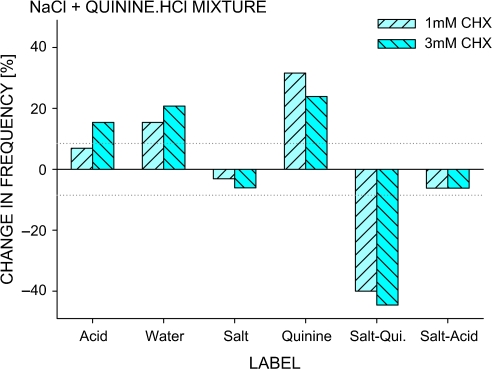
Basic H2O control misidentification of NaCl as “salt–acid” is an example of a “side taste” and misidentification of the NaCl-quinine·HCl mixture as salt or quinine is an example of unprompted “mixture analysis.” CHX treatment was predicted to lead to decreases in correct identification and increases in misidentification as water, but other changes also occurred (Figure 1A). First, salt–acid tastes of NaCl and the mixture were replaced by acid tastes, that is, with salt taste weakened by CHX treatment, an acid taste component emerged. Second, as shown in Figure 7, the mixture was increasingly identified as quinine rather than water. When the stimuli were individually presented after CHX, subjects identified the quinine taste of quinine·HCl about twice as frequently as the salt taste of NaCl (χ2 = 20.8, P = 0.00001). Likely, the quinine component, weakened less than the salt component by CHX, emerged from the mixture, an example of release from “mixture suppression.”
Figure 7.
Percent change in identification frequency of the NaCl–quinine·HCl mixture on CHX treatment trials. The mixture's quinine component and the acid component of NaCl's salt–acid side taste emerged after CHX rinse. Dotted lines at ±8.5% indicate significant changes in identification. Control = H2O(1) rinse trials. This figure appears in color in the online version of Chemical Senses.
NaCl or quinine·HCl mixed with sucrose or citric acid, mixture analysis, side tastes, and mixture suppression
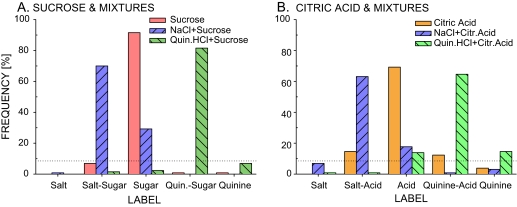
If prototypic stimuli were processed independently, CHX, besides decreasing correct identification of mixtures, would increase identifications of sucrose-containing mixtures as sugar and identifications of citric acid–containing mixtures as acid. However, this straightforward prediction overlooks consistent misidentifications of some of the mixtures in controls (Figure 1B).
With no consistent mislabeling of either component (Figure 8A), identifications of the quinine·HCl + sucrose mixture fit the simple model. After CHX, subjects correctly identified the mixture less often and mislabeled it as sugar more often. However, when mixture interactions occurred and/or side tastes were present, the basic model proved insufficient. Unprompted mixture analysis prevailed in controls. The NaCl + sucrose mixture was identified as sugar, the NaCl + citric acid mixture was identified as acid, and the quinine·HCl–citric acid mixture was identified as quinine and acid (Figure 8). CHX treatment decreased salt–sugar responses but failed to substantially increase an already high number of sugar responses; 3 mM CHX decreased salt–acid responses and increased acid responses but 1 mM CHX failed to do so; and 3 mM CHX treatment increased acid responses but correct identification as quinine–acid did not decrease significantly, perhaps because of the relatively weak inhibition of quinine. Side tastes not blunted by CHX, the acid taste of NaCl and the combined salt–acid and quinine–acid tastes of citric acid, may contribute to the persisting accurate identification of the NaCl + citric acid and quinine·HCl + citric acid mixtures after CHX treatment.
Figure 8.
Control percent identification of (A) sucrose mixtures and components and (B) citric acid mixtures and components. (A) Sucrose and the quinine·HCl–sucrose mixture were accurately identified. The NaCl–sucrose mixture was misidentified as salt. (B) Citric acid was misidentified as the 2 mixtures containing acid. The NaCl–citric acid mixture was misidentified as citric acid. The quinine·HCl–citric acid mixture was misidentified as both components. The dotted horizontal line indicates a significant 8.5% identification.
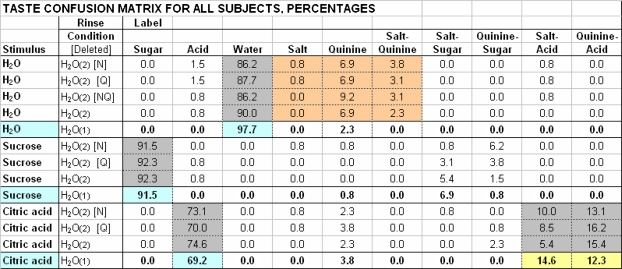
Sucrose and citric acid and the misidentification of H2O
Identification of sucrose and citric acid was unchanged by CHX (Figure 2) and response frequencies for sucrose and citric acid in the 2 water controls were indistinguishable (gray cells in Figure 9). Sucrose had no consistent side tastes, but citric acid was misidentified as salt–acid and quinine–acid in both H2O control sessions, and the quinine–acid side taste of citric acid even emerged from the NaCl + citric acid mixture after 3 mM CHX inhibited NaCl's taste (Figure 1B).
Figure 9.
H2O(2) Control TCM: Sucrose, Citric Acid and H2O, w/o-NQ Stimuli.
Values for H2O, sucrose, and citric acid are aggregate (N = 13) response percentages for rinse-conditions of H2O(2) without NaCl and quinine·HCl (deleted components in brackets) or H2O(1) with complete set of 10 distinct stimuli. H2O(2) values in gray cells correspond to on-label and off-label responses subjects used in H2O(1) control condition (taken from Figure 2); H2O(2) values in tan cells were used to evaluate mislabeling of H2O as salt, quinine or salt-quinine. As in Figure 2, H2O(1) correct values are in blue cells and consistent off-label values in yellow cells.
H2O was misidentified as quinine after 3 mM CHX treatment (Figure 2). Misidentifications may have, in part, arisen because CHX reduced detection of quinine and salt tastes to create a “tasteless” context. With multiple presentations of tasteless H2O in the positive control, erroneous choices were made (Figure 9). H2O was identified as water 98% of the time when 10 different stimuli were presented but just 88% of the time (gray cells) when there were only 3 different stimuli (P = 0.002). The decrease in correct water responses was accompanied by increased mislabeling of H2O as salt, quinine, or salt–quinine (tan cells), the missing stimuli, from a median 4% to 14% (P = 0.006). Even though subjects were told that all stimuli may not appear, a tendency to use all responses on the list likely led to “false positives” for the tastes inhibited by CHX.
Discussion
Identification of 100 mM NaCl as salt more than identification of 0.1 mM quinine·HCl as quinine were reduced by treatment with 1 and 3 mM chlorhexidine, without affecting identification of the tastes of 3 mM citric acid or 300 mM sucrose. The chlorhexidine concentration used in mouth rinses, 1.34 mM, has the same restricted effect, impacting neither acid, sugar, nor even glutamate (100 mM sodium glutamate) tastes (Frank et al. 2001; Gent et al. 2002). Chlorhexidine's selective inhibition and its relationship to the independence of basic tastes in humans are the topics of this discussion.
Chlorhexidine, a selective taste inhibitor
Increasing CHX from 1 to 3 mM strengthens selective inhibition of NaCl and quinine·HCl tastes
With an increase in CHX concentration, correct identification of stimuli containing NaCl and/or quinine·HCl decreased by an additional 10%. However, mistaken identification of H2O appeared (Figure 2), as it had in previous TCM studies with 1.34 mM CHX (Gent et al. 2002; Johar 2006). Mislabeling of H2O in the TCM-experimental context was also evident in results of the positive control in which NaCl and quinine·HCl were replaced with water.
After CHX treatment (Figure 2) and in the positive control (Figure 9), the quinine label was most often mistakenly used for H2O. The taste attributed to H2O following CHX treatment is weak (Breslin and Tharp, 2001), its intensity rated “barely detectable,” 0.6 ± 0.3 after 3 mM CHX compared to 0.9 ± 0.5 after H2O rinse (Johnson EA, Frank ME, unpublished observations). This weakness combined with results of the positive control suggest mislabeling H2O after CHX reflects a decisional bias, with subjects trying to use all responses that they were trained to use when few stimuli were recognizable. Labels for “missing” stimuli were attributed to tasteless samples. Perhaps these mistakes could be minimized with suitable subject instructions in future TCM studies with more potent CHX concentrations.
Relationships between NaCl and bitter tastes have been observed. Bitter tastes appear when H2O is introduced to the lingual surface adapted to NaCl (Bartoshuk 1974), citric acid and KCl taste bitterer after NaCl adaptation (Smith and van der Klaauw 1995), and the addition of sodium salts inhibits bitterness (Breslin and Beauchamp 1997; Keast et al. 2004). NaCl adaptation and CHX inhibition block salty taste; might they also disable “bitter” inhibition by Na+?
NaCl may elicit several tastes via several receptors
The mixed salt–acid side taste of NaCl is converted to acid taste after CHX (Figure 1A; Gent et al. 2002), which is consistent with emergence of a sour taste quality after treatment with CHX (Grover and Frank 2008). It is this sour side taste of NaCl that amiloride inhibits in humans (Ossebaard and Smith 1995, 1996). As in rodents (Frank and Nowlis 1989; Hettinger and Frank 1990; Hill et al. 1990), the taste of NaCl may be dually mediated by the gustatory system in humans, with salty CHX sensitive and sour amiloride sensitive. In this way, multiple receptors for NaCl may explain partial yet specific effects of inhibitors on the taste of NaCl.
Correct identification of NaCl as salt was a quite low 26% after 3 mM CHX treatment, having dropped to 41% after 1 mM CHX from the control 79%, and some of the remaining salt identity may arise from slow recovery from CHX inhibition during lengthy testing (Frank et al. 2001; Gent et al. 2002). Perhaps an increase to 5 mM CHX would bring salt identification of 100 mM NaCl down to chance levels, without signs of nonspecific general aguesia (Flötra et al. 1971; Shaupp and Whonaut 1978; Gürgan et al. 2006), yet leave an amilioride-inhibitable acid side taste unaffected.
Quinine·HCl may elicit its bitter taste via several receptors
Quinine·HCl, which had no consistent side tastes, was correctly identified as quinine on 54% of presentations after 3 mM CHX treatment, having dropped to 63% after 1 mM CHX from a control 89%. Quinine was identified well above chance levels after CHX treatment. CHX-sensitive receptors accounted for most of the quinine·HCl response elicited from anterior lingual and palatal taste-bud fields in humans (Grover and Frank 2008). After CHX, the intensity of the potent 1 mM quinine·HCl was typically reduced to zero and unidentifiable when locally applied to CN VII sites. In contrast, its bitter quality was often still identified from CN IX posterior taste receptive fields.
Posterior oral receptors are more responsive to quinine than anterior receptors in rodents (Frank 1991), and there may also be highly sensitive quinine receptors in human posterior taste-bud fields. Several distinct quinine-sensing T2R receptors (Boughter et al. 1992, 2005; Bufe et al. 2002; Kuhn et al. 2004; Nelson et al. 2005; Wooding et al. 2006) apparently all code for bitter taste. Perhaps some T2R, especially those in anterior taste-bud fields, are very sensitive to CHX inhibition, whereas others, in the posterior taste-bud fields, are CHX insensitive.
Independent tastes
Except for losses in NaCl and quinine·HCl identity, the perceptual order is unchanged with CHX treatment
On control trials, identification of the 4 taste prototypes was imperfect. Identifications of sucrose as sugar and quinine·HCl as quinine were 90%, but identifications of NaCl as salt, 80%, and citric acid as acid, 70%, were less accurate. Confusions were not random; rather, subjects confused the taste of NaCl and citric acid with a mixed salt–acid taste and also confused the taste of citric acid with a mixed quinine–acid taste. Sharing salt–acid confusions, citric acid and NaCl, are closely spaced by MDS (Figure 6A). NaCl and citric acid may both have several sensory facets (McBurney and Shick 1971; McBurney et al. 1972; van der Klaauw and Smith 1995), with perceptual borders waffling from trial to trial. That is, single-compound MDS proximity may represent stimulus confusions rather than quality or semantic confusions.
On 1 mM CHX trials (Figure 6B), distances between stimuli within the sucrose-containing cluster, citric acid–containing cluster, and H2O were unchanged, whereas within-cluster distances were halved compared to basic controls (Table 2, rows 3–7). The latter reflects increased misidentification of sucrose or citric acid mixed with the inhibited NaCl or quinine·HCl as sugar or acid, respectively. One stimulus trio is sugar-like, the other acid-like. There is also a halving of distances between inhibited NaCl, quinine·HCl, and NaCl + quinine·HCl after 1 mM CHX treatment (Table 2, row 2), but even more dramatic is the 70% shrinking of distances between NaCl, quinine·HCl, or NaCl + quinine·HCl and H2O (Table 2, row 1). The inhibited NaCl–quinine·HCl trio is water-like. In spite of drastic changes in tastes of 2 of 4 taste prototypes, human gustatory perceptual order did not fall apart but sugar and acid tastes remained recognizable and as distinct from water as in controls.
Cross labeling of electrolytes (NaCl, citric acid, and quinine·HCl) change with CHX treatment
Replacement of the salt–acid side taste of NaCl by an acid taste following CHX suggests that salt and acid identities of NaCl are independent. Blockage of the sour quality of NaCl in humans by the ENaC inhibitor, amiloride, without any reduction in salty (Ossebaard and Smith 1995, 1996) is consistent with this idea. CHX blocked neither acid, salt–acid, nor quinine–acid identities of citric acid, and its sour quality (McBurney and Shick 1971; van der Klaauw and Smith 1995) was unchanged by amiloride treatment (Ossebaard and Smith 1996). Humans apparently recognize independent acid identities (sour qualities) in NaCl and citric acid. Perhaps the labels made available to subjects failed to discriminate between 2 pharmacologically separable sharp tastes.
Overlapping sensory identities of electrolyte prototypes help explain why T2 values for particular stimulus pairs, low on control H2O trials, improved after CHX. Each of the 4 pairs contained 1 citric acid (NaCl vs. NaCl + citric acid, quinine·HCl vs. quinine·HCl + citric acid, NaCl + quinine·HCl vs NaCl + citric acid, NaCl + quinine·HCl vs. quinine·HCl + citric acid). Controls were difficult discriminations between electrolytes with shared identities; CHX treatment shifted them toward easier discriminations between citric acid and the water-like NaCl and quinine·HCl. This kind of detail illustrates the rich information on the concurrent discrimination of multiple stimuli in TCM data.
Another 4 stimulus pairs, among those predicted to be indistinguishable after CHX, were poorly discriminated on control trials (Figure 5). They were pairwise comparisons of a mixture and 1 component of the mixture. In 3 of the 4 pairs, shared side tastes of electrolytes (salt–acid or quinine–acid) and in all 4 pairs, unprompted mixture analysis (acid for citric acid + NaCl and citric acid + quinine·HCl, salt for NaCl + quinine·HCl, or sugar for sucrose + NaCl) may have contributed. TCM off-label “errors” provide convincing evidence of the value of considering “prototype” side tastes and unprompted mixture analysis, also seen in hamsters’ generalizations of conditioned taste aversions (Frank et al. 2003, 2008).
Unprompted mixture analysis and mixture suppression are evident in mistaking components for mixtures
Control misidentification of the components NaCl and quinine·HCl (Figure 1A) exemplifies unprompted mixture analysis. Following CHX, the NaCl + quinine·HCl mixture continued to be mislabeled salt but was most often mislabeled quinine. An explanation for dominance of the quinine identity lies in component intensity (Laing et al. 2002). By weakening NaCl more than quinine·HCl, CHX left the mixture with a weak salt taste, allowing the stronger quinine taste to dominate. Similar interactions explain suppressed recognition of mixture components in hamsters (Frank et al. 2003).
Unprompted mixture analysis, perhaps spontaneous fluctuation between mixture and component percepts, also occurs as the mislabeling of a binary mixture as one of the mixture components. Confusions between mixture and component are reflected in the MDS configurations for control trials (Figure 6A). The proximity of sucrose + NaCl to sucrose and NaCl + citric acid to citric acid reflects frequent identification of NaCl + sucrose as sugar and NaCl + citric acid as acid. Neither mixture was consistently identified as the salt component (Figure 8). The taste of NaCl appears especially vulnerable to asymmetric mixture suppression in humans. Sucrose reduces salt identification in sucrose + NaCl mixtures without salt reciprocally reducing sugar identification (Watson et al. 2001) and citric acid may similarly dominate citric acid + NaCl mixtures (Oram et al. 2001). In golden hamsters, behavioral mixture analysis is also influenced by quality selective and general, intensity-based suppression (Frank et al. 2003, 2008). An example of selective neural suppression is inhibition of the hamster's chorda tympani responses to sucrose by quinine in mixtures (Frank et al. 2008).
Conclusion
Unlike color perception, which is fundamentally reorganized with the functional loss of 1 cone receptor (because of loss of input to at least 1 subsequent opponent-color mechanism), the tastes that remain after CHX survive essentially undisturbed. Misidentifications are not random, as might be expected if a new taste had appeared, but represent residual tastes of NaCl and quinine·HCl, mixture–component intensity interactions, or, when there are many H2O-like stimuli, decisional processes. Discriminations among prototypic stimuli with varying strengths were consistent with a gustatory system that evaluates a small number of independent taste qualities.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/.
Funding
National Institutes of Health (5R01 DC004849 to M.E.F.).
Acknowledgments
Contributions to the initial design, analysis, and final interpretation of this TCM experiment by Janneane F. Gent and Thomas P. Hettinger are much appreciated, as are the really helpful comments of 2 anonymous reviewers.
References
- AlKadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53:385–391. doi: 10.1159/000109767. [DOI] [PubMed] [Google Scholar]
- Attneave F. Applications of information theory to psychology. New York: Holt, Rinehart & Winston; 1959. [Google Scholar]
- Bartoshuk LM. NaCl thresholds in man: thresholds for water taste or NaCl taste? J Comp Physiol Psychol. 1974;87:310–325. doi: 10.1037/h0036788. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM. Taste mixtures: is mixture suppression related to compression? Physiol Behav. 1975;14:643–649. doi: 10.1016/0031-9384(75)90193-6. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Murphy C, Cleveland CT. Sweet taste of dilute NaCl: psychophysical evidence for a sweet stimulus. Physiol Behav. 1978;21:609–613. doi: 10.1016/0031-9384(78)90138-5. [DOI] [PubMed] [Google Scholar]
- Bateman DN, Dyson EH. Quinine toxicity. Adverse Drug React Acute Poisoning Rev. 1986;5:215–233. [PubMed] [Google Scholar]
- Boughter JD, Jr, Harder DB, Capeless CG, Whitney G. Polygenic determination of quinine aversion among mice. Chem Senses. 1992;17:427–434. [Google Scholar]
- Boughter JD, Jr, Raghow S, Nelson TM, Munger SD. Inbred mouse strains C57BL/6J and DBA/2J vary in sensitivity to a subset of bitter stimuli. BMC Genet. 2005;6:36. doi: 10.1186/1471-2156-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand JG, Teeter JH, Silver WL. Inhibition by amiloride of chorda tympani responses evoked by monovalent salts. Brain Res. 1985;334:207–214. doi: 10.1016/0006-8993(85)90212-4. [DOI] [PubMed] [Google Scholar]
- Breslin PA, Beauchamp GK. Salt enhances flavour by suppressing bitterness. Nature. 1997;387:563. doi: 10.1038/42388. [DOI] [PubMed] [Google Scholar]
- Breslin PA, Tharp CD. Reduction in saltiness and bitterness after a chlorhexidine rinse. Chem Senses. 2001;26:105–116. doi: 10.1093/chemse/26.2.105. [DOI] [PubMed] [Google Scholar]
- Briner WW, Kayrouz GA, Chanak MX. Comparative antimicrobial effectiveness of a substantive (0.12% chlorhexidine) and a nonsubstantive (phenolic) mouthrinse in vivo and in vitro. Compendium. 1994;15 1158, 1160, 1162 passim. [PubMed] [Google Scholar]
- Bufe B, Hofmann T, Krautwurst D, Raguse J-D, Meyerhof W. The human TAS2R16 receptor mediates bitter taste in response to β-glucopyranosides. Nat Genet. 2002;32:397–401. doi: 10.1038/ng1014. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Delwiche JF, Buletic Z, Breslin PA. Covariation in individuals′ sensitivities to bitter compounds: evidence supporting multiple receptor/ transduction mechanisms. Percept Psychophys. 2001;63:761–776. doi: 10.3758/bf03194436. [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Ferrell F. Analysis of amiloride inhibition of chorda tympani taste response of rat to NaCl. Am J Physiol. 1985;249:R52–R61. doi: 10.1152/ajpregu.1985.249.1.R52. [DOI] [PubMed] [Google Scholar]
- Flötra L, Gjermo P, Rölla G, Waerhaug J. Side effects of chlorhexidine mouth washes. Scand J Dent Res. 1971;79:119–125. doi: 10.1111/j.1600-0722.1971.tb02001.x. [DOI] [PubMed] [Google Scholar]
- Formaker BK, Hill DL. Lack of amiloride sensitivity in SHR and WKY glossopharyngeal taste responses to NaCl. Physiol Behav. 1991;50:765–769. doi: 10.1016/0031-9384(91)90015-g. [DOI] [PubMed] [Google Scholar]
- Frank ME. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J Neurophysiol. 1991;65:1452–1463. doi: 10.1152/jn.1991.65.6.1452. [DOI] [PubMed] [Google Scholar]
- Frank ME. A perspective on chemosensory coding. In: Basbaum AI, Kanako A, Shepherd GM, Westheimer G, editors. The senses: a comprehensive reference, Vol. 4, Olfaction & taste, Firestein S, Beauchamp GK. San Diego (CA): Academic Press; 2008. pp. 339–344. [Google Scholar]
- Frank ME, Bouverat BP, MacKinnon BI, Hettinger TP. The distinctiveness of ionic and nonionic bitter stimuli. Physiol Behav. 2004;80:421–431. doi: 10.1016/j.physbeh.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Frank ME, Formaker BK, Hettinger TP. Taste responses to mixtures: analytic processing of quality. Behav Neurosci. 2003;117:228–235. doi: 10.1037/0735-7044.117.2.228. [DOI] [PubMed] [Google Scholar]
- Frank ME, Gent JF, Hettinger TP. Effects of chlorhexidine on human taste perception. Physiol Behav. 2001;74:85–99. doi: 10.1016/s0031-9384(01)00558-3. [DOI] [PubMed] [Google Scholar]
- Frank ME, Lundy RF, Jr, Contreras RJ. Cracking taste codes by tapping into sensory neuron impulse traffic. Prog Neurobiol. 2008;86:245–263. doi: 10.1016/j.pneurobio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME, Nowlis GH. Learned aversions and taste qualities in hamsters. Chem Senses. 1989;14:379–394. [Google Scholar]
- Frijters JE, Schifferstein HN. Perceptual interactions in mixtures containing bitter tasting substances. Physiol Behav. 1994;56:1243–1249. doi: 10.1016/0031-9384(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Gent JF, Hettinger TP, Frank ME, Marks LE. Taste confusions following gymnemic acid rinse. Chem Senses. 1999;24:393–403. doi: 10.1093/chemse/24.4.393. [DOI] [PubMed] [Google Scholar]
- Gent JF, Frank ME, Hettinger TP. Taste confusions following chlorhexidine treatment. Chem Senses. 2002;27:73–80. doi: 10.1093/chemse/27.1.73. [DOI] [PubMed] [Google Scholar]
- Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol. 2006;96:2513–2527. doi: 10.1152/jn.00607.2006. [DOI] [PubMed] [Google Scholar]
- Gilbert P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol. 2005;99:703–715. doi: 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- Grover R, Frank ME. Regional specificity of chlorhexidine effects on taste perception. Chem Senses. 2008;33:311–318. doi: 10.1093/chemse/bjm095. [DOI] [PubMed] [Google Scholar]
- Gürgan CA, Zaim D, Bakirsoy I, Soykan E. Short-term side effects of 0.2% alcohol-free chlorhexidine mouthrinse used as an adjunct to non-surgical periodontal treatment: a double-blind clinical study. J Periodontol. 2006;77:370–384. doi: 10.1902/jop.2006.050141. [DOI] [PubMed] [Google Scholar]
- Hansen JL, Reed DR, Wright MJ, Martin NG, Breslin PA. Heritability and genetic covariation of sensitivity to PROP, SOA, quinineHCl, and caffeine. Chem Senses. 2006;31:403–413. doi: 10.1093/chemse/bjj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JA, Della-Fera MA, Mott AE, Frank ME. Effects of chlorhexidine on human taste perception. Arch Oral Biol. 1995;40:913–920. doi: 10.1016/0003-9969(95)00062-t. [DOI] [PubMed] [Google Scholar]
- Hettinger TP, Formaker BK, Frank ME. Gustatory effects of chlorhexidine in hamsters [abstract 287.5] Soc Neurosci. 2001 [Google Scholar]
- Hettinger TP, Formaker BK, Frank ME. Cycloheximide: no ordinary bitter stimulus. Behav Brain Res. 2007;180:4–17. doi: 10.1016/j.bbr.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger TP, Frank ME. Specificity of amiloride inhibition of hamster taste responses. Brain Res. 1990;513:24–34. doi: 10.1016/0006-8993(90)91085-u. [DOI] [PubMed] [Google Scholar]
- Hettinger TP, Frank ME. Salt taste inhibition by cathodal current. Brain Res Bull. 2009 doi: 10.1016/j.brainresbull.2009.06.019. Advance Access published July 1, 2009, doi:10.1016/j.brainresbull.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger TP, Gent JF, Marks LE, Frank ME. A confusion matrix for the study of taste perception. Percept Psychophys. 1999;61:1510–1521. doi: 10.3758/bf03213114. [DOI] [PubMed] [Google Scholar]
- Hill DL, Formaker BK, White KS. Perceptual characteristics of the amiloride-suppressed sodium chloride taste response in the rat. Behav Neurosci. 1990;104:734–741. doi: 10.1037//0735-7044.104.5.734. [DOI] [PubMed] [Google Scholar]
- Johar AO. [CT (USA)]: Biomedical Sciences, University of Connecticut; 2006. Effects of chlorhexidine rinse on salt taste in human and hamster: psychophysical, immunohistochemical and electrophysiological studies [PhD Thesis] [Google Scholar]
- Jones CG. Chlorhexidine: is it still the gold standard? Periodontol. 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- Keast RS, Breslin PA. Cross-adaptation and bitterness inhibition of L-tryptophan, L-phenylalanine and urea: further support for shared peripheral physiology. Chem Senses. 2002;27:123–131. doi: 10.1093/chemse/27.2.123. [DOI] [PubMed] [Google Scholar]
- Keast RS, Canty TM, Breslin PA. The influence of sodium salts on binary mixtures of bitter-tasting compounds. Chem Senses. 2004;29:431–439. doi: 10.1093/chemse/bjh045. [DOI] [PubMed] [Google Scholar]
- Kitada Y, Mitoh Y, Hill DL. Salt taste responses of the IXth nerve in Sprague–Dawley rats: lack of sensitivity to amiloride. Physiol Behav. 1998;63:945–949. doi: 10.1016/s0031-9384(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Kruskal JB, Wish M. Multidimesional scaling. Beverly Hills (CA): Sage Publications; 1978. [Google Scholar]
- Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtschenko T, Slack JP, Ward CD, Meyerhof W. Bitter taste receptors for saccharin and acesulfame K. J Neurosci. 2004;24:10260–10265. doi: 10.1523/JNEUROSCI.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing DG, Link C, Jinks AL, Hutchinson I. The limited capacity of humans to identify the components of taste mixtures and taste–odour mixtures. Perception. 2002;31:617–635. doi: 10.1068/p3205. [DOI] [PubMed] [Google Scholar]
- Lundy RF, Jr, Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J Neurophysiol. 1999;82:2970–2988. doi: 10.1152/jn.1999.82.6.2970. [DOI] [PubMed] [Google Scholar]
- Marshall K, Laing DG, Jinks AL, Effendy J, Hutchinson I. Perception of temporal order and the identification of components in taste mixtures. Physiol Behav. 2005;83:673–681. doi: 10.1016/j.physbeh.2004.08.038. [DOI] [PubMed] [Google Scholar]
- McBurney DH, Shick TR. Taste and water taste of twenty-six compounds for man. Percept Psychophys. 1971;10:249–252. [Google Scholar]
- McBurney DH, Smith DV, Shick TR. Gustatory cross adaptation: sourness and bitterness. Percept Psychophys. 1972;11:228–232. [Google Scholar]
- Nelson TM, Munger SD, Boughter JD., Jr Haplotypes at the Tas2r locus on distal chromosome 6 vary with quinine taste sensitivity in inbred mice. BMC Genet. 2005;6:32. doi: 10.1186/1471-2156-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Funakoshi M. Amiloride inhibition of responses of rat single chorda tympani fibers to chemical and electrical tongue stimulations. Brain Res. 1988;451:319–325. doi: 10.1016/0006-8993(88)90777-9. [DOI] [PubMed] [Google Scholar]
- Nowlis GH, Frank ME. Quality coding in gustatory systems of rats and hamsters. In: Norris DM, editor. Perception of behavioral chemicals. Amsterdam: Elsevier/No. Holland Biomedical Press; 1981. pp. 58–80. [Google Scholar]
- Oram N, Laing DG, Freeman MH, Hutchinson I. Analysis of taste mixtures by adults and children. Dev Psychobiol. 2001;38:67–77. doi: 10.1002/1098-2302(2001)38:1<67::aid-dev6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Ossebaard CA, Smith DV. Effect of amiloride on the taste of NaCl, Na-gluconate and KCl in humans: implications for Na+ receptor mechanisms. Chem Senses. 1995;20:37–46. doi: 10.1093/chemse/20.1.37. [DOI] [PubMed] [Google Scholar]
- Ossebaard CA, Smith DV. Amiloride suppresses the sourness of NaCl and LiCl. Physiol Behav. 1996;60:1317–1322. doi: 10.1016/s0031-9384(96)00258-2. [DOI] [PubMed] [Google Scholar]
- Schaup H, Wohnaut H. Geschmackstörungen durch Munddesinfizientien. Ein Beitrag zur Erkennung von Nebenwirkungen hexetidin- und chlorhexidin-haltiger Präparate. HNO. 1978;26:335–341. [PubMed] [Google Scholar]
- Schiffman SS, Gatlin LA, Sattely-Miller EA, Graham BG, Heiman SA, Stagner WC, Erickson RP. The effect of sweeteners on bitter taste in young and elderly subjects. Brain Res Bull. 1994;35:189–204. doi: 10.1016/0361-9230(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Suggs MS, Cragoe EJ, Jr, Erickson RP. Inhibition of taste responses to Na+ salts by epithelial Na+ channel blockers in gerbil. Physiol Behav. 1990;47:455–459. doi: 10.1016/0031-9384(90)90108-g. [DOI] [PubMed] [Google Scholar]
- Shi P, Zhang J. Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Mol Biol Evol. 2006;23:292–300. doi: 10.1093/molbev/msj028. [DOI] [PubMed] [Google Scholar]
- Smith DV, McBurney DH. Gustatory cross-adaptation: does a single mechanism code the salty taste? J Exp Psychol. 1969;80:101–105. doi: 10.1037/h0027112. [DOI] [PubMed] [Google Scholar]
- Smith DV, Theodore RM. Conditioned taste aversions: generalization to taste mixtures. Physiol Behav. 1984;32:983–989. doi: 10.1016/0031-9384(84)90289-0. [DOI] [PubMed] [Google Scholar]
- Smith DV, van der Klaauw NJ. The perception of saltiness is eliminated by NaCl adaptation: implications for gustatory transduction and coding. Chem Senses. 1995;20:545–557. doi: 10.1093/chemse/20.5.545. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Hill DL. Taste responses in the greater superficial petrosal nerve: substantial sodium salt and amiloride sensitivities demonstrated in two rat strains. Behav Neurosci. 1998;112:991–1000. doi: 10.1037//0735-7044.112.4.991. [DOI] [PubMed] [Google Scholar]
- Travers SP, Geran SC. Bitter-responsive brainstem neurons: characteristics and functions. Physiol Behav. 2009;97:592–603. doi: 10.1016/j.physbeh.2009.02.042. [DOI] [PubMed] [Google Scholar]
- van der Klaauw NJ, Smith DV. Taste quality profiles for fifteen organic and inorganic salts. Physiol Behav. 1995;58:295–306. doi: 10.1016/0031-9384(95)00056-o. [DOI] [PubMed] [Google Scholar]
- Watson WL, Laing DG, Hutchinson I, Jinks AL. Identification of the components of taste mixtures by adults and children. Dev Psychobiol. 2001;39:137–145. doi: 10.1002/dev.1037. [DOI] [PubMed] [Google Scholar]
- Wooding S, Bufe B, Grassi C, Howard MT, Stone AC, Vazquez M, Dunn DM, Meyerhof W, Weiss RB, Bamshad MJ. Independent evolution of bitter-taste sensitivity in humans and chimpanzees. Nature. 2006;440:930–934. doi: 10.1038/nature04655. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Johnson BA, Leon M, Sheehe PR, Kent PF. Predicting odorant quality perceptions from multidimensional scaling of olfactory bulb glomerular activity patterns. Behav Neurosci. 2006;120:1337–1345. doi: 10.1037/0735-7044.120.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.