Abstract
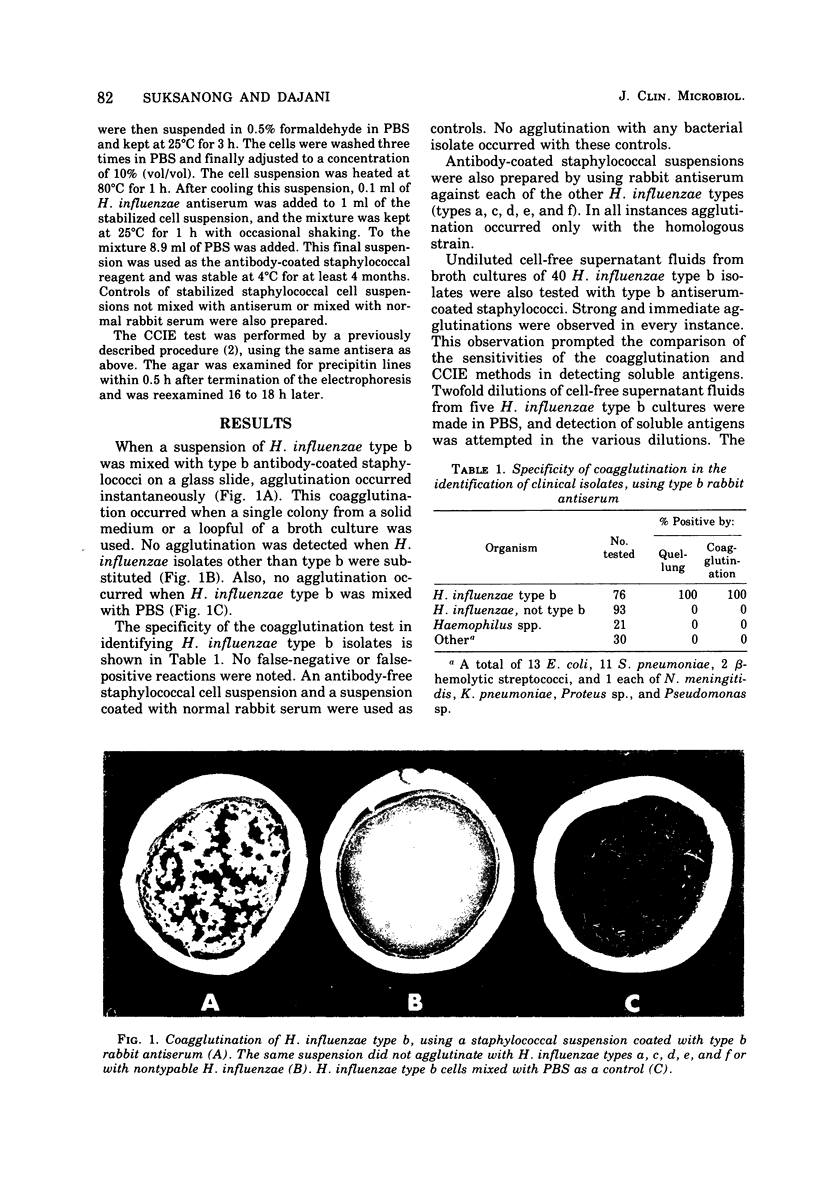
Protein A-rich staphylococci coated with Haemophilus influenzae type b antiserum agglutinate specifically with homologous bacterial cells or with cell-free supernatant fluids of cultures of the organism. Antibody-coated staphylococci were used to detect soluble antigens in body fluids of patients infected with H. influenzae type b. Cerebrospinal fluid from 36 cases of meningitis caused by this orgainsm showed positive coagglutination tests in 86% of patients prior to initiation of therapy. Antigens could be detected in 46% of sterile cerebrospinal fluid specimens obtained from the same cases 1 to 10 days after therapy. Soluble antigens were also detectable in sera (58%) and urine specimens (67%) of patients with H. influenzae type b septicemia, when such specimens were tested within 10 days of onset of illness. No antigen could be detected in body fluids beyond 10 days. The coagglutination test was positive in 57% of all body fluids examined; contercurrent immunoelectrophoresis (CCIE) was positive in only 27%. All specimens positive by CCIE were also positive by coagglutination. No false-positive reactions were noted by either test in body fluids from controls. The coagglutination test is simple, specific, and more sensitive than the CCIE method and could be a valuable tool for detecting antigens in body fluids of patients with various infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradshaw M. W., Schneerson R., Parke J. C., Jr, Robbins J. B. Bacterial antigens cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b. Lancet. 1971 May 29;1(7709):1095–1096. doi: 10.1016/s0140-6736(71)91837-x. [DOI] [PubMed] [Google Scholar]
- Dajani A. S. Rapid identification of beta hemolytic streptococci by counterimmunoelectrophoresis. J Immunol. 1973 Jun;110(6):1702–1705. [PubMed] [Google Scholar]
- Danielsson D., Kronvall G. Slide agglutination method for the serological identification of Neisseria gonorrhoeae with anti-gonococcal antibodies adsorbed to protein A-containing staphylococci. Appl Microbiol. 1974 Feb;27(2):368–374. doi: 10.1128/am.27.2.368-374.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria P., Smith E. W., Ingram D., Sade R. M., Gardner P. Hemophilus influenzae b pericarditis in children. Pediatrics. 1975 Nov;56(5):808–818. [PubMed] [Google Scholar]
- Edwards E. A., Hilderbrand R. L. Method for identifying Salmonella and Shigella directly from the primary isolation plate by coagglutination of protein A-containing staphylococci sensitized with specific antibody. J Clin Microbiol. 1976 Mar;3(3):339–343. doi: 10.1128/jcm.3.3.339-343.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E. A., Larson G. L. New method of grouping beta-hemolytic streptococci directly on sheep blood agar plates by coagglutination of specifically sensitized protein A-containing staphylococci. Appl Microbiol. 1974 Dec;28(6):972–976. doi: 10.1128/am.28.6.972-976.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin R. D., Stechenberg B. W., Chang M. J., Dunkle L. M., Wong M. L., Palkes H., Dodge P. R., Davis H. Prospective evaluation of treatment of Hemophilus influenzae meningitis. J Pediatr. 1976 Apr;88(4 Pt 1):542–548. doi: 10.1016/s0022-3476(76)80002-9. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- Ingram D. L., Anderson P., Smith D. H. Countercurrent immunoelectrophoresis in the diagnosis of systemic diseases caused by Hemophilus infleunzae type b. J Pediatr. 1972 Dec;81(6):1156–1159. doi: 10.1016/s0022-3476(72)80252-x. [DOI] [PubMed] [Google Scholar]
- Kronvall G. A rapid slide-agglutination method for typing pneumococci by means of specific antibody adsorbed to protein A-containing staphylococci. J Med Microbiol. 1973 May;6(2):187–190. doi: 10.1099/00222615-6-2-187. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Frommel D. Definition of staphylococcal protein A reactivity for human immunoglobulin G fragments. Immunochemistry. 1970 Jan;7(1):124–127. doi: 10.1016/0019-2791(70)90036-4. [DOI] [PubMed] [Google Scholar]
- Newman R. B., Stevens R. W., Gaafar H. A. Latex agglutination test for the diagnosis of Haemophilus influenzae meningitis. J Lab Clin Med. 1970 Jul;76(1):107–113. [PubMed] [Google Scholar]
- Smith E. W., Ingram D. L. Counterimmunoelectrophoresis in Hemophilus influenzae type b epiglottitis and pericarditis. J Pediatr. 1975 Apr;86(4):571–573. doi: 10.1016/s0022-3476(75)80151-x. [DOI] [PubMed] [Google Scholar]
- Wald E. R., Levine M. M. Frequency of detection of Hemophilus influenzae type b capsular polysaccharide in infants and children with pneumonia. Pediatrics. 1976 Feb;57(2):266–268. [PubMed] [Google Scholar]



